行政院國家科學委員會專題研究計畫 成果報告
中空型奈米銀/鈀合金奈米殼粒子之開發及其應用於化學鍍
活化液之研究
研究成果報告(精簡版)
計 畫 類 別 : 個別型 計 畫 編 號 : NSC 96-2218-E-151-001- 執 行 期 間 : 96 年 08 月 01 日至 97 年 07 月 31 日 執 行 單 位 : 國立高雄應用科技大學化學工程與材料工程系 計 畫 主 持 人 : 李建良 計畫參與人員: 碩士班研究生-兼任助理人員:曾竣民 碩士班研究生-兼任助理人員:吳士齊 報 告 附 件 : 出席國際會議研究心得報告及發表論文 處 理 方 式 : 本計畫涉及專利或其他智慧財產權,2 年後可公開查詢中 華 民 國 97 年 10 月 28 日
行政院國家科學委員會補助專題研究計畫成果報告
中空型奈米銀鈀合金奈米殼粒子之開發及其應用於化學鍍活
化液之研究
計畫類別:■ 個別型計畫 □ 整合型計畫
計畫編號:NSC 96-2218-E-151-001
執行期間: 96 年 8 月 1 日至 97 年 7 月 31 日
計畫主持人:李建良
計畫參與人員: 計畫兼任助理,碩士班研究生- 曾竣民、吳士齊
成果報告類型(依經費核定清單規定繳交):■精簡報告 □完整報告
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
■涉及專利或其他智慧財產權,□一年■二年後可公開查詢
執行單位:國立高雄應用科技大學
中 華 民 國 97 年 7 月 30 日
Abstract:
Ag/Pd triangular nanoshells of different sizes are prepared for catalyzing electroless copper deposition. As supported by the analysis of quartz crystal microbalance and mixed potential theory, the alloy nanoshells can be successfully used as new activators and have the excellent activity for electroless copper bath. For a comparison of deposition rates with a system of triangular Ag/Pd nanoshells, the large Ag/Pd nanoshell is found to have the maximum activity toward electroless bath.
Keyword:
nanoparticle; shape; catalyst; printed circuit board; ULSI
Text:
1. Introduction:
Electroless copper deposition (ECD) is an important technology in advanced electronic circuit industries, and is used for printing circuit board [1-2] and manufacturing semiconductors [3-6]. Although the composition of the deposition bath [7-9] and the choice of ligands [10, 11] can influence the performance of an electroless process, the activation is a determinant key on the deposition rate [12] and the surface roughness of deposited layers [13]. Activation, which is a catalytic reaction, is triggered with active colloids on the surface of the substrates dipped in an electroless deposition bath. Therefore, the active catalyst plays a role as an electron carrier that supports the transfer of electrons from the reducer to the metal ions.
Metallic nanomaterials are gained of interest because of their optical [14, 15], magnetic [16] and catalytic properties [17-19]. In particular, for the catalytic reaction, metallic nanomaterials of various size [18] and shape [20] apparently alter the catalysis rate. In the early report, we have prepared spherical Pd nanoparticles and apply them as an activator for ECD [21]. This study further explores that electrochemical activity of the Ag/Pd triangular nanoshells (Ag/Pdshell) with
different sizes for catalyzing ECD bath. MPT and QCM are applied in cases of various sizes of Ag/Pdshell to compare theoretical and actual ECD kinetics. The ECD deposition rates activated by
these Ag/Pdnanoshells can thus be fairly compared.
