1. Title Page Article category: Original article Article title:
Effect of premature serum progesterone rise on embryo transfer outcomes and the role of blastocyst culture and transfer in assisted reproductive technology cycles with premature progesterone rise
Authors:
Pei-Chen Huang a, b, 1, Ming-Jer Chen a, c, 1 *, Hwa-Fen Guu a, Yu-Chiao Yi a, Jason Yen-Ping Ho a,
Ya-Fang Chen a, Li-Yu Chen a, Min-Min Chou a
Author affiliations:
a Division of Reproductive Medicine, Department of Obstetrics and Gynecology, Taichung
Veterans General Hospital, Taiwan
b School of Medicine, China Medical University, Taichung, Taiwan c School of Medicine, National Yang-Ming University, Taipei, Taiwan 1 These authors contributed equally as first authors for this article.
*Corresponding author: Dr. Ming-Jer Chen
Department of Obstetrics and Gynecology, Taichung Veterans General Hospital 1650, Taiwan Boulevard, Sec. 4, Taichung, Taiwan 40705, ROC
Tel: 886-4-23592525 ext. 5801, 5836 Fax: 886-4-23503021 E-mail: mingjer_chen@yahoo.com.tw 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
2. Abstract Objective:
In 1991, researchers reported that a modest pre-ovulatory increase in serum progesterone levels is associated with lower pregnancy rates and higher incidence of pregnancy loss in in vitro fertilization (IVF).
We wonder whether embryo transfer (ET) in assisted reproductive technology (ART) cycles in patients with premature progesterone rise (PPR) have a negative impact on the clinical pregnancy rates (CPRs) and/or live birth rates (LBRs) in our series. Consequently, will blastocyst transfer reverse the negative impact?
Materials and methods:
This non-interventional, retrospective, observational tertiary center study was conducted between January 2010 and December 2012. All fresh ET cycles with serum progesterone levels measured (n= 599) on the day of hCG administration were analyzed.
Results:
Sera LH, E2, and P were measured and analyzed. The CPRs of cycles in patients with P≤ 1.5 ng/ml (low) versus those with P> 1.5 ng/ml (high) were 37.04% versus 41.03% (OR: 1.18, 95% CI: 0.728-1.920; P= 0.50). The LBRs of cycles in patients with low progesterone level versus those with PPR were 30.52% versus 34.62% (OR: 1.21, 95% CI: 0.729-1.992; P= 0.47). No statistically significant association was detected. We further analyzed the outcomes according to different stages ET and found that blastocyst (D5) ET significantly increase the LBRs as compare to cleavage stage (D2/D3) ET in the PPR group (44.44% versus 21.43%; P= 0.043).
Conclusion:
PPR did not significantly compromise the clinical outcomes in this series. However, shifting to blastocyst transfer probably could increase the live birth in cycles with PPR.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24
3. Key words
assisted reproductive technology, blastocyst transfer, clinical pregnancy rates, live birth rates, premature progesterone rise
1 2 3
4. Introduction
Many researchers have adopted the term ‘premature progesterone rise (PPR)’ or ‘premature luteinization (PL)’ for patients with progesterone elevation on the day of human chorionic gonadotropin (hCG) administration for final oocyte maturation [1]. In 1991, Schoolcraft et al. [2] and Silverberg et al. [3] reported that a modest pre-ovulatory increase in serum progesterone levels was associated with lower pregnancy rates and higher incidence of pregnancy loss in ovarian stimulation for IVF, but the pathogenesis and effects of PPR or PL on IVF outcomes remain controversial.
Several authors have failed to demonstrate any negative impact of PPR on ART outcomes [4-10] while others reported that pregnancy rates [11-13] or live birth rates [14] have been inversely related to serum progesterone levels or duration of elevation [15] on the day of hCG administration.
Furthermore, Ou et al. [16] suggested that ovarian response or reserve may be of critical importance when considering PL or PPR. However, Xu et al. [12] reported that elevated serum progesterone had no adverse effect on pregnancy rates in fresh embryo-transfer cycles within different ovarian responses.
Papanikolaou et al. [17] and Ochsenkuhn et al. [14] concluded that blastocyst transfer (D5) was more effective than early cleavage-stage embryo transfer (D2/D3) for improving pregnancy rates and live birth rates.
The purpose of the present study is to review from our own series the impacts of premature elevated serum progesterone levels on the pregnancy outcomes of fresh embryo transfer cycles. We also wanted to find out whether the ovarian responses play a role in these phenomena. Furthermore, we retrospectively investigated whether D5 blastocyst transfer (D5-ET) could improve the clinical outcomes, both in clinical pregnancy rates (CPRs) and live birth rates (LBRs). 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24
5. Materials and Methods Trial Design
This non-interventional, retrospective, observational tertiary center study, which enrolled women treated for infertility, was conducted in the Center for Reproductive Medicine of Taichung Veterans General Hospital between January 2010 and December 2012.
Participants
A total of 777 cycles undergoing assisted reproduction (in vitro fertilization [IVF] and/or intracytoplasmic sperm injection [ICSI]) with controlled ovarian hyperstimulation (COH), suppression of premature ovulation by GnRH agonists [leuprolide acetate or triptorelin acetate (50.08%)], antagonists [cetrotide (34.06%)] or other protocols [including mild stimulation, natural cycle or modified natural cycle (15.86%)], were included for chart review in the study period. Because we did not routinely check pre-ovulatory progesterone levels until August 2010, there were 69 cycles been excluded initially. We also excluded cases (109 cycles) which did not receive fresh ET. The reasons included 27 cycles of cancelled oocyte retrieval, 24 cycles of fertilization failure, 10 cycles of very poor embryos development, 31 cycles of planned oocytes and/or embryos cryopreservation, and 17 cycles of postponement for ET due to very high serum E2 (>8000 pg/ml) and/or risk of ovarian hyperstimulation syndrome. Finally a total of 599 non-selective fresh ET cycles with serum progesterone levels measured on the day of hCG injection were analyzed.
