RESEARCH PAPER
Validation of a multi-analyte LC
–MS/MS method for screening
and quantification of 87 psychoactive drugs and their metabolites
in hair
Marco Fisichella&Luca Morini&Cristina Sempio&
Angelo Groppi
Received: 29 January 2014 / Revised: 6 March 2014 / Accepted: 14 March 2014 # Springer-Verlag Berlin Heidelberg 2014
Abstract A multi-analyte method for the detection and quan-tification of 87 psychoactive drugs (antidepressants, antipsy-chotics, benzodiazepines, and z-drugs) in human hair has been developed and fully validated using the liquid chromatogra-phy–tandem mass spectrometry system. Due to the remark-able increase in requests of hair sample tests (such as for driver’s license renewals, child custody, DFA cases, and post-mortem toxicology), we focused on the development of a rapid sample preparation. About 20 mg of hair samples, previously washed and cut into snippets, was ultrasonicated with 700μl of methanol. Samples were then directly analyzed using a 4000 QTRAP (AB SCIEX, Foster City, CA, USA) with an electrospray ionization (ESI) Turbo VTMIon Source. The validation criteria parameters were satisfactory and in accordance with the international guidelines. All the com-pounds tested were successfully detected. One important as-pect is the LODs in the low picogram per milligram concen-tration which may suggest a potential use of this method in cases of detection of single drug exposure. However, the LC– MS/MS method has been successfully applied for the analysis of postmortem cases (n=9).
Keywords Psychoactive drugs . Antidepressants . Benzodiazepines . Hair . LC–MS/MS
Introduction
Identification of drug exposure based on a hair test is of great interest in the forensic field especially due to the large window
of detection that the keratin matrix guarantees [1,2]. Drugs of abuse in hair like cocaine, opiates, amphetamines, amphet-amine derivatives, cannabis, and many others are routinely detected by laboratories of forensic medicine for many differ-ent purposes, such as driver’s license renewal, workplace drug testing, organ transplantation, child custody, and divorces [3–5]. In the last years, psychoactive drugs are also under investigation, and the requests for their detection in this alter-native matrix are extensively increasing [6–9]. In particular, benzodiazepine determination in hair is routinely requested by addiction service staff, in order to monitor patients under therapy. So far, there are not many screening procedures in the literature. Generally, only methods for the detection of a single substance [6–9], or a single class of drugs in hair [10–13], are developed and reported. Even when an ana-lytical approach that aims at identifying and quantifying different classes of pharmaceutical products and their me-tabolites is settled, a time-consuming sample preparation is needed [14–17]. The aim of this study was to fully validate a method for the screening and quantification in human hair samples of 87 drugs, among benzodiazepines, hypnotics, antidepressants, and antipsychotics, which are commonly prescribed in different therapies in Italy. The LC–MS/MS assay has proven to be useful not only for the potential detection of a single-dose drug exposure, assessed by the high analytical sensitivity, but also for chronic use monitoring, especially in drug users who are frequently poly-drug consumers. It is also our opinion that a multi-analyte method concerning the detection and quan-tification of psychoactive drugs could find other possible applications such as in clinical toxicology (for intoxication cases) and in psychiatry especially because new commer-cial drugs can be easily added to the method. Finally, an interesting case regarding long-term changes in psychoac-tive drug therapy is described.
M. Fisichella
:
L. Morini (*):
C. Sempio:
A. GroppiDepartment of Public Health, Experimental and Forensic Medicine, University of Pavia, via forlanini 12, 27100 Pavia, Italy
e-mail: luca.morini@unipv.it DOI 10.1007/s00216-014-7763-2
Material and methods Reagents
Eighty-seven analytes among benzodiazepines, antidepressants, antipsychotics, and their metabolites were analyzed. Amilsulpride, clozapine, quetiapine, and risperidone were purchased by Sandoz (Sandoz Industrial Products, Trento, Italy); amitriptyline, asenapine, halazepam SI, mianserin, mirtazapine, and perphena-zine were obtained by Merck & Co. (MSD Italia, Pavia, Italy); bromperidol, clomipramine, desipramine, desmethylclozapine, desmethylvenlafaxine, droperidol, duloxetine, hydroxyzine, imip-ramine, levomepromazine, meprobamate, olanzapine, paliperidone, phenelzine, pimozide, and trazodone were pur-chased by LGC Standards (Milan, Italy); buspirone was obtained by Menarini (Gruppo Menarini, Florence, Italy); chlorpromazine, fluoxetine, and haloperidol were purchased by Lusofarmaco ( G r u p p o M e n a r i n i , M i l a n , I t a l y ) ; c i t a l o p r a m , desmethylcitalopram, fluphenazine, nortriptyline, and protryptiline were obtained by Lundbeck (Lundbeck Italia SPA, Milan, Italy); clothiapine, dibenzepin, maprotiline, trimipramine, and veralipride were purchased by Novartis (Novartis Farma, Varese, Italy); dixyrazine and tranylcypromine were obtained by Laboratorio Farmacuetico S.I.T. (Pavia, Italy); dothiepin, levosulpiride, pericyazine, sultopride, and tiapride were purchased by Teofarma (Pavia, Italy); fluvoxamine was obtained by Duphar B.V. (Solvay, Weesp, Holland); paroxetine was purchased by SmithKline Bee-cham Pharmaceuticals (GSK, Verona, Italy); promazine was ob-tained by Pierrel (Pierrel SPA, Milan, Italy); reboxetine, sertraline, venlafaxine, and ziprasidone were purchased by Pfizer Roerig (Pfizer SPA, Milan, Italy). Alprazolam, bromazepam, camazepam, chlordiazepoxide, clobazepam, clonazepam, 7-amino-clonazepam, demoxepam, diazepam, desmethyldiazepam, flunitrazepam, 7-amino-flunitrazepam, flurazepam, α-hydroxy-ethylflurazepam, desalkylflurazepam, lorazepam, lormetazepam, medazepam, midazolam, nitrazepam, oxazepam, prazepam, temazepam, α-hydroxy-triazolam, triazolam, zolpidem, and zopiclone were purchased by Lipomed (Nova Chimica, Milan, Italy); clotiazepam, estazolam, and etizolam were obtained by Formenti (Formenti SPA, Milan, Italy); brotizolam and chlordesmethyldiazepam were purchased by Ravizza (Ravizza Farmaceutici SPA, Milan, Italy); and ketazolam and pinazepam were obtained by Ciba Geigy (Basel, Switzerland). Water was purified by filtering deionized water on a Milli-Q Simplicity 185 filtration system from Millipore (Bedford, MA, USA). Formic acid for mass spectrometry was obtained from Sigma-Aldrich (St. Louis, MI, USA). HPLC-grade methanol and acetonitrile were purchased from Mallinkrodt Baker (Milan, Italy).