2. Experimental:
2-1. Preparation of Ag/Pd triangular nanoshells with different sizes. The synthesis of
Ag/Pd triangular nanoshells with different sizes in aqueous solution is described below. The synthesis method of the dispersed Ag triangular nanotemplates is modified according to the seed growth method [22]. Initially, ~50 μl of a ~0.05 M silver nitrate (AgNO3) aqueous
solution was added to 10 ml of a 2.5 ×10-4 M sodium citrate aqueous solution. Then, ~25 μl of ~0.1 M NaBH4 solution was gradually added to a stirred mixed solution of sodium citrate and
AgNO3. An Ag seed solution of light yellow was then obtained. Furthermore, 100μl of ~0.05
M AgNO3 was added to 20 ml of 0.1 M hexadecyltrimethy ammonium bromide (C16TAB)
aqueous solution. 1 ml of ~ 0.1 M ascorbic acid and ~100μl of the prepared Ag seed solution was slowly dropped into the C16TAB aqueous solution. The Ag nanotemplates of middle size
were then prepared after 80 μl of 2M NaOH aqueous solution was added. If the amounts of the seed solution are changed to ~50μl and ~200μl in the synthesis pathway, the Ag nanoplates of large and small size are then obtained, respectively. Furthermore, 20 ml of the synthesized Ag triangular nanoplates solution of middle size was precipitated by centrifugation at 4,000 rpm
and redispersed using 3 ml deionized water to reduce the interaction of free C16TAB upon the
synthesis of the Ag/Pd triangular nanoshells. ~1.25 mg palladium acetate (Pd(OAc)2) was
added and slowly dissolved in 1 ml pure acetate acid to produce Pd2+ ion solution for the
following galvanic displacement reaction. ~50μl Pd(OAc)2 solution was added to 3 ml of
stirred solution of Ag nanotemplates with middle size at a fixed controlled temperature, 60 ℃. After 60 min, dispersed middle Ag/Pdshells were obtained. Experiment detail has been
presented elsewhere [23]. Based on the same method, the large and small Ag/Pdshell are then
obtained if large and small Ag nanoplates were used as templates in galvanic displacement reaction, respectively.
The characteristic size and microstructure of the prepared Ag/Pdshell solution, which were
dipped onto the copper grid that was covered with a carbon film and dried naturally, were observed under a high-resolution transmission electron microscope (HR-TEM; JEOL JEM-2000EX).
2-2. Electrochemical measurement. The synthesized Ag/Pd nanoshells of different sizes
were then applied as activators on ECD. The bath compositions of the ECD for quartz crystal microbalance (QCM) experiment are listed in Table 1. All electrochemical experiments were carried out in the baths controlled at 30℃and firstly bubbled with N2 gas for 15 min
prior to the measurement.
For the QCM experiment, the working electrode in ECD measurement was prepared by applying 3µl solution of the small, middle and large Ag/Pdshell uniformly on a 0.159 cm2 Au
surface of the QCM substrate, respectively. The QCM substrate was sputtered with gold on top of 100 Å titanium film from both sides and was connected to a home-build oscillator. The reference electrode (Hg/Hg2Cl2) was separated from the main solution compartment by a
Luggin capillary, which was filled with saturated KCl solution.
For linear scanning voltammetry (LSV) and mixed-potential theory analysis, the resulting small, middle and large Ag/Pdshell aqueous solutions of 3µl was uniformly dropped onto 0.07
cm2 glassy carbon electrode (GCE), respectively, and heated at 70℃ to evaporate H2O for 5
min. In order to prevent the catalyst from falling off the electrode, the glass carbon electrode was rinsed by 3 µl 5wt% Nafion solution and heated at 70℃ for 10 min. LSV measurement was carried out by using a potentiostat (Autolab PGSTAT30) incorporating a rotation disk electrode (RDE). A three-electrode cell, consisting of a GCE working electrode, a Pt counter electrode and a Hg/Hg2Cl2 reference electrode, was used for the measurement. The LSV
experiment was performed in anodic or cathodic reaction (seeing Table 1) at a scan rate of 5 mV/s and a rotation speed of working electrode of 3600 rpm.
Table 1. The composition of electroess copper deposition for analyses of linear sweep voltammetry (LSV), mixed potential theory (MPT) and quartz crystal microbalance (QCM).