The mean age of patients was 35.21 years (range 23-49 years). The primary or combined indications for fertility treatment were male subfertility (29.33%), tubal pathology (24.52%), endometriosis (15.63%), polycystic ovarian syndrome (PCOS) (2.40%), adenomyosis (1.68%) and other causes (0.48%), including malignancy or immunology. Detailed patient characteristics with different progesterone levels are listed in Table I.
All patients signed a written informed consent document for the ART treatment. Institutional review board approval was not mandatory, because all women in the study underwent the routine 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
IVF/ICSI-ET clinical treatment performed in our unit and no additional intervention or blood sampling was performed.
Controlled Ovarian Hyperstimulation Protocol
Briefly, patients in the GnRH agonist group received either leuprolide acetate (Leuprolide, 0.1 mg/d; Famar L’Aigle) or triptorelin acetate (Decapeptyl SR, 0.1 mg/d; Ipsen Pharmaceuticals, Ltd.), consisting of a daily low dose of GnRHa, subcutaneously administrated for at least 10 days before the onset of ovarian stimulation. On the other hand, participants in the antagonist group received the GnRH antagonist cetrorelix acetate (Cetrotide, 0.25 mg/d SC; Merck Serono) starting flexibly on stimulation days 5 ~ 7 by ultrasound monitoring 5 days after the onset of COH with gonadotrophins.
The types and dosages of gonadotropin administration were individualized by the attending physician for each participant according to her age, body mass index, antimullerian hormone level, FSH level/antral follicle counts on cycle day 2~3 and previous response to ovarian stimulation. Doses were adjusted according to ovarian response as monitored by means of vaginal ultrasound folliculometry and serum E2 level testing.
When two or more follicles reached a mean diameter of 18 mm, 10,000 IU of hCG (Pregnyl; Organon) or 500 ug of recombinant hCG (Ovidrel; Merck-Serono) was injected for the oocyte retrieval 35~36 hours later. Progesterone 25mg/amp, 1-2 amp/day (Astar Co.), was injected intramuscularly starting from the day of oocyte retrieval and continued or shifted to topical progesterone (Crinone; Merck-Serono) 1 tube/day on the day of embryo transfer, then maintained till the day of serum β-hCG check-up (14 days after ovum pick-up) for luteal support (LS). In cases of ICSI treatment, 0.1 mg Decapetyl was also administered 6 days after ICSI as a measure of additional luteal support. If pregnancy was confirmed, LS was maintained till gestational week 8. The embryo transfers were carried out on day 2, 3 or day 5 of culture.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Hormone Assays
Sera were obtained on the day of hCG administration for oocyte retrieval; LH, E2, and P were measured and analyzed by Immulite 2000 (Euro Diagnostic Products Corporation, Ltd.). The intra- and inter-assay coefficients of variation, respectively, were 3.71% and 6.2% for LH, 4.9% and 7.1% for E2, and 7.0% and 9.5% for P. The sensitivity for progesterone was 0.2 ng/ml and the range of measurement was 0.2-40 ng/ml.
Patients were first categorized into 5 different groups according to serum progesterone levels as follows: ≤ 0.5 ng/ml, 0.5001~1.0 ng/ml, 1.001~1.5 ng/ml, 1.5001~2.0 ng/ml and > 2 ng/ml and the pregnancy outcomes of each group were analyzed. Then patients were further categorized into 2 serum progesterone levels: ≤ 1.5 ng/ml (low) versus > 1.5 ng/ml (high), according to the cutoff value reported from the previous literature. The clinical pregnancy and live birth rates were statistically compared according to the day of embryo transfer (D2/ D3 and D5 ET).
Outcomes Measurement
The serum β-hCG was measured 14 ~ 16 days following oocyte retrieval and was checked as necessary. When the gestational sac was detected by transvaginal ultrasonographic evaluation at gestational week 5, it indicated the clinical pregnancy. Furthermore, we followed all the on-going pregnancies till live delivery, which indicated live birth.
Grouping of Poor, Intermediate, and High Ovarian Responders
We categorized ovarian responses into three arbitrary groups according to the number of oocytes retrieved [12]: poor ovarian response (≤ 4 oocytes retrieved), intermediate ovarian response (5–19 oocytes retrieved), and high ovarian response (≥ 20 oocytes retrieved). We explored the relationship between serum progesterone levels on the day of hCG administration and the IVF outcomes in different ovarian responders.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Statistics
Analyses were performed using the Statistical Package for the Social Sciences (version 15.1; SPSS Inc., Chicago, IL, USA). Mann-Whitney U test, Chi-square test (Fisher’s test) and logistic regression were used for statistical analyses as appropriate. The significance level was set at p < 0.05. 1 2 3 4 5 6
6. Results
The CPRs in each progesterone level group (≤ 0.5 ng/ml, 0.5001-1.0 ng/ml, 1.001-1.5 ng/ml, 1.5001-2.0 ng/ml and > 2 ng/ml) were 35.71% (45/126), 34.13% (86/252), 43.36% (62/143), 40.91% (18/44) and 41.18% (14/34), respectively. The LBRs in each progesterone level group were 30.95% (39/126), 25.79% (65/252), 38.47% (55/143), 31.82% (14/44) and 38.24% (13/34), respectively. There were no statistically significant differences in CPRs and LBRs among the 5 groups in our series (P= 0.42 and P= 0.10, respectively).