Instrumentation
LC–MS/MS analyses were performed with an Agilent 1100– 1200 Series system (Agilent Technologies, Palo Alto, CA,
USA) interfaced to a 4000 QTRAP (AB SCIEX, Foster City, CA, USA) with an electrospray ionization (ESI) Turbo VTM Ion Source. The LC instrumentation was composed of a vacuum degasser, a binary pump, an isocratic pump, and an autosampler maintained at 4 °C. The injector needle was externally washed with methanol prior to any injection. A Hypersil GOLD column (100 mm, 2.1 mm i.d., 3 mm particle size) (Thermo Scientific, MI, Italy) was kept at 25 °C during the analysis. The mobile phase consisted of formic acid 0.1 % (A) and methanol (B). Chromatographic gradient elution was the following: constant flow of 0.2 ml/min; 95 % phase A maintained for 2.5 min, then decreased up to 30 % A in 0.5 min and successively declined to 5 % A in 2.5 min, maintained at 5 % A for 7 min and re-equilibrated for 8 min.
The 87 substances monitored were then divided into four groups (antidepressants, antipsychotics, and two subgroups for benzodiazepines). Each sample was then injected four times, in order to maintain a high analytical sensitivity. The ESI source settings were ion spray voltage, +5,500 V; source temperature, 350 °C; and nebulization and heating gas (air), 20 and 25, respectively. Multiple reaction monitoring was optimized using nitrogen as collision gas (with pressure set at level 8) and a dwell time of 30 ms. Two transitions for each substance were chosen for identification; the most intense was used for quantifi-cation purposes. All the transitions and MS parame-ters are listed in Table 1. Data acquisition and elab-oration were performed by the Analyst software (ver-sion 1.5, AB SCIEX).
Sample treatment Procedure
About 20 mg of hair was washed twice with organic solvent (dichloromethane, methanol), taken to dryness under a nitrogen stream, and cut into small pieces. Then, 20 μl of halazepam (I.S. 100 ng/ml) was added to the sample, together with 700 μl of methanol. After 1 h of ultrasonication, 5 μl was directly injected in the LC–MS/MS system.
Calibration standards and quality control samples Standards were prepared by dissolution of each com-pound in methanol at the concentration of 1 mg/ml. Working solutions were prepared in methanol at five different concentrations ranging from the limit of quan-tification (LOQ) to 1,000 ng/ml by independent dilution.
QC samples were prepared by a different operator by independent dilution at three concentration levels: LOQ,
20 ng/ml, and 100 ng/ml. All standard solutions were stored at −20 °C.