Composition of electroless copper bath
MPT and LSV
QCM
Oxidation Reaction Reduction Reaction
CuSO4 0.05 mol/dm3 HCOH 0.27 mol/dm3 CuSO4 0.05 mol/dm3 HCOH 0.27 mol/dm3 EDTA 0.1 mol/dm3 EDTA 0.27 mol/dm3 EDTA 0.1 mol/dm3 pH (adjustment with NaOH) 12.3 pH (adjustment with NaOH) 12.3 pH (adjustment with NaOH) 12.3
3. Results and discussion:
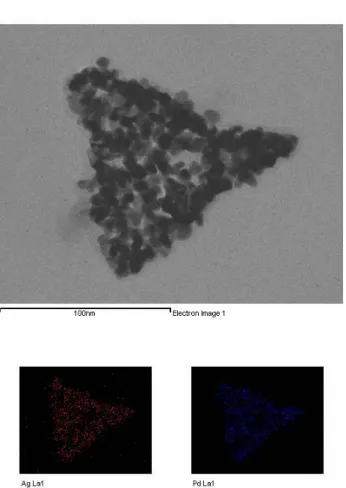
When the Ag/Pd nanoshells were formed, HR-TEM was employed to measure the morphology and size of the prepared Ag/Pdshell, which are displayed on copper grids. Figure 1 presents
HR-TEM images of the prepared Ag/Pdshell of different sizes, which were prepared as description
in the experimental section. The Ag/Pdshell of small, middle and large size are detected and
shown in Figure 1A-C, respectively.
Fig 1. The Pictures of transmission electron microscope of the prepared Ag/Pd triangular
nanoshells of different sizes. (A) Small Ag/Pdshell;(B) Middle Ag/Pdshell; (C) Large Ag/Pdshell.
Based on HR-TEM results, the lengths of triangular edge on small, middle and large Ag/Pdshell are calculated and summarized in Fig. 2. The mean edge lengths upon small and
middle Ag/Pdshell are 82.8 nm and 133.28 nm, respectively, and 193.29 nm is at the large case.
This indicates that the sizes of Ag/Pd triangle nanoshells which are applied as new ECD activators can be tuned by Ag nanotemplate’s size.
small middle large 50 100 150 200 250 300 L en g th of ed ge
Triangular Ag/Pd nanoshells
Fig. 2. The statistical calculation of length of the triangular edge on the prepared Ag/Pd
nanoshells with different sizes.
Element mapping of energy dispersive X-ray spectroscopic analysis upon the prepared Ag/Pd nanoshells yields information on the composition architecture and the atomic distribution. Figure 3 presents the EDX patterns obtained by mapping element of the large Ag/Pd nanoshells, as the image of the triangular nanoshell is captured. The two signals with uniform distribution from the nanomaterials identify them as silver and palladium. The exact composition and mixed alloy of the prepared nanoshells is thus determined.
Fig 3. Element mapping pattern of EDX analysis at the hollow Ag/Pd triangular nanoshell with
As for the catalytic potential on electroless copper deposition, the activity of triangular Ag/Pd nanoshells are measured, which increase with increasing hollow nanocatalyst size of small Ag/Pdshell < middle Ag/Pdshell < large Ag/Pdshell. Theoretically, for electroless copper deposition,
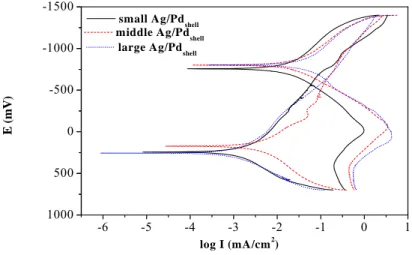
the catalytic particles play roles as carriers in the path of transfer of electrons from reducing agent to copper ions in the ECD bath. The solid catalysts are thus the import factor for the kinetics. Mixed potential theory (MPT), which yields two Tafel curves of the anodic and cathodic reactions,is frequently adopted to analyze the kinetics of electroless deposition [13, 24]. The points of intersection between the anodic and cathodic tafel curves yield the deposition current and the mixed potential associated with a deposition reaction in the steady state. Furthermore, quartz crystal microgravimetry is a novel method for analyzing in-situ the observed electrochemical kinetics, based on the change in frequency of the vibration at the electrode [21]. Figure 4 presents the electrochemical current-potential curves of the Tafel equation of ECD catalyzed by small, middle and large Ag/Pdshell, which analyzed by using potentiostate.