In all patients, serum progesterone levels on the day of hCG administration ranged from 0.2 ng/mL to 14.30 ng/mL. Patients were assigned to group 1 [≤ 1.5 ng/ml (low)] or group 2 [> 1.5 ng/ml (high)] based on the serum progesterone values. Statistical distribution of the progesterone levels in group 1 was as follows: mean, 0.78 ng/ml; standard deviation, 0.34 ng/ml; maximum, 1.50 ng/ml; 25th percentile, 0.51 ng/ml; 50th percentile, 0.76 ng/ml; and 75th percentile, 1.03 ng/ml. Statistical distribution of the progesterone levels in group 2 was as follows: mean, 2.34 ng/ml; standard deviation, 1.66 ng/ml; maximum, 14.30 ng/ml; 25th percentile, 1.71 ng/ml; 50th percentile, 1.93 ng/ml; and 75th percentile, 2.30 ng/ml (P< 0.001). Group 2 were considered to show a premature progesterone rise.
The total incidence of PPR in our cycles was 13.02%. Based on different treatment protocols, the incidences of PPR were 18.00% (54/300) in the GnRH agonist subgroup, 9.31% (19/204) in the GnRH antagonist subgroup and 5.26% (5/95) in the other protocols subgroup. There were no statistically significant differences in PPR incidence between the antagonist subgroup and the other protocols subgroup (P= 0.3312). However, the PPR incidence of the agonist subgroup was significantly higher than that of the antagonist and the other protocols subgroups (P= 0.01 and P= 0.004, respectively).
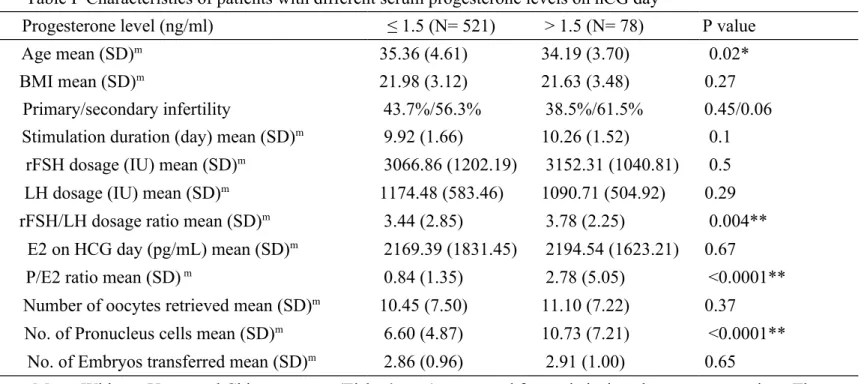
Female and male infertility status, mean number of stimulation days, total dose of administered rFSH and LH, serum E2 level, number of retrieved oocytes and number of transferred embryos did not differ between these two progesterone-level groups (Table I). 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
However, the mean age of group 2 patients was lower than that of group 1 patients (35.36 versus 34.19 years old, respectively, correlation coefficient: −0.080; P< 0.05). Furthermore, the gonadotropin rFSH/LH ratio, the number of pronucleus cells (2PN) and the P/E2 ratio in group 2 were significantly increased. Progesterone level on the day of hCG administration was positively and significantly correlated with the number of pronucleus cells (correlation coefficient: 0.197; P< 0.001) and the P/E2 ratio (correlation coefficient: 0.481; P< 0.001).
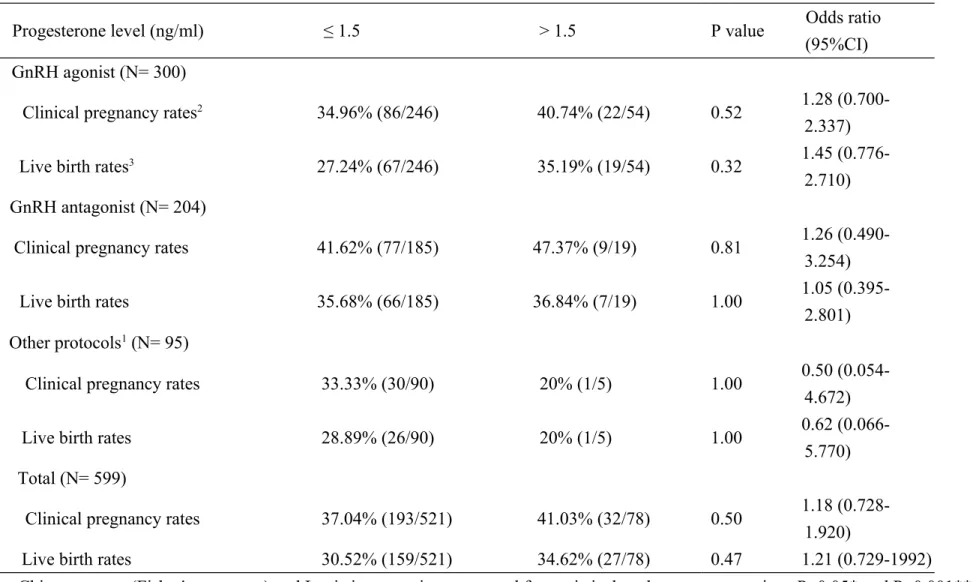
The mean number of transferred embryos was similar in both groups, 2.86 versus 2.91, respectively. The CPRs of the low progesterone group versus that of the high progesterone group was 37.04% versus 41.03%, respectively (OR: 1.18, 95%, CI: 0.728-1.920; P= 0.50). The LBRs of the low progesterone group versus that of the high progesterone group was 30.52% versus 34.62%, respectively (OR: 1.21, 95%, CI: 0.729-1.992; P= 0.47). No statistically significant association between progesterone elevation and the probability of CPRs or LBRs was detected (Table II).