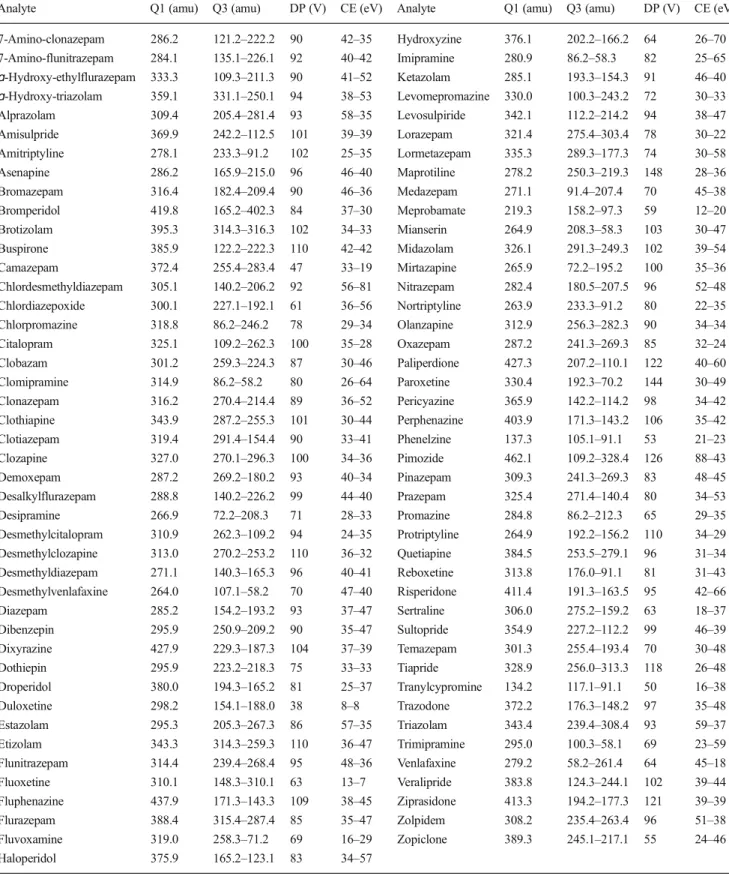
Table 1 MRM parameters
Analyte Q1 (amu) Q3 (amu) DP (V) CE (eV) Analyte Q1 (amu) Q3 (amu) DP (V) CE (eV)
7-Amino-clonazepam 286.2 121.2–222.2 90 42–35 Hydroxyzine 376.1 202.2–166.2 64 26–70 7-Amino-flunitrazepam 284.1 135.1–226.1 92 40–42 Imipramine 280.9 86.2–58.3 82 25–65 ɑ-Hydroxy-ethylflurazepam 333.3 109.3–211.3 90 41–52 Ketazolam 285.1 193.3–154.3 91 46–40 ɑ-Hydroxy-triazolam 359.1 331.1–250.1 94 38–53 Levomepromazine 330.0 100.3–243.2 72 30–33 Alprazolam 309.4 205.4–281.4 93 58–35 Levosulpiride 342.1 112.2–214.2 94 38–47 Amisulpride 369.9 242.2–112.5 101 39–39 Lorazepam 321.4 275.4–303.4 78 30–22 Amitriptyline 278.1 233.3–91.2 102 25–35 Lormetazepam 335.3 289.3–177.3 74 30–58 Asenapine 286.2 165.9–215.0 96 46–40 Maprotiline 278.2 250.3–219.3 148 28–36 Bromazepam 316.4 182.4–209.4 90 46–36 Medazepam 271.1 91.4–207.4 70 45–38 Bromperidol 419.8 165.2–402.3 84 37–30 Meprobamate 219.3 158.2–97.3 59 12–20 Brotizolam 395.3 314.3–316.3 102 34–33 Mianserin 264.9 208.3–58.3 103 30–47 Buspirone 385.9 122.2–222.3 110 42–42 Midazolam 326.1 291.3–249.3 102 39–54 Camazepam 372.4 255.4–283.4 47 33–19 Mirtazapine 265.9 72.2–195.2 100 35–36 Chlordesmethyldiazepam 305.1 140.2–206.2 92 56–81 Nitrazepam 282.4 180.5–207.5 96 52–48 Chlordiazepoxide 300.1 227.1–192.1 61 36–56 Nortriptyline 263.9 233.3–91.2 80 22–35 Chlorpromazine 318.8 86.2–246.2 78 29–34 Olanzapine 312.9 256.3–282.3 90 34–34 Citalopram 325.1 109.2–262.3 100 35–28 Oxazepam 287.2 241.3–269.3 85 32–24 Clobazam 301.2 259.3–224.3 87 30–46 Paliperdione 427.3 207.2–110.1 122 40–60 Clomipramine 314.9 86.2–58.2 80 26–64 Paroxetine 330.4 192.3–70.2 144 30–49 Clonazepam 316.2 270.4–214.4 89 36–52 Pericyazine 365.9 142.2–114.2 98 34–42 Clothiapine 343.9 287.2–255.3 101 30–44 Perphenazine 403.9 171.3–143.2 106 35–42 Clotiazepam 319.4 291.4–154.4 90 33–41 Phenelzine 137.3 105.1–91.1 53 21–23 Clozapine 327.0 270.1–296.3 100 34–36 Pimozide 462.1 109.2–328.4 126 88–43 Demoxepam 287.2 269.2–180.2 93 40–34 Pinazepam 309.3 241.3–269.3 83 48–45 Desalkylflurazepam 288.8 140.2–226.2 99 44–40 Prazepam 325.4 271.4–140.4 80 34–53 Desipramine 266.9 72.2–208.3 71 28–33 Promazine 284.8 86.2–212.3 65 29–35 Desmethylcitalopram 310.9 262.3–109.2 94 24–35 Protriptyline 264.9 192.2–156.2 110 34–29 Desmethylclozapine 313.0 270.2–253.2 110 36–32 Quetiapine 384.5 253.5–279.1 96 31–34 Desmethyldiazepam 271.1 140.3–165.3 96 40–41 Reboxetine 313.8 176.0–91.1 81 31–43 Desmethylvenlafaxine 264.0 107.1–58.2 70 47–40 Risperidone 411.4 191.3–163.5 95 42–66 Diazepam 285.2 154.2–193.2 93 37–47 Sertraline 306.0 275.2–159.2 63 18–37 Dibenzepin 295.9 250.9–209.2 90 35–47 Sultopride 354.9 227.2–112.2 99 46–39 Dixyrazine 427.9 229.3–187.3 104 37–39 Temazepam 301.3 255.4–193.4 70 30–48 Dothiepin 295.9 223.2–218.3 75 33–33 Tiapride 328.9 256.0–313.3 118 26–48 Droperidol 380.0 194.3–165.2 81 25–37 Tranylcypromine 134.2 117.1–91.1 50 16–38 Duloxetine 298.2 154.1–188.0 38 8–8 Trazodone 372.2 176.3–148.2 97 35–48 Estazolam 295.3 205.3–267.3 86 57–35 Triazolam 343.4 239.4–308.4 93 59–37 Etizolam 343.3 314.3–259.3 110 36–47 Trimipramine 295.0 100.3–58.1 69 23–59 Flunitrazepam 314.4 239.4–268.4 95 48–36 Venlafaxine 279.2 58.2–261.4 64 45–18 Fluoxetine 310.1 148.3–310.1 63 13–7 Veralipride 383.8 124.3–244.1 102 39–44 Fluphenazine 437.9 171.3–143.3 109 38–45 Ziprasidone 413.3 194.2–177.3 121 39–39 Flurazepam 388.4 315.4–287.4 85 35–47 Zolpidem 308.2 235.4–263.4 96 51–38 Fluvoxamine 319.0 258.3–71.2 69 16–29 Zopiclone 389.3 245.1–217.1 55 24–46 Haloperidol 375.9 165.2–123.1 83 34–57
Validation
The method was initially tested for sensitivity. Limits of detection (LOD) and LOQ were measured by evaluating the signal/noise (S/N) ratio of three replicates for each compound at proper concentrations. LOD was fixed at the concentration with a S/N>3 while concentrations of analytes with a S/N>10 were chosen as LOQ.