-6 -5 -4 -3 -2 -1 0 1 1000 500 0 -500 -1000 -1500
small Ag/Pdshell
E (
m
V
)
middle Ag/Pdshell
log I (mA/cm2) large Ag/Pdshell
Fig 4. Cathodic and anodic polarization curves of ECD catalyzed by the small, middle and
large Ag/Pd nanoshells in the absence of either formaldehyde or Cu2+.
According to the mixed potential theory, the coordinates of the intersection point of anodic and cathodic curve are the deposition current (Ideposition) and the mixed potential, respectively, at
which the deposition reaction steady-stately occurs. Firstly, it can be seen that the peak current of small Ag/Pdshell < middle Ag/Pdshell < large Ag/Pdshell in the anodic I-E curve are measured and
observed. This indicates that large Ag/Pdshell has the maximum catalytic power toward the
oxidation of reducing agent. The ECD deposition rate (Rmp) by mixed potential theory can be
expressed by Faraday’s law [13] depositon
mp I
R = 181. ×
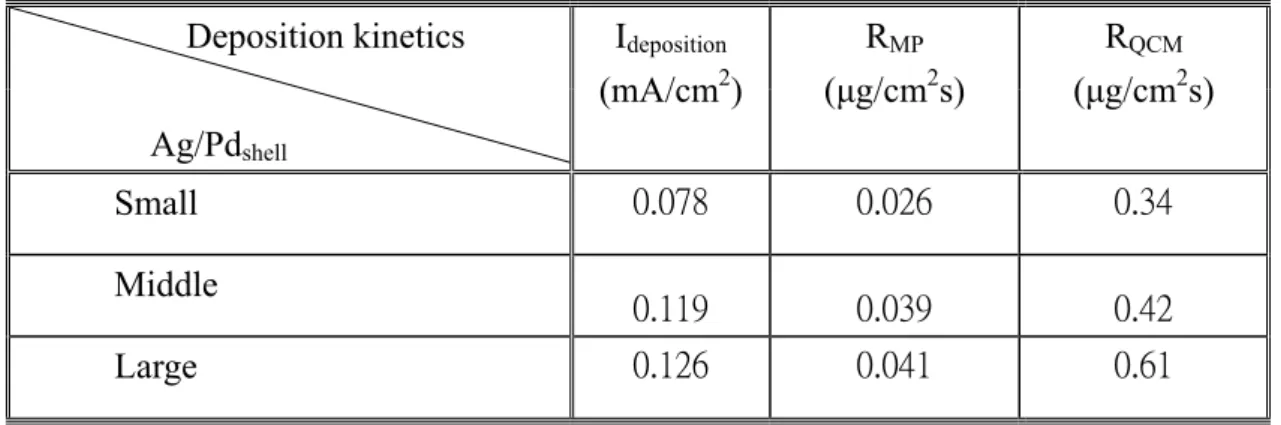
The measured results are summarized in Table 2. The Rmp on small Ag/Pdshell, middle Ag/Pdshell
and large Ag/Pdshell are about ~0.026, ~0.039 and ~0.041 μg/cm2⋅s, respectively, showing the
order of small Ag/Pdshell < middle Ag/Pdshell < large Ag/Pdshell. The large Ag/Pdshell with
lengthening edges shows the maximum activity. The activity of Ag/Pd triangle nanoshells increases with the lengthening of edge lengths. It is plausible that the edge sites of Ag/Pd nanoshells play as an important role as active sites on electroless copper bath.
Table 2: The summary for ECD kinetics measured by MPT and QCM.
Deposition kinetics
Fig. 5 shows the results of QCM upon in-situ measuring the catalytic activity of corresponding nanoshells in the ECD bath. The results are summarized in Table 2. The induction periods, defined as the time necessary for occurring steady-state deposition, for small, middle, and large Ag/Pdshell were observed in Figure 5.