We further analyzed the ART outcomes according to the different treatment protocols (Table II). However, no statistical significance was found both in CPRs and LBRs within different protocols.
Moreover, we analyzed our results according to the ovarian responses (Table III). In the poor responder subgroup, the CPRs of the low progesterone group versus that of the high progesterone group was 23.62% versus 22.22%; the LBRs of the 2 groups were 18.90% versus 16.67%, respectively, and neither group reached statistical significance. The results were similar, and without statistical significance, in both the intermediate and high responder subgroups.
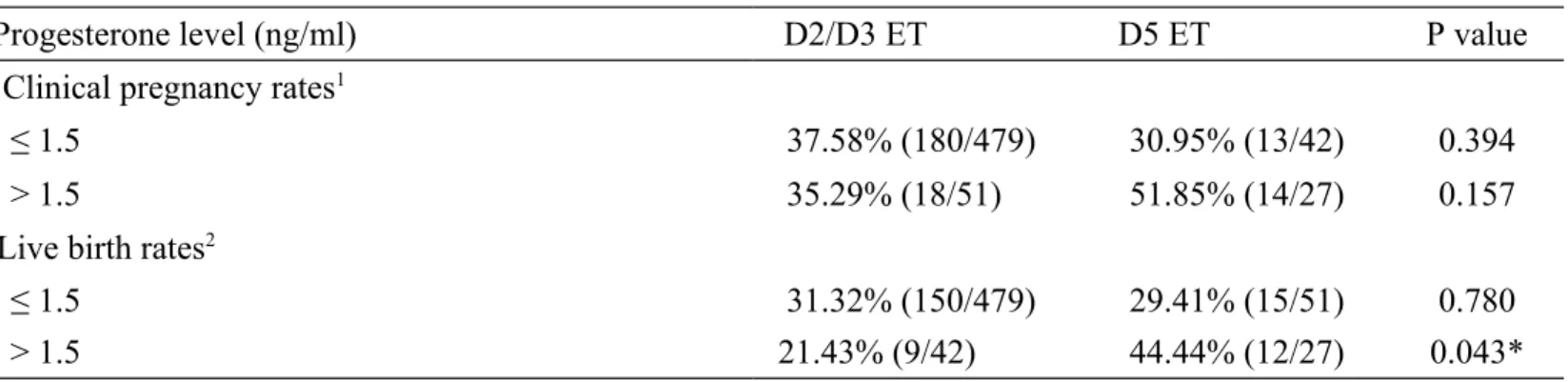
According to our data, 69 cycles received blastocyst (D5) transfer instead of early cleavage stage (D2 or D3) embryo transfer (Table IV). The CPRs of D2/D3 ET versus that of D5 ET was 35.29% versus 51.85% in PPR group (P= 0.157). However, D5 ET could significantly increase the LBRs in the PPR group as compared to D2/D3 ET (44.44% versus 21.43%, P< 0.05). There were two cases, on D5 ET, in which a pregnancy failed to carry to term in the PPR group. One of them was an ectopic pregnancy and the other was a missed abortion at gestation week 10.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
7. Discussion
The definition of premature luteinization (PL) or premature progesterone rise (PPR) differed and was believed to be responsible for the variable pregnancy outcome assessment in previously published literature. Most studies used an absolute progesterone level on the day of hCG administration as an indicator of PPR, and the cut-off values ranged from 0.8 to 2 ng/ml [5-7, 18-19]. In recently published studies, which used new methods of serum progesterone assessment, this cut-off concentration was usually set at 1.5 ng/ml [20]. The selection of this cut-off is supported by evidence showing a marked difference in endometrial gene expression profiles between patients with a serum progesterone concentration above and below the threshold of 1.5 ng/ml on the day of hCG administration [20-21].
Recently, Ou et al. [16] suggested that ovarian response or reserve may be of critical importance when considering PPR. More follicles produce more serum progesterone. Therefore, Younis et al. [22] defined PPR as a P/E2 ratio >1. This criterion could differentiate between the progesterone level secretion from immature follicles and the physiologic secretion from multiple healthy mature follicles [1].
There is a marked variation in the incidence of PPR due to discrepancies in definition, population characteristics, and/or treatment protocols among studies. The reported incidence of PPR varies from 13% to 71%, when an absolute progesterone level is used to define PPR. The incidence of PPR using the criterion of P/E2 ratio >1 was 41% in the report by Younis et al [22]. It should be noted that the proportion of patients with progesterone elevation varies widely even among studies in which the same serum progesterone threshold and the same type of GnRH agonist were chosen.
The total incidence of PPR in our study was 13.02%. The incidence rates for the treatment protocols were 18.00% in the GnRH agonist subgroup, 9.31% in the GnRH antagonist subgroup and 5.26% in the other protocols subgroup. In comparison, the incidences of a progesterone rise > 1.5ng/ml were 24.1% and 23.0% in the agonist and antagonist groups, respectively, in the study by 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
Papanikolaou et al. [13]. The discrepancies between our series and that study were probably due to the use of a different gonadotropin or GnRH analogue, different population characteristics and different sample sizes (n= 599 in our series vs. n= 190 in the study by Papanikolaou et al.).
