行政院國家科學委員會專題研究計畫 期中進度報告
B 型肝炎病毒基因型及表面抗原 T 細胞抗原決定部位基因變
異慢性 B 型肝炎病毒感染病程的影響(2/3)
計畫類別: 個別型計畫 計畫編號: NSC91-2314-B-002-149-執行期間: 91 年 08 月 01 日至 92 年 07 月 31 日 執行單位: 國立臺灣大學醫學院小兒科 計畫主持人: 張美惠 報告類型: 精簡報告 報告附件: 出席國際會議研究心得報告及發表論文 處理方式: 本計畫可公開查詢中
華
民
國 92 年 5 月 29 日
行政院國家科學委員會補助專題研究計畫
□ 成 果 報
告 □期
中進度
報
告
B 型肝炎病毒基因型及表面抗原 T 細胞抗原決定部位基因變異慢性 B
型肝炎病毒感染病程的影響(2/3)
(計畫名稱)
計畫類別:□ 個別型計畫
□ 整合型計畫
計畫編號:NSC 91
-
2314
-
B -
002
-149
-
執行期間:
91
年
8
月
1
日至
92
年
7
月
31
日
計畫主持人:張美惠
共同主持人:
計畫參與人員:
成果報告類型(依經費核定清單規定繳交):□精簡報告 □完整
報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
附件一處理方式:除產學合作研究計畫、提升產業技術及人才培育研究
計畫、列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開
查詢
執行單位:台大醫學院小兒科
中 華 民 國 92 年 5 月 28 日
中文摘要 在慢性 B 型肝炎病毒感染之自然過程中,其臨床過程及預後因宿主及病毒而 異。若能瞭解影響其臨床過程及預後之因子,有助於決定肝炎防治之策略。本計 畫之目的在 :(一)瞭解在長程追蹤中,帶原兒童 B 型肝炎病毒表面抗原 T 細胞 與 B 細胞抗原決定點基因變化的情形,以及其對臨床病程,B 型肝炎病毒標記等 變化之影響。並與 B 肝最嚴重的合併症,亦即肝細胞癌之兒童做比較。(二)比 較 B 型肝炎病毒表面抗原 T 細胞及 B 細胞抗原決定點基因變異對臨床病程,及 B 型肝炎病毒標記等變化之影響在未接受疫苗者與疫苗失敗者的異同。 我們比較長程追蹤的 97 名 B 型肝炎帶原兒童,及另 15 名肝細胞癌兒童之 B 型肝炎病毒表面抗原 T 細胞及 B 細胞抗原決定點基因在長程追蹤中變異的情 形,以及其對臨床病程,及 B 型肝炎病毒標記等變化的影響。另比較未接受疫苗 者與在嬰兒期接受 B 型肝炎預防注射者,其 B 型肝炎病毒表面抗原 T 細胞抗原 決定點基因變異之情形。結果發現肝細胞癌兒童之 B 型肝炎病毒表面抗原 T 細 胞抗原決定點基因之變異率比慢性 B 型肝炎病毒感染但無肝癌之兒童較高 (46.7% v.s. 6.2%, p=0.0013).此現象並未發生於 B 型肝炎病毒表面抗原 B 細胞抗原 決定點基因。B 型肝炎病毒表面抗原 B 細胞抗原決定點基因之突變則較常發生於 接受過 B 肝預防注射的兒童。 總之, 肝細胞癌兒童之 B 型肝炎病毒表面抗原 T 細胞抗原決定點基因比慢性 B 型肝炎病毒感染但無肝癌之兒童有較高的變異率. 此間差異比成人之肝癌與非 肝癌帶原者相比更加顯著。 兒童肝癌之早期癌形成與此 B 型肝炎病毒表面抗原 T 細胞抗原決定點基因變異可能有關, 然而是因是果仍待進一步研究. 關鍵詞 :慢性 B 型肝炎病毒感染、肝細胞癌兒童、B 型肝炎病毒表面抗原 T 細 胞抗原決定點基因、B 型肝炎病毒表面抗原 B 細胞抗原決定點基因
SUMMARY
The aims of this study are (1) to investigate the role of mutation at the HLA-class I restricted T cell epitope and B cell epitope of hepatitis B surface antigen (HBsAg) gene in the natural course of children with chronic hepatitis B virus ( HBV) infection with and
without hepatocellular carcinoma (HCC), and (2) to compare the mutation rates at the T cell epitope and the B cell epitope in children with chronic HBV infection and HCC with and without hepatitis B immunoprophylaxis in infancy.