Fifteen blank hair samples from ten adults and five children were analyzed for possibly interfering peaks during the early validation phase of the method. Linearity was evaluated through the analysis of five replicates for each calibration level. The calibration curve was estimated by least-squares regression.
Intra-day imprecision, expressed as relative standard devi-ation (RSD), was calculated analyzing the QC samples (20/ LOQ and 100 pg/mg) in five replicates, while inter-day pre-cision was measured analyzing the QC samples in duplicate on five different days over a 3-week period. The concentration of the analytes in the QC samples was calculated versus the daily calibration curves. Accuracy was determined for all analytes as the percentage deviation of the average of results from the corresponding nominal value.
Five different blank hair samples and five methanolic so-lutions were spiked at two levels (20/LOQ and 100 pg/mg), processed separately with the described procedure, and the absolute peak areas were compared. Experiments were carried out in triplicate. Results were calculated as the percentage of the mean deviation of drug response in hair samples from the response measured in methanol at the same concentration level. Matrix effects were then expressed as ion suppression or enhancement.
Several postmortem hair samples of known diazepam users were collected together, and a homogeneous sample of washed and cut hair was created. This sample was analyzed over a 5-week period to monitor reproducibility and robust-ness of the method.
Application on real samples
The procedure was applied to healthy volunteers and to pa-tients under treatment with psychoactive drugs who have given an informed consent before sample collection, as well as to four different hair samples collected from autopsy cases. For healthy volunteers, 15 hairs were analyzed. Six were collected among the authors’ colleagues and friends. All the subjects did not consume any psychoactive substances during the 9 months before sample collection. The 9-cm proximal hair segment was used for the analysis. Nine samples were collected from the patients under pharmacological treatment. All the substances consumed in the last 9 months were regis-tered, and the 9-cm proximal segment was submitted to the
analytical procedure. In one case, hair was divided in three aliquots of 3 cm length. Finally, analyses of four hair samples collected from autopsy cases were carried out.
Results and discussion
A multi-analyte method in hair for simultaneous screening and quantification of psychoactive drugs frequently pre-scribed in Italy was developed by LC–MS/MS and fully validated according to international guidelines [18,19]. Spe-cifically, 87 substances were identified through one extraction procedure and the direct injection of the methanolic solution using the same chromatographic conditions over four consec-utive injections. The liquid chromatographic system is equipped with a secondary isocratic pump and a Valco valve. This is not used during routine analysis, but it could be of interest in case of a great batch of samples. In fact, using a secondary isocratic pump, the run time of a single injection lasted 12 min, allowing to save the column equilibration and to obtain a total run time of 48 min, as already observed in previously published methods [20].
During the development of the method, zuclopenthixol and flupenthixol have also been evaluated, but they did not fulfill the validation acceptance criteria, and they have been re-moved from the validation procedure, but still remaining on the list of transitions detected. Olanzapine provided a good sensitivity and specificity, but it is rather stable on the solution and was validated only for qualitative purposes. The quantifi-cation for this molecule is performed, whenever necessary, using an ad hoc method. The phenothiazine group generally provided the worst sensitivity, mainly due to the bad chro-matographic retention. However, all the validation parameters were within the acceptance range, and they were maintained in the list of the detected compounds. All the LODs and LOQs are listed in Table2. The method showed a very high sensi-tivity for the majority of the molecules evaluated and provided LODs generally lower than those previously published by other authors [15,21].
The procedure was validated using a five-point calibration curve ranging from LOQ to 1,000 pg/mg. A good linearity was assessed by a regression coefficient always higher than 0.99. A weighted regression (1/X) was used to calculate the concentrations. Accuracy and imprecision were measured at two different quality control levels (20 pg/mg, except for the molecules with higher LOQ, and 100 pg/mg) and fulfilled the acceptance criteria. Ion suppression and enhancement were found to be negligible for all the compounds. The quantitative determination of diazepam in a real pool sample was found to be reproducible over a period of 5 weeks.
The method was entirely validated using halazepam as internal standard. Halazepam is no more prescribed and used in Italy; therefore, there is no chance to have false-positive and
real interferences. However, before any calibration curve, the blank hair samples were tested for the presence of this
substance. Recently, we have purchased d5-diazepam, and we are using both the compounds as internal standards.