Since the early 1990s, the impacts of PPR on ART outcomes have remained controversial [23, 24]. Several authors have failed to demonstrate any negative impact of this rise on ART outcomes [4-10], while others reported that pregnancy rates were inversely associated with serum progesterone concentrations on the day of hCG administration [3, 11, 25-27].
In an attempt to resolve this controversy, Venetis et al. [28] conducted a meta-analysis of published studies (five studies; 700 patients). In this meta-analysis, a lower pregnancy rate was found in patients with elevated progesterone on the day of hCG administration; however, no statistically significant association between progesterone elevation and the probability of clinical pregnancy was detected (OR: 0.75, 95% CI: 0.53-1.06; P= 0.10).
In a subsequent meta-analysis, regarding the impact of progesterone on GnRH antagonist cycles alone (five studies; 585 patients), progesterone elevation on the day of hCG administration was significantly associated with a lower probability of clinical pregnancy (-9%, 95% CI: -17 to -2%, fixed model effects; P< 0.02) [29].
Following their previous meta-analysis, Venetis et al. [30] conducted a more comprehensive systemic review and published a meta-analysis in 2013, which evaluated 63 studies (n= 55,199 cycles) in fresh IVF cycles over a range of progesterone elevation thresholds (0.4–3.0 ng/ml). They concluded that progesterone diminishes the probability of that women undergoing fresh IVF cycles will achieve pregnancy, even at concentrations in the range of 0.8~ 1.1 ng/ml (OR: 0.79), and this likelihood appears to be increased when the progesterone concentration reaches 1.2~ 1.4 ng/ml (OR: 0.67) or higher. Interestingly, this effect appears to be relatively stable at concentrations above 1.2 ng/ml.
In our series, there were no statistically significant differences in IVF/ICSI outcomes, either in CPRs or LBRs, in the < 1.5 ng/ml and the > 1.5 ng/ml groups. The differences between our 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
study results and those of a recent meta-analysis probably were due to the limited number of patients, the different COH protocols used and the methods of progesterone measurement in this series. Furthermore, the younger age of patients in the PPR group in our study might have had a positive impact on the IVF/ICSI outcomes. Moreover, the mean progesterone level was 0.78 ng/ml (n= 521) in our non- PPR group; according to the meta-analysis of Venetis et al. [30], there was a slightly detrimental effect on ART outcomes at this threshold level.
Although a significant inverse relationship between serum progesterone on the day of hCG administration and the success of IVF was established in many programs, the involved endocrinological mechanism was unclear. It may involve an ovarian event, with adverse effects on oocyte maturation, fertilization, or early cleavage [2, 24-26, 31]. On the other hand, poorer embryo quality was not reported in other studies [4, 7, 18, 32]. In our series, we did not find adverse effects of PPR on oocyte maturation, number of oocytes retrieved and fertilization results. These findings suggested that PPR may impact the success of IVF, not via an ovarian event, but through its influence on the endometrium, possibly leading to impaired endometrial receptivity. Melo et al. [33] retrospectively analyzed 240 oocyte-donation cycles in which 120 women donated twice, with elevated progesterone levels in the first donation cycle and no progesterone elevation in the following one. The results showed that progesterone elevation did not have a negative impact on ongoing pregnancy rates. In a study by Xu et al. [12], the implantation potential of frozen-thaw ET cycles for the embryos derived from cycles with prematurely elevated progesterone was not impaired.
In light of these observations, it would be better to take into account both the ovarian response and the serum progesterone level when considering the reasons for this phenomenon [1]. Similar to the study by Xu et al. [12], our series showed that elevated progesterone had no negative effect on pregnancy rates in fresh embryo transfer cycles in all groups with different ovarian responses (Table III).
The risk of PPR appears to be associated with the number and size of follicles and the 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
intensity of FSH stimulation. Elevated progesterone may lead to embryo/endometrial asynchrony, reducing the probability of implantation. It might be worth evaluating the possibility of cryopreserving the resulting embryos and their transfer in a subsequent frozen-thawed cycle [3, 18, 34] or alternatively, administering hCG at an earlier time in the follicular phase, prior to progesterone elevation [31].
Papanikolaou et al. [17] suggested that on the fifth luteal day, the endometrium has already significantly recovered from the disruption induced by the supraphysiologic progesterone levels. Using a progesterone cut-off level of 1.5 ng/ml, they found that elevated progesterone had a significant negative effect on the pregnancy outcome when cleavage-stage embryos were transferred. However, no negative effect on blastocyst stage transfers was observed, supporting the idea that the blastocyst transfer strategy could potentially overcome the detrimental effect of progesterone elevation.
In 1997, Fanchin et al. [23] co-cultured embryos up to the blastocyst stage and reported similar blastulation rates in low and high progesterone groups, but patients in the high progesterone group had significantly lower clinical and ongoing pregnancy rates. Three other studies reported that fresh D5 blastocyst transfer could not completely overcome the detrimental effect of elevated progesterone levels on IVF/ICSI cycles on the day of hCG administration [14, 35, 36]. In a recent meta-regression analysis, Venetis et al. [30] did not find evidence of a significant moderating effect of the developmental stage of embryo at transfer (cleavage vs. blastocyst stage) on the association of progesterone elevation with the probability of pregnancy achievement, after controlling for the effect of the progesterone elevation thresholds employed in the various datasets analyzed (coeff: +0.28, 95%, CI: -0.17 to +0.74; overall model: P= 0.15).