0 100 200 300 400 500 0 10 20 30 40 50
Deposition Time (Sec)
m a ss change ( μ g)
small Ag/Pdshell middle Ag/Pdshell
large Ag/Pdshell
Fig 5. The in situ QCM analyses of the deposition kinetics of electroless copper deposition
catalyzed with the small, middle and large Ag/Pd nanoshells.
The deposition rates are recalculated from the electrode frequency changes according to the Sauerbrey’s equation [25]. The average deposition rate (RQCM) on small Ag/Pdshell is about ~0.34
μg/cm2⋅s, about ~0.42 μg/cm2⋅s on middle Ag/Pd
shell and about ~0.61 μg/cm2⋅s on large
Ag/Pdshell, as shown in Table 2. Note that the deposition rate linearly increases with increase
size of triangular alloy nanoactivators, which is consisting with the result trend analyzed by mixed potential theory. It strongly indicates again that the high activity exists in these prepared alloy nanocatalysts with a hollow structure. Additionally, according to the HR-TEM image shown in Fig. 1C, it can be seen that the edges of large Ag/Pdshells are constructed from many
particles. It is reasonable that small particles could be active center when catalyzing electroless copper bath. Ag/Pdshell Ideposition (mA/cm2) RMP (μg/cm2s) RQCM (μg/cm2s) Small 0.078 0.026 0.34 Middle 0.119 0.039 0.42 Large 0.126 0.041 0.61
4. Conclusion:
Ag/Pd triangular nanoshells of different size are successfully applied as activators for electroless copper deposition. As supported by the analysis of QCM and mixed potential theory, the activity of hollow Ag/Pd triangular nanoshells shows the order of small Ag/Pdshell < middle
Ag/Pdshell < large Ag/Pdshell on electroless copper bath. A comparison of deposition rate with a
system of triangular Ag/Pd nanoshells, the large Ag/Pdshell is found to exhibit maximum activity
toward oxidation of formaldehyde, reducing agent of ECD.
Acknowledgements
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC 96-2218-E-151-001.
5. Reference
1. S. Abe, M. Ohkubo, T. Fujinami, H. Honma, Trans. Inst. Met. Finish. 69 (1991) 140. 2. H. Meyer, R.J. Nichols, D. Schröer, L. Stamp, Electrochim. Acta 39 (1994) 1325. 3. Y. Schacham-Diamand, V. Dubin, M. Angyal, Thin Solid Film 262 (1995) 93.
4. J.P. O’Kelly, K.F. Mongery, Y. Gobil, J. Torres, P.V. Kelly, G.M. Grean, Microelectron. Eng. 50 (2000) 473.
5. V.M. Dubin, Y.Y. Schacham-Diamand, J. Electrochem. Soc. 144 (1997) 898.
6. S. Shingubara, Z. Wang, O. Yaegashi, R. Obata, H. Sakaue, T. Takahagi, Electrochem. Solid State Lett. 7 (2004) C78.
7. K.G. Mishra, R.K. Paramguru, Metall. Mater. Trans. B 30b (1999) 223. 8. T.M. Tam, J. Electrochem. Soc. 132 (1985) 806.
9. M. Wanner, H. Wies, K.G. Weil, Ber. Bunsenges. Phys. Chem. 92 (1988) 736. 10. M. Paunovic, J. Electrochem. Soc. 124 (1977) 349.
11. Z. Jusys , R. Pauliukaité, A. Vaškelis, Phys. Chem. Chem. Phys. 1 (1999) 313. 12. J.F. Hamilton, R.C. Baetzold, Science 205 (1979) 1213.
13. M. Paunovic, in: M. Schlesinger, M. Paunovic (Ed.), Modern Electroplating, John Wiely & Sons, USA, 2000 (Chapter 17).