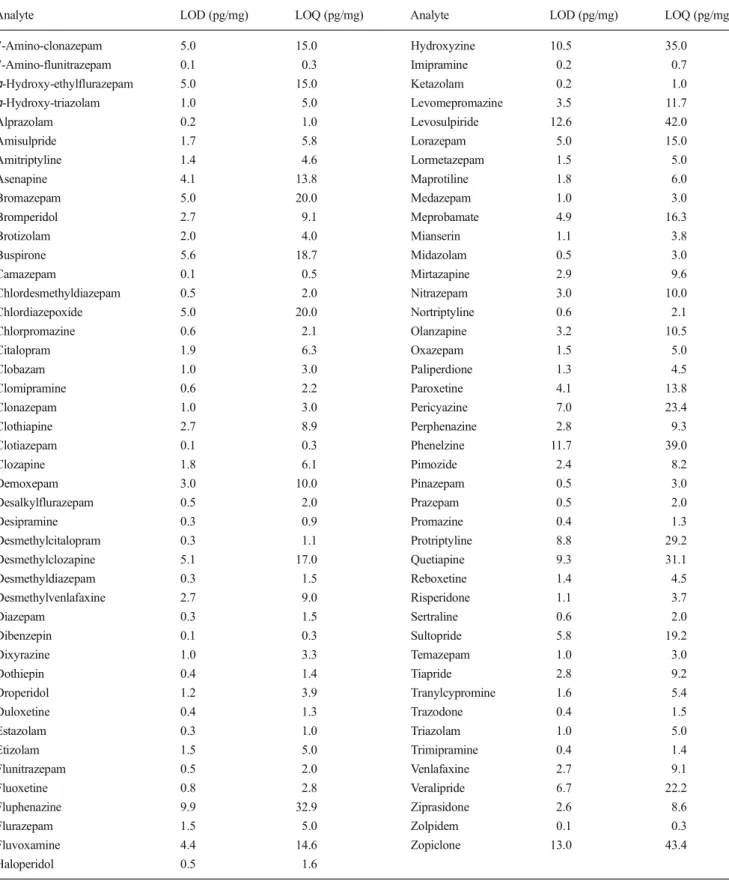
Table 2 Limits of detection and limits of quantification
Analyte LOD (pg/mg) LOQ (pg/mg) Analyte LOD (pg/mg) LOQ (pg/mg)
7-Amino-clonazepam 5.0 15.0 Hydroxyzine 10.5 35.0 7-Amino-flunitrazepam 0.1 0.3 Imipramine 0.2 0.7 ɑ-Hydroxy-ethylflurazepam 5.0 15.0 Ketazolam 0.2 1.0 ɑ-Hydroxy-triazolam 1.0 5.0 Levomepromazine 3.5 11.7 Alprazolam 0.2 1.0 Levosulpiride 12.6 42.0 Amisulpride 1.7 5.8 Lorazepam 5.0 15.0 Amitriptyline 1.4 4.6 Lormetazepam 1.5 5.0 Asenapine 4.1 13.8 Maprotiline 1.8 6.0 Bromazepam 5.0 20.0 Medazepam 1.0 3.0 Bromperidol 2.7 9.1 Meprobamate 4.9 16.3 Brotizolam 2.0 4.0 Mianserin 1.1 3.8 Buspirone 5.6 18.7 Midazolam 0.5 3.0 Camazepam 0.1 0.5 Mirtazapine 2.9 9.6 Chlordesmethyldiazepam 0.5 2.0 Nitrazepam 3.0 10.0 Chlordiazepoxide 5.0 20.0 Nortriptyline 0.6 2.1 Chlorpromazine 0.6 2.1 Olanzapine 3.2 10.5 Citalopram 1.9 6.3 Oxazepam 1.5 5.0 Clobazam 1.0 3.0 Paliperdione 1.3 4.5 Clomipramine 0.6 2.2 Paroxetine 4.1 13.8 Clonazepam 1.0 3.0 Pericyazine 7.0 23.4 Clothiapine 2.7 8.9 Perphenazine 2.8 9.3 Clotiazepam 0.1 0.3 Phenelzine 11.7 39.0 Clozapine 1.8 6.1 Pimozide 2.4 8.2 Demoxepam 3.0 10.0 Pinazepam 0.5 3.0 Desalkylflurazepam 0.5 2.0 Prazepam 0.5 2.0 Desipramine 0.3 0.9 Promazine 0.4 1.3 Desmethylcitalopram 0.3 1.1 Protriptyline 8.8 29.2 Desmethylclozapine 5.1 17.0 Quetiapine 9.3 31.1 Desmethyldiazepam 0.3 1.5 Reboxetine 1.4 4.5 Desmethylvenlafaxine 2.7 9.0 Risperidone 1.1 3.7 Diazepam 0.3 1.5 Sertraline 0.6 2.0 Dibenzepin 0.1 0.3 Sultopride 5.8 19.2 Dixyrazine 1.0 3.3 Temazepam 1.0 3.0 Dothiepin 0.4 1.4 Tiapride 2.8 9.2 Droperidol 1.2 3.9 Tranylcypromine 1.6 5.4 Duloxetine 0.4 1.3 Trazodone 0.4 1.5 Estazolam 0.3 1.0 Triazolam 1.0 5.0 Etizolam 1.5 5.0 Trimipramine 0.4 1.4 Flunitrazepam 0.5 2.0 Venlafaxine 2.7 9.1 Fluoxetine 0.8 2.8 Veralipride 6.7 22.2 Fluphenazine 9.9 32.9 Ziprasidone 2.6 8.6 Flurazepam 1.5 5.0 Zolpidem 0.1 0.3 Fluvoxamine 4.4 14.6 Zopiclone 13.0 43.4 Haloperidol 0.5 1.6
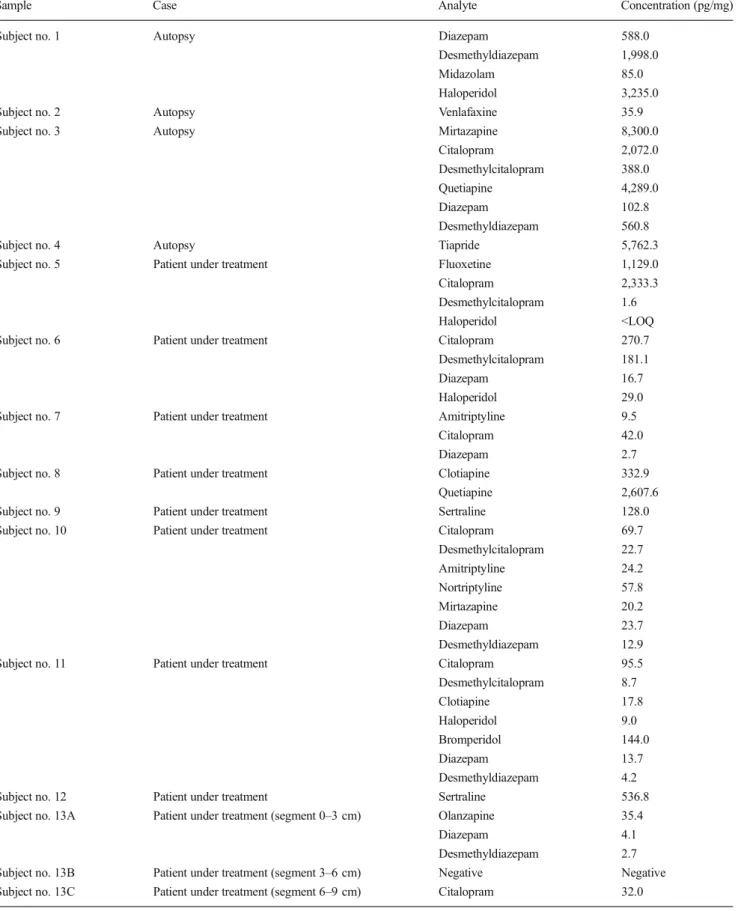
Table 3 Concentrations measured in real samples