In our study, 69 patients received blastocyst transfer (D5 ET) instead of early cleavage embryo transfer (D2/D3 ET). In the PPR group, the LBRs were statistical significantly decreased by D2/D3, but not D5, ET (Table IV). Although D5 blastocyst transfer did not induce any statistically significant improvement in the CPRs in the PPR group, it did significantly enhance the 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
LBRs. However, additional case series are needed to support this finding.
In conclusion, our analysis of the outcomes of 599 ART cycles in a three-year period revealed that PPR did not significantly compromise the clinical outcomes in this series. However, shifting to D5 blastocyst transfer probably could enhance the LBRs in cycles with PPR.
1 2 3 4
8. Authors’ roles
Pei-Chen Huang analyzed the database and prepared the manuscript. Ming-Jer Chen (corresponding author) initiated and supervised the study, performed the ART treatments and finalized the manuscript. Hwa-Fen Guu performed the laboratory work. Ya-Fang Chen helped analyze the database. Yu-Chiao Yi and Jason Yen-Ping Ho helped recruit patients for the study. Li-Yu Chen acted as coordinator. Min-Min Chou provided invaluable research support and advice. 1 2 3 4 5 6
9. Acknowledgements
The authors thank the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan, R.O.C., for their excellent statistical assistance.
1 2 3
10. Funding
No specific funding was obtained for this study. 1
11. Conflict of interest None declared.
1 2
12. Reference
1. Elnashar AM. Progesterone rise on the day of HCG administration (premature luteinization) in
IVF: an overdue update. J Assist Reprod Genet 2010;27:149-155.
2. Schoolcraft W, Sinton E, Schlenker T, Huynh D, Hamilton F, Meldrum DR. Lower pregnancy
rate with premature luteinization during pituitary suppression with leuprolide acetate. Fertil
Steril 1991;55:563-566.
3. Silverberg KM, Burns WN, Olive DL, Riehl RM, Schenken RS. Serum progesterone levels
predict success of in vitro fertilization/embryo transfer in patients stimulated with leuprolide
acetate and human menopausal gonadotropins. J Clin Endocrinol Metab 1991;73:797-803. 4. Bustillo M, Stern JJ, Coulam CB. Serum progesterone at the time of human chorionic
gonadotrophin does not predict pregnancy in in-vitro fertilization and embryo transfer. Hum
Reprod 1995;10:2862-2867.
5. Edelstein MC, Seltman HJ, Cox BJ, Robinson SM, Shaw RA, Muasher SJ. Progesterone levels
on the day of human chorionic gonadotropin administration in cycles with
gonadotropin-releasing hormone agonist suppression are not predictive of pregnancy outcome. Fertil Steril
1990;54:853-857.
6. Givens CR, Schriock ED, Dandekar PV, Martin MC. Elevated serum progesterone levels on
the day of human chorionic gonadotropin administration do not predict outcome in assisted
reproduction cycles. Fertil Steril 1994;62:1011-1017.
7. Hofmann GE, Bentzien F, Bergh PA, Garrisi GJ, Williams MC, Guzman I, Navot D. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
Premature luteinization in controlled ovarian hyperstimulation has no adverse effect on oocyte
and embryo quality. Fertil Steril 1993;60:675-679.
8. Hofmann GE, Khoury J, Johnson CA, Thie J, Scott RT Jr. Premature luteinization during
controlled ovarian hyperstimulation for in vitro fertilization-embryo transfer has no impact on
pregnancy outcome. Fertil Steril 1996;66:980-986.
9. Ubaldi F, Smitz J, Wisanto A, Joris H, Schiettecatte J, Derde MP et al. Oocyte and embryo
quality as well as pregnancy rate in intracytoplasmic sperm injection are not affected by high
follicular phase serum progesterone. Hum Reprod 1995;10:3091-3096.
10. Urman B, Alatas C, Aksoy S, Mercan R, Isiklar A, Balaban B. Elevated serum progesterone
level on the day of human chorionic gonadotropin administration does not adversely affect
implantation rates after intracytoplasmic sperm injection and embryo transfer. Fertil Steril
1999;72:975-979.
11. Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J et al. Circulating progesterone
levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro
fertilization: analysis of over 4000 cycles. Hum Reprod 2010;25:2092-2100.
12. Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J et al. Serum progesterone level effects on the outcome
of in vitro fertilization in patients with different ovarian response: an analysis of more than
10,000 cycles. Fertil Steril 2012;97:1321-1327.
13. Papanikolaou EG, Pados G, Grimbizis G, Bili E, Kyriazi L, Polyzos NP et al. GnRH-agonist
versus GnRH-antagonist IVF cycles: is the reproductive outcome affected by the incidence of 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
progesterone elevation on the day of HCG triggering? A randomized prospective study. Hum
Reprod 2012;27:1822-1828.
14. Ochsenkühn R, Arzberger A, von Schönfeldt V, Gallwas J, Rogenhofer N, Crispin A et al.
Subtle progesterone rise on the day of human chorionic gonadotropin administration is
associated with lower live birth rates in women undergoing assisted reproductive technology: a
retrospective study with 2,555 fresh embryo transfers. Fertil Steril 2012;98:347-354. 15. Huang CC, Lien YR, Chen HF, Chen MJ, Shieh CJ, Yao YL et al. The duration of
pre-ovulatory serum progesterone elevation before hCG administration affects the outcome of
IVF/ICSI cycles Hum Reprod 2012;27:2036-2045.