14. C. J. Orendorff, T. K. Sau, C. J. Murphy, Small 2 (2006) 636. 15. Y. He, G. Shi, J. Phys. Chem. B 109 (2005) 17503.
16. O. Margeat, D. Ciuculescu, P. Lecante, M. Respaud, C. Amiens, B. Chaudret, Small 3 (2007) 451.
17. F. Liu, J. Y. Lee, W. J. Zhou, Small 2 (2006) 121. 18. I. Zuburtikudis, H. Saltsburg, Science 258 (1992) 1337. 19. C. T. Campbell, Science 306 (2004) 234.
20. C. Burda, X. Chen, R. Narayanan, M. A. El-Sayed, Chem. Rev. 105 (2005) 1025
21. C.L. Lee, Y.C. Huang, C.C. Wan, Y.Y. Wang, Y.J. Ju , L.C. Kuo, J.C. Oung, J. Electrochem. Soc 152 (2005) C520.
22. S. Chen, D.L. Carrol, Nano Lett. 2 (2003) 1002.
23. C. L. Lee, C. M. Tseng, S. C. Wu, R. B. Wu, Electrochem. Solid-State Lett. 11 (2008) D27. 24. Y. Sverdlov, V. Bogush, H. Einati, Y. Shacham-Diamand, J. Electrochem. Soc 152 (2005)
C631.
6. 近三年發表期刊論文與計畫成果
1. C-L Lee* and C-M Tseng (2008) “Ag-Pt nanoplates: Galvanic Displacement Preparation
and Their Applications as Electrocatalysts.” J. Phys. Chem. C, 112, 13342-13345.
(NSC96-2218-E-151-001)
2. C-L Lee*, C-M Tseng, R-B Wu and K-L Yang (2008) “Hollow Ag/Pd triangular
nanoplate : A novel Activator for electroless nickel deposition” Nanotechnology, 19, 215709 (SCI, Impact Factor: 3.3) (NSC96-2218-E-151-001)
3. C-L Lee*, C-M Tseng, S-C Wu, R-B Wu and K-L Yang (2008) “Activation of Ag/Pd
triangular nanoshells with different szies on deposition electrolessly copper” Electrochim.
Acta, 53, 5905-5908. (SCI, Impact Factor: 2.8) (NSC96-2218-E-151-001)
4. C-L Lee*, C-M Tseng, S-C Wu and R-B Wu “Activator for Electrolessly Depositing Copper from Triangular Ag/Pd Nanoshells” Electrochem. Solid State Lett. 11, D27-D29 (2008) . (SCI, Impact Factor: 2.00) ( been selected for Virtual Journal of NanoScale Science
& Technology v.16, I.24 (2007))(NSC96-2218-E-151-001)
5. C-L Lee*, (2007) “Synthesis and Electroactivity of Carbon-nanomaterials-supported Pd
nanoparticles from self-regulated reduction of sodium n-dodecyl sulfate” J. Solid State
Electrochem. 11, 1313-1317. (SCI, Impact Factor: 1.542)
6. S-C Chung, Y-L Tsai, T-C Lin, K-L Change, M-J Chien and C-L Lee* (2007) “Electroless Preparation of Macroporous Cu Thin Film from Large-Scale-Orderly Colloid Monolayer”
Mater. Lett. 61, 1929-1932. (SCI, Impact Factor: 1.353)
7. C-L Lee*, Y-C Huang and L-C Kuo, (2007) “Catalytic Effect of Pd nanoparticles on Electroless Copper Deposition” J. Solid State Electrochem.11, 639-646. (SCI, Impact Factor: 1.542)
8. C-L Lee*, Y-C Huang, L-C Kuo and Y-W Lin, (2007) “Preparation of Carbon nanotube-supported palladium nanoparticles by self-regualted reduction of surfactant”
Carbon , 45, 203-206 . (SCI, Impact Factor: 3.884 )
9. C-L Lee* and Y-C Huang, (2006) “Electroless Activators for Depositing Copper from Self-assembled Palladium Nanospheres with Mesopores” Electrochem. Solid State Lett. 9, C196-C198. (SCI, Impact Factor: 2.00) ( been selected for Virtual Journal of NanoScale Science & Technology v.14, I.17 (2006))