Sample Case Analyte Concentration (pg/mg)
Subject no. 1 Autopsy Diazepam 588.0
Desmethyldiazepam 1,998.0
Midazolam 85.0
Haloperidol 3,235.0
Subject no. 2 Autopsy Venlafaxine 35.9
Subject no. 3 Autopsy Mirtazapine 8,300.0
Citalopram 2,072.0
Desmethylcitalopram 388.0
Quetiapine 4,289.0
Diazepam 102.8
Desmethyldiazepam 560.8
Subject no. 4 Autopsy Tiapride 5,762.3
Subject no. 5 Patient under treatment Fluoxetine 1,129.0
Citalopram 2,333.3
Desmethylcitalopram 1.6
Haloperidol <LOQ
Subject no. 6 Patient under treatment Citalopram 270.7
Desmethylcitalopram 181.1
Diazepam 16.7
Haloperidol 29.0
Subject no. 7 Patient under treatment Amitriptyline 9.5
Citalopram 42.0
Diazepam 2.7
Subject no. 8 Patient under treatment Clotiapine 332.9
Quetiapine 2,607.6
Subject no. 9 Patient under treatment Sertraline 128.0
Subject no. 10 Patient under treatment Citalopram 69.7
Desmethylcitalopram 22.7 Amitriptyline 24.2 Nortriptyline 57.8 Mirtazapine 20.2 Diazepam 23.7 Desmethyldiazepam 12.9
Subject no. 11 Patient under treatment Citalopram 95.5
Desmethylcitalopram 8.7 Clotiapine 17.8 Haloperidol 9.0 Bromperidol 144.0 Diazepam 13.7 Desmethyldiazepam 4.2
Subject no. 12 Patient under treatment Sertraline 536.8
Subject no. 13A Patient under treatment (segment 0–3 cm) Olanzapine 35.4
Diazepam 4.1
Desmethyldiazepam 2.7
Subject no. 13B Patient under treatment (segment 3–6 cm) Negative Negative
The procedure was applied to several hair samples, collect-ed from autopsies, healthy volunteers, and the patients under pharmacological treatment. The four postmortem cases regarded subjects that were under therapeutic treatment with
psychoactive substances before death, and their blood samples provided positive results. The first hair sample was collected from a woman, 47 years old, who was suffering from anxiety and psychosis and who committed suicide by precipitation.
Benzodiazepines and haloperidol were found in the keratin matrix. The second case regarded a man, 33 years old, found dead after a fatal intoxication with methadone and heroin.