16. Ou YC, Lan KC, Chang SY, Kung FT, Huang FJ. Increased progesterone/estradiol ratio on the
day of HCG administration adversely affects success of in vitro fertilization-embryo transfer in
patients stimulated with gonadotropin-releasing hormone agonist and recombinant
follicle-stimulating hormone. Taiwan J Obstet Gyncol 2008;47:168-174.
17. Papanikolaou EG, Kolibianakis EM, Pozzobon C, Tank P, Tournaye H, Bourgain C et al.
Progesterone rise on the day of human chorionic gonadotropin administration impairs
pregnancy outcome in day 3 single-embryo transfer, while it has no effect on day 5 single
blastocyst transfer. Fertil Steril 2009;91:949-952.
18. Silverberg KM, Martin M, Olive DL, Burns WN, Schenken RS. Elevated serum progesterone
levels on the day of human chorionic gonadotropin administration in in vitro fertilization
cycles do not adversely affect embryo quality. Fertil Steril 1994;61:508-513. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
19. Ubaldi F, Camus M, Smitz J, Bennink HC, Van Steirteghem A, Devroey P. Premature
luteinization in in vitro fertilization cycles using gonadotropin-releasing hormone agonist
(GnRH-a) and recombinant follicle-stimulating hormone (FSH) and GnRH-a and urinary FSH.
Fertil Steril 1996;66:275-280.
20. Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In't Veld P, Schuit F,
Kolibianakis EM, Devroey P, Bourgain C. Progesterone rise on HCG day in GnRH
antagonist/rFSH stimulated cycles affects endometrial gene expression. Reprod Biomed Online
2011;22:263-271.
21. Labarta E, Martínez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, Simón C, Bosch E.
Endometrial receptivity is affected in women with high circulating progesterone levels at the
end of the follicular phase: a functional genomics analysis. Hum Reprod 2011;26:1813-1825. 22. Younis JS, Matilsky M, Radin O, Ben-Ami M. Increased progesterone/estradiol ratio in the late
follicular phase could be related to low ovarian reserve in in vitro fertilization-embryo transfer
cycles with a long gonadotropin-releasing hormone agonist. Fertil Steril 2001;76:294-299. 23. Fanchin R, Hourvitz A, Olivennes F, Taieb J, Hazout A, Frydman R. Premature progesterone
elevation spares blastulation but not pregnancy rates in in vitro fertilization with coculture.
Fertil Steril 1997;68:648-652.
24. Shulman A, Ghetler Y, Beyth Y, Ben-Nun I. The significance of an early (premature) rise of
plasma progesterone in in vitro fertilization cycles induced by a "long protocol" of
gonadotropin releasing hormone analogue and human menopausal gonadotropins. J Assist 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
Reprod Genet 1996;13:207-211.
25. Bosch E, Valencia I, Escudero E, Crespo J, Simón C, Remohí J, Pellicer A. Premature
luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with
in vitro fertilization outcome. Fertil Steril 2003;80:1444-1449.
26. Fanchin R, de Ziegler D, Taieb J, Hazout A, Frydman R. Premature elevation of plasma
progesterone alters pregnancy rates of in vitro fertilization and embryo transfer. Fertil Steril
1993;59:1090-1094.
27. Hamori M, Stuckensen JA, Rumpf D, Kniewald T, Kniewald A, Kurz CS. Premature
luteinization of follicles during ovarian stimulation for in-vitro fertilization. Hum Reprod
1987;2:639-643.
28. Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is
progesterone elevation on the day of human chorionic gonadotrophin administration associated
with the probability of pregnancy in in vitro fertilization? A systematic review and
meta-analysis. Hum Reprod Update 2007;13:343-355.
29. Kolibianakis EM, Venetis CA, Bontis J, Tarlatzis BC. Significantly lower pregnancy rates in
the presence of progesterone elevation in patients treated with GnRH antagonists and
gonadotrophins: a systematic review and meta-analysis. Curr Pharm Biotechnol
2012;13:464-470.
30. Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and
probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
cycles. Hum Reprod Update 2013;19:433-457.
31. Harada T, Yoshida S, Katagiri C, Takao N, Ikenari T, Toda T, Mio Y, Terakawa N. Reduced
implantation rate associated with a subtle rise in serum progesterone concentration during the
follicular phase of cycles stimulated with a combination of a gonadotrophin-releasing hormone
agonist and gonadotrophin. Hum Reprod 1995;10:1060-1064.
32. Fanchin R, Righini C, Olivennes F, de Ziegler D, Selva J, Frydman R. Premature progesterone
elevation does not alter oocyte quality in in vitro fertilization. Fertil Steril 1996;65:1178-1183. 33. Melo MA, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohí J. The significance of
premature luteinization in an oocyte-donation programme. Hum Reprod 2006;21:1503-1507. 34. Legro RS, Ary BA, Paulson RJ, Stanczyk FZ, Sauer MV. Premature luteinization as detected
by elevated serum progesterone is associated with a higher pregnancy rate in donor oocyte
in-vitro fertilization. Hum Reprod 1993;8:1506-1511.
35. Elgindy EA, Abou-Setta AM, Mostafa MI. Blastocyst-stage versus cleavage-stage embryo
transfer in women with high oestradiol concentrations: a randomized controlled trial. Reprod
Biomed Online 2011;23:789-798.