10. C-L Lee*, Y-C Huang, and L-C Kuo, (2006) “High catalytic potential of Ag/Pd nanoparticles from self-regulated reduction method on electroless Ni deposition“ Electrochem. Comm. 8, 1021-1026. (SCI, Impact Factor: 3.484)
11. L-C Kuo, Y-C Huang, C-L Lee* and Y-W Yan, (2006) “The Activation Effect of Pd
nanoparticles on Electroless Nickel-Phosphorous Deposition” Electrochim. Acta 52, 353-360 (SCI, Impact Factor: 2.955)
12. C-L Lee*, L-C Kuo, Y-C Huang and Y-W Yan, (2006) “Mesoporous, Self-assembled Palladium Nanospheres: High Efficiency Activator for Electroless Nickel Deposition”
Electrochem. Comm. 8, 697-702. (SCI, Impact Factor: 3.484)
13. C-L Lee*, Y-C Huang, L-C Kuo, J-C Oung and F-C Wu, (2006) “Preparation and
high electroactivities toward oxygen reduction” Nanotechnology, 17, 2390-2395. (SCI, Impact Factor: 3.037)
14. W-L Wang, Y-Y Wang, C-C Wan* and C-L Lee, (2006) “Self-assembly of Pd nanoparticles in deodecanol in situ generated from sodium dodecyl sulfate and its potential applications”
Colloids and Surfaces A: Physicochem. Eng. Aspects 275, 11-16 (SCI, Impact Factor:
1.611)
15. F-T Huang, R S. Liu*, S-F Hu, C-L Lee and A. K. Li (2006) ” Formation of nanostructured cobalt wires with Chinese caterpillar type structure” J. Vacuum Sci. Tech. B, 24, 1440-1443 (SCI, Impact Factor: 1.597)
16. H-M Chen, H-C Peng, R-S Liu*, K. Asakura and C-L Lee, J-F Lee, S-F Hu, (2005) “Controlling the Length and sharp of Gold nanorods” J. Phys. Chem. B, (Letter) 109, 19553-19555. (SCI, Impact Factor: 4.115)
17. C-L Lee*, Y-C Huang, C-C Wan, Y-Y Wang, Y-C Ju and J-C Oung, (2005) “Synthesis of hydrophilic and hydrophobic Pd nanoparticles with in situ generated reducing agent and its application as activator for electroless copper and nickel depositions” J. Electrochem.
Soc. 152, C520-C524. (SCI, Impact Factor: 2.387)
18. C-L Lee*, Y-C Ju, P-T Chou, Y-C Huang, L-C Kuo and J-C Oung (2005) “Preparation of Pt nanoparticles on Carbon nanotubes and graphite nanofibers via self-regulated reduction of surfactanted reduction of surfactants and their application as electrochemical catalyst”
Electrochem. Comm. 7, 453-458 (SCI, Impact Factor: 3.484)
7. 專利
1.
C-L Lee and J-C Oung” The method of preparing nanometal solution” TW Patent200538398
2. C-L Lee and C-C Wan, “一種活化非導體基板之方法” Japan Patent JP2002322565
3. C-L Lee and C-C Wan, “ Method of activating non-conductive substrate for use in electroless deposition ” U.S. Patent 20021974004
4. C-L Lee and C-C Wan, “The process for preparing noble metal nanoparticles” U.S. Patent 6572673.
附件一
可供推廣之研發成果資料表
■可申請專利 □ 可技術移轉 日期:97 年 7 月 30 日國科會補助計畫
計畫名稱:中空型奈米銀鈀合金奈米殼粒子之開發及其應用於化學 鍍活化液之研究 計畫主持人:李建良 計畫編號:NSC96-2218-E-151-001 學門領域:化學工程技術/創作名稱
一種中空銀鈀三角板奈米觸媒製備法發明人/創作人
李建良 中文: 在本研究中係開發具有高活性之中空型銀鈀奈米殼高效能粒子溶 液,並作為化學鍍觸媒。在本製備法中利用奈米三角銀板粒子與鈀 離子反應後形成中空多孔型銀鈀奈米板,合成之銀鈀奈米板粒子晶 測試後具有高活性觸媒功能。技術說明
英文:One novel method to prepare hollow Ag/Pd nanoplates is proposed. Triangular Ag/Pd nanoplates were prepared by using a triangular Ag nanoplate as a displacement template and introducing Pt ions.