Venlafaxine was detected in hair. The third postmortem case concerned a man, 52 years old, found dead in his apartment, apparently from natural causes, and that provided positive
results for several psychoactive substances. The fourth sample was collected from a man, 40 years old, found dead after a fatal alcohol intoxication; hair tested positive for tiapride. All
the 15 hair samples collected from volunteers provided nega-tive results. All the psychoacnega-tive drugs used by nine patients under treatment were identified in hair samples. All the
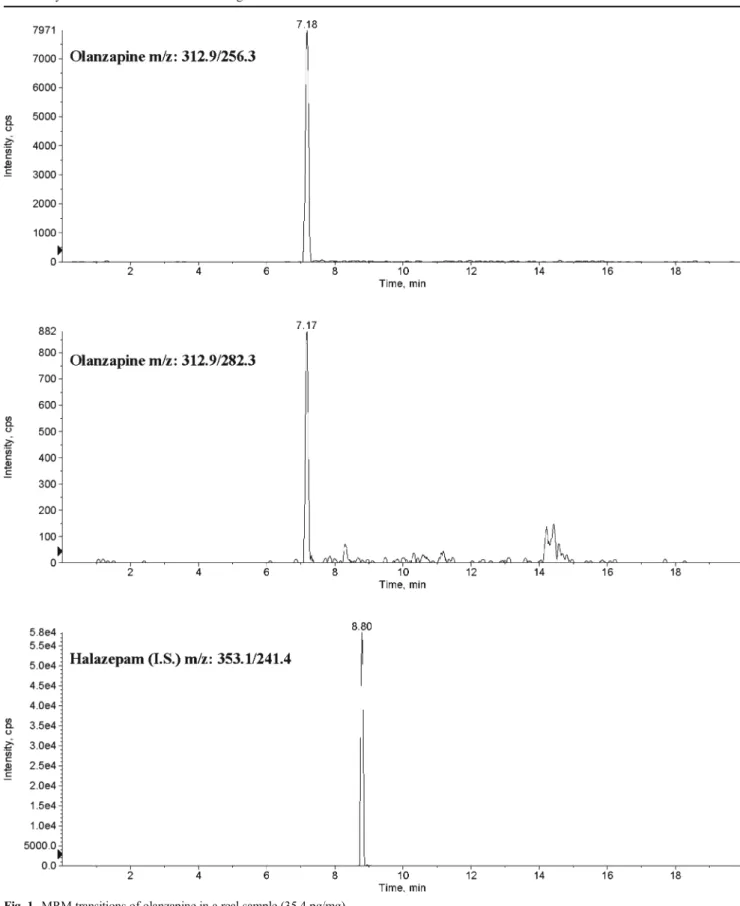
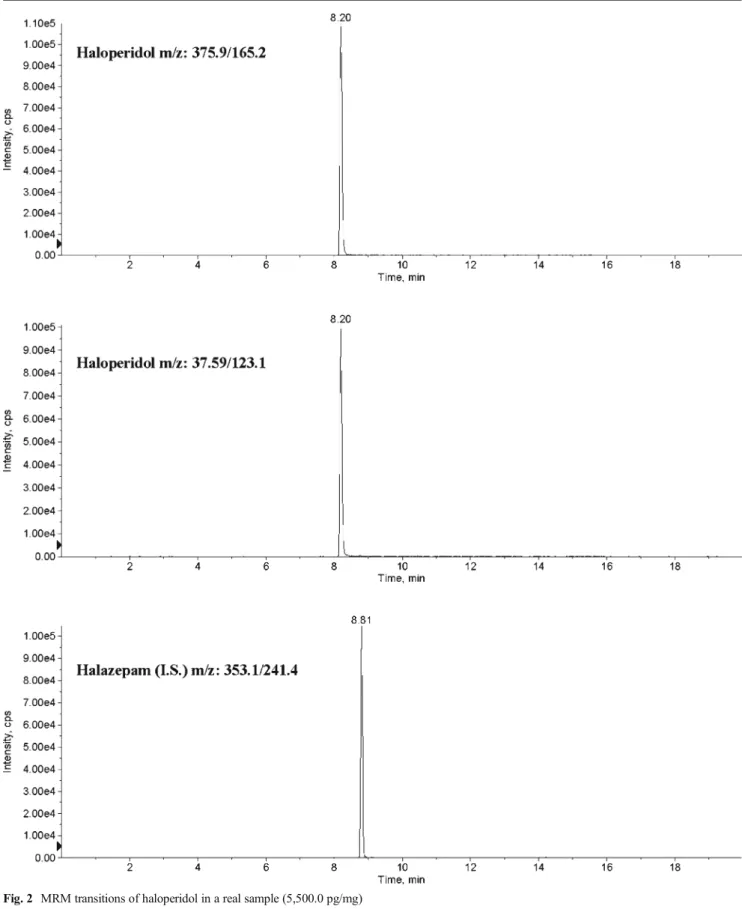
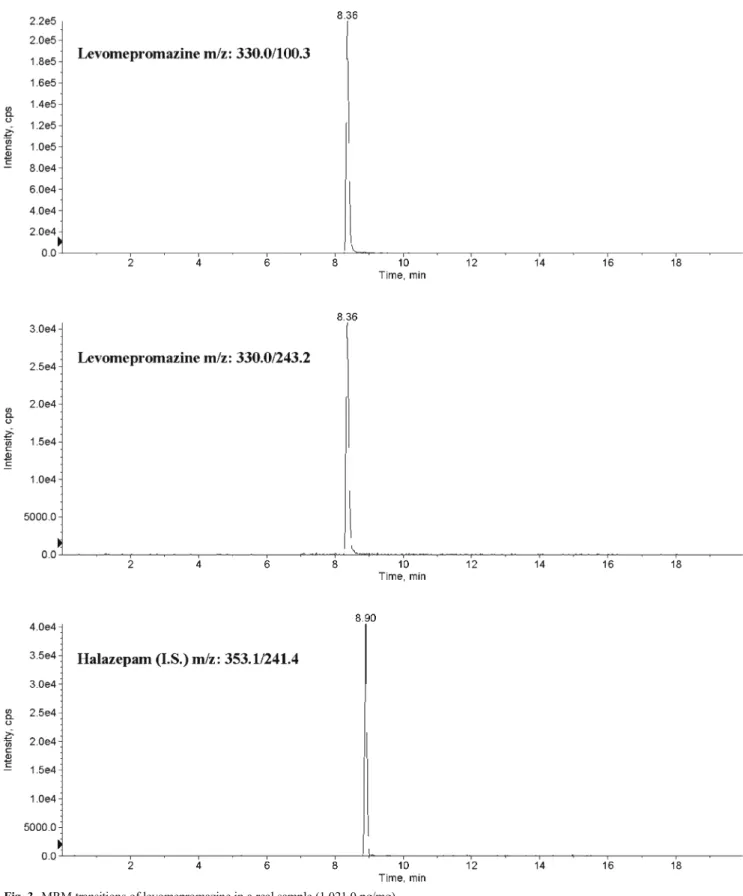
molecules and concentrations measured are listed in Table3. Of considerable interest is the case reported as subject no. 13. This patient declared that he has been under citalopram ther-apy until 5 months before sample collection, but after a period of abstinence, not properly specified, he has been taking olanzapine and diazepam within the 3 months before sample collection. Hair samples were cut to 9 cm proximal hair segment and then divided in three segments of 3-cm length. The results assessed the patient’s changing of therapy. Figures1,2, and3show (MRM) transitions of olanzapine in real samples.
Conclusion
The LC–MS/MS method here presented allows the iden-tification and quaniden-tification of 87 psychoactive drugs in hair. The assay was successfully developed and fully validated. The direct injection of the sample after one single extraction step has been proven to be the best in the evaluation of a therapeutic use of these medica-ments. The procedure was successfully applied to hair collected from autopsy samples and from the patients under treatment, where all the substances prescribed were easily detected. Due to the high sensitivity of the method, it is our opinion that it also could be used in cases of drug-facilitated crime related with the use of these substances efficient to incapacitate the victim. Referring to these specific cases, a future perspective would be to develop a screening method in keratin matrix including drugs of abuse and other molecules of potential interest in the forensic field.