36. Corti L, Papaleo E, Pagliardini L, Rabellotti E, Molgora M, La Marca A, Vigano P, Candiani
M. Fresh blastocyst transfer as a clinical approach to overcome the detrimental effect of
progesterone elevation at hCG triggering: a strategy in the context of the Italian law. Eur J
Obstet Gynecol Reprod Biol 2013;171:73-77. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19
Table I Characteristics of patients with different serum progesterone levels on hCG day
Progesterone level (ng/ml) ≤ 1.5 (N= 521) > 1.5 (N= 78) P value
Age mean (SD)m 35.36 (4.61) 34.19 (3.70) 0.02*
BMI mean (SD)m 21.98 (3.12) 21.63 (3.48) 0.27
Primary/secondary infertility 43.7%/56.3% 38.5%/61.5% 0.45/0.06 Stimulation duration (day) mean (SD)m 9.92 (1.66) 10.26 (1.52) 0.1
rFSH dosage (IU) mean (SD)m 3066.86 (1202.19) 3152.31 (1040.81) 0.5
LH dosage (IU) mean (SD)m 1174.48 (583.46) 1090.71 (504.92) 0.29
rFSH/LH dosage ratio mean (SD)m 3.44 (2.85) 3.78 (2.25) 0.004**
E2 on HCG day (pg/mL) mean (SD)m 2169.39 (1831.45) 2194.54 (1623.21) 0.67
P/E2 ratio mean (SD) m 0.84 (1.35) 2.78 (5.05) <0.0001**
Number of oocytes retrieved mean (SD)m 10.45 (7.50) 11.10 (7.22) 0.37
No. of Pronucleus cells mean (SD)m 6.60 (4.87) 10.73 (7.21) <0.0001**
No. of Embryos transferred mean (SD)m 2.86 (0.96) 2.91 (1.00) 0.65
m Mann-Whitney U test and Chi-square test (Fisher’s test) were used for statistical analyses as appropriate. The
significance level was set at P< 0.005* and P< 0.001** 1
Table II Assisted reproductive technology outcomes grouped by controlled ovarian hyperstimulation protocols
Progesterone level (ng/ml) ≤ 1.5 > 1.5 P value Odds ratio
(95%CI) GnRH agonist (N= 300)
Clinical pregnancy rates2 34.96% (86/246) 40.74% (22/54) 0.52 1.28
(0.700-2.337)
Live birth rates3 27.24% (67/246) 35.19% (19/54) 0.32 1.45
(0.776-2.710) GnRH antagonist (N= 204)
Clinical pregnancy rates 41.62% (77/185) 47.37% (9/19) 0.81 1.26 (0.490-3.254)
Live birth rates 35.68% (66/185) 36.84% (7/19) 1.00 1.05
(0.395-2.801) Other protocols1 (N= 95)
Clinical pregnancy rates 33.33% (30/90) 20% (1/5) 1.00 0.50
(0.054-4.672)
Live birth rates 28.89% (26/90) 20% (1/5) 1.00 0.62
(0.066-5.770) Total (N= 599)
Clinical pregnancy rates 37.04% (193/521) 41.03% (32/78) 0.50 1.18 (0.728-1.920)
Live birth rates 30.52% (159/521) 34.62% (27/78) 0.47 1.21 (0.729-1992)
Chi-square test (Fisher's exact test) and Logistic regression were used for statistical analyses as appropriate. P<0.05* and P<0.001**
1. Other protocols group included participants who received controlled ovarian hyperstimulation with mild stimulation, natural cycle or modified natural cycle. 1
2. Clinical pregnancy rates (CPRs) was defined as the appearance of gestational sac as revealed by transvaginal ultrasonography.
3. Live birth rates (LBRs) was defined as live delivery. 1
Table III Assisted reproductive technology outcomes grouped by different ovarian responses
Progesterone level (ng/ml) ≤ 1.5 > 1.5 P
value Odds ratio (95%CI) Oocytes retrieved ≤ 4 (N= 145)
Clinical pregnancy rates1 23.62% (30/127) 22.22% (4/18) 1.00 0.92 (0.283-3.019)
Live birth rates2 18.90% (24/127) 16.67% (3/18) 1.00 0.86 (0.230-3.203)
5≤ Oocytes retrieved ≤ 19 (N= 372)
Clinical pregnancy rates 40.56% (131/323) 44.90% (22/49) 0.67 1.19 (0.652-2.187) Live birth rates 32.2% (104/323) 36.73% (18/49) 0.64 1.22 (0.654-2.286) Oocytes retrieved ≥ 20 (N= 82)
Clinical pregnancy rates 45.07% (32/71) 54.55% (6/11) 0.79 1.46 (0.408-5.237) Live birth rates 43.66% (31/71) 54.55% (6/11) 0.53 1.55 (0.432-5.547)
Chi-square test (Fisher's exact test) and Logistic regression were used for statistical analyses as appropriate. P<0.05* and P<0.001**
1. Clinical pregnancy rates (CPRs) was defined as the appearance of gestational sac as revealed by transvaginal ultrasonography.
2. Live birth rates (LBRs) was defined as live delivery. 1
Table IV Assisted reproductive technology outcomes according to different embryo transfer days
Progesterone level (ng/ml) D2/D3 ET D5 ET P value
Clinical pregnancy rates1
≤ 1.5 37.58% (180/479) 30.95% (13/42) 0.394
> 1.5 35.29% (18/51) 51.85% (14/27) 0.157
Live birth rates2
≤ 1.5 31.32% (150/479) 29.41% (15/51) 0.780
> 1.5 21.43% (9/42) 44.44% (12/27) 0.043*
Chi-square test (Fisher's exact test) was used for statistical analyses as appropriate. *P<0.05
1. Clinical pregnancy rates (CPRs) was defined as the appearance of gestational sac as revealed by transvaginal ultrasonography.
2. Live birth rates (LBRs) was defined as live delivery.
1