可利用之產業
及
可開發之產品
化學鍍活化液、鹼性燃料電池觸媒以及化學催化觸媒技術特點
1. 可大容積製造 2. 可製作出多孔、高活性觸媒推廣及運用的價值
以此合成法生產之中空銀鈀奈米觸媒可廣泛應用於燃料電池、石油 化學觸媒以及化學鍍活化液,尤其對電化學能源觸媒上發展有很大 的助益。 ※ 1.每項研發成果請填寫一式二份,一份隨成果報告送繳本會,一份送 貴單位 研發成果推廣單位(如技術移轉中心)。 ※ 2.本項研發成果若尚未申請專利,請勿揭露可申請專利之主要內容。附件二 本計劃發表期刊論文成果 1.
國際學術會議心得報告
計畫編號 NSC96-2218-E-151-001 計畫名稱 中空型奈米銀/鈀合金奈米殼粒子之開發及其應用於化學鍍活化液之研究 與會人員及 服務機關 李建良 助理教授,國立高雄應用科技大學 會議時間地點 2008/7/13-16, Singapore會議名稱 The 4th International Conference on Technological Advances of Thin Films & Surface Coatings
發表論文題目 One- Pot Synthesis of Pt/Carbon Nanoparticles by Self-Regulated Reduction Method for Electrocatalysis
本次會議,結合了奈米薄膜材料與表面處理技術以及奈米材料製程兩大
主題,會議地點在新加坡管理大學,本實驗室在”Coating for Clean Energy”
的 section 中發表論文” One- Pot Synthesis of Pt/Carbon Nanoparticles by
Self-Regulated Reduction Method for Electrocatalysis “,此論文探討了鉑
奈米觸媒的合成且可直接沉積在奈米碳管表面,奈米碳管表面可先不經由酸
洗官能基化,簡化合成製程,並把所合成的 Pt/CNT 奈米材料作為燃料電池
電化學反應觸媒,探討所合成奈米觸媒材料與電化學特性。
本次參與的研討會為一國際性研討會,會議期間,除了發表論文,以及
與相關學者討論外,亦參加了多場專題演講。此次會議中,接觸到了不少有
關材料在電化學能源或光電產業應用方面的研究,從中吸收到奈米材料在許
多新領域的應用與相關知識,例如太陽能電池等,收穫良多。
One-Pot Synthesis of Pt/Carbon nanotubes by Self-Regulated reduction
Method for Electrocatalysis
Chien-Liang Lee, * and Shi-Chi Wu
Department of Chemical and Materials Engineering, National Kaohsiung University of Applied Science, Kaohsiung 807, Taiwan, R. O. C.
A method for the size-controlled synthesis of Pt nanoparticles and attaching them to the sidewalls of
multiwall carbon nanotubes without any surface pretreatment via the self-regulated reduction of
sodium n-dodecyl sulfate (SDS) is described. In the method, the sizes of the Pt nanoparticles
decorated at multiwall carbon nanotubes are controlled by adjustment of SDS concentration.
Simultaneously, the Pt nanochains with/without small islands on the CNTs are found after Pt/CNTs
are heated to 500 °C upon N2 atmosphere. As supported by electrochemical measurements, the
electroactivities of the prepared Pt/CNT nanocatalysts are enhanced with decreasing sizes of Pt
nanoparticles. Additionally, comparing with the heated Pt/CNT nanocatalysts contained smooth
Pt nanochains, the heated Pt/CNT nanocatalysts contained Pt nanochains with small Pt islands show
higher activities and stabilities on the electrocatalysis.
This work is funded by the National Science Council of Taiwan under grant # NSC96-2218-E-151-001.