References
1. Nielsen MK, Johansen SS, Linnet K (2014) Forensic Sci Int 234:16–21 2. Pichini S, Gottardi M, Marchei E, Svaizer F, Pellegrini M, Rotolo
MC, Algar OG, Pacifici R (2013) Clin Chem Lab Med 28:1–8 3. Pragst F, Broecker S, Hastedt M, Herre S, Andresen-Streichert H,
Sachs H, Tsokos M (2013) Ther Drug Monit 35:737–752
4. Vignali C, Stramesi C, Morini L, Pozzi F, Collo G, Groppi A (2013) Drug Test Anal 5:208–212
5. Stramesi C, Polla M, Vignali C, Zucchella A, Groppi A (2008) Forensic Sci Int 176:34–37
6. Frison G, Favretto D, Vogliardi S, Terranova C, Ferrara SD (2008) Ther Drug Monit 30:467–473
7. Favretto D, Stocchero G, Vogliardi S, Frison G, Trevisanuto D, Castagna F, Ferrara SD (2010) Ther Drug Monit 32:30–39 8. Kintz P (2013) Drug Test Anal doi:10.10027dta.1596
9. Laloup M, Fernandez Mdel M, Wood M, Maes V, De Boeck G, Vanbeckevoort Y, Samyn N (2007) Anal Bioanal Chem 388:1545–1556 10. Morini L, Vignali C, Polla M, Sponta A, Groppi A (2012) Forensic
Sci Int 218:53–6
11. Rust KY, Baumgartner MR, Meggiolaro N, Kraemer T (2012) Forensic Sci Int 215:64–72
12. Villain M, Concheiro M, Cirimele C, Kintz P (2005) J Chromatogr B Anal 825:72–78
13. Kim J, Lee S, In S, Choi H, Chung H (2011) J Chromatogr B Anal Technol Biomed Life Sci 879:876–878
14. Müller C, Vogt S, Goerke R, Kordon A, Weinmann W (2000) Forensic Sci Int 113:415–421
15. Lendoiro E, Quintela O, de Castro A, Cruz A, Lopez-Rivadulla M, Concheiro M (2012) Forensic Sci Int 217:207–215
16. Montesano C, Johansen SS, Klose Nielsen MK (2014) J Pharm Biomed Anal 87:295–306
17. Maurer HH (2007) Anal Bioanal Chem 387:1315–1325
18. Peters FT, Drummer OH, Musshoff F (2007) Forensic Sci Int 165: 216–224
19. Peters FT (2006) Applications of liquid chromatography-mass spec-trometry in toxicology. Pharmaceutical Press, London
20. Politi L, Morini L, Polettini A (2007) Clin Chim Acta 386:46–52 21. Klose Nielsen MK, Johansen SS, Dalsgaard PW, Linnet K (2010)