We have longitudinally followed 97 HBsAg carrier children, 53 did and 44 did not receive hepatitis B immunoprophylaxis during infancy. T cell epitope amino acids 28-51 at the HBV surface gene has been studied in those 97 children and another 15 children with HBV related HCC. Children with HCC showed a significantly higher rate of T cell epitope mutation at amino acid residue 40-49 than children without HCC (46.7% v.s. 6.2%, p=0.0013). No difference in the rate of B cell epitope mutation was found between children with and without HCC (p > 0.1). A trend of higher mutation rate in the B-cell epitope was observed in children who received HBV vaccination than in children without vaccination (22.4% v.s. 9.3%, p=0.063). No difference in the mutation rate at T cell epitope domain of the HBsAg gene was found between those who were unvaccinated and those who were vaccinated during infancy (p > 0.1 ).
In conclusion, Mutations at the T cell epitope amino acid residue 40-49 were much more frequently found in childhood HCC than HBV carriers children. Its relationship with early hepatocarcinogenesis requires further investigation.
Key words:chronic hepatitis B virus infection, hepatocellular carcinoma in children, T cell epitope on hepatitis B surface gene, B cell epitope on hepatitis B surface gene
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a world wide health problem. It may leads to chronic hepatitis , liver cirrhosis and hepatocellular carcinoma (HCC) (1). In hyperendemic areas, chronic HBV infection begins mainly during infancy and early childhood (2,3). HBV in early childhood tends to cause persistent infection (4). However, the mechanism remains unclear.
During the natural history of HBV infection, clinical course and outcome differ in different individuals. Factors influencing the clinical course, degree of liver damage and fibrosis, outcome, and response to treatment have been extensively investigated in adults. Yet the fact found in adults are the later part of the natural history of HBV infection. Although complications of chronic HBV infection manifest mostly during adulthood, liver histologic changes begin early since childhood (5). Study in children is therefore very important to enhance the understanding of the mechanism of
persistent HBV infection and the clinical course shortly after primary infection. HCC is the most serious consequence of persistent HBV infection, and the determinant factor toward this complication is still largely unclear. The host and virus interaction is very likely the most important determinant for the persistent HBV infection, its disease courses and complication. We have previously demonstrated the
importance of transplacental hepatitis B e antigen as the immunomodulatory agent which makes the maternal transmitted neonates become tolerant to HBV(7). We also found that host factors may affect the clinical course : such as HLA DRB1*14 predicts earlier HBeAg seroconversion with higher amino- transferase levels, while HLA DRB1*11 had a protective role in aminotransferase activities during HBeAg seroconversion. (Chang MH et al. unpublished data).
We have also investigated the viral role of HBV in the long -term follow-up course of chronic HBV infection in children. According to our previous longitudinal follow-up studies, mutation of the precore gene (10), basal core promoter gene, and core gene deletion (11) precedes or are accompanied with HBeAg seroconversion, some with more severe liver diseases. But debates remain in that whether the mutants or deletions may develop as a consequence of immune pressure or an initiating event of HBeAg seroconversion and liver damage. Additional determinant factors have to be explored.
Genetic variabilities of HBV may influence the viral antigen expression, which will affect the host immune response to HBV. Antibody to hepatitis B surface antigen (anti-HBs) can provide protective immunity to HBV infection and help to clear HBV. Mutations in the surface gene of HBV may result in amino acid
substitutions in the common “a” antigenic determinant (amino acids 124 to 147) (12), which can alter B-cell epitopes of HBsAg leading to immunologic escape from the host immunity elicited by either vaccination or previous infection. In addition to the mutation at the B-cell epitope as mentioned above, a novel mechanism of persistent infection which may escape T-cell recognition was demonstrated in lymphochorio-meningitis virus infection (13). This negative stranded RNA virus has a high natural mutation rate, and may escape MHC-dependent immune surveillance in the host by in vivo selection of virus mutants that are resistant to recognition by cytotoxic T cells. A novel mutation domain at the amino acid 29 to 53 of the HBV surface protein
coincides with a T-cell epitope was found in HBV infected individuals (14). In a study among six chronic HBsAg carriers and six adult patients, 83% had mutation within this T-cell epitope region in both carriers and HCC adults,but with no difference between carriers and HCC adults(15). Mutations were particularly frequent at amino acid position 40, 47, and 53, which are highly evolutionarily conservative. These mutational hot spots coincide with a mapped HLA-A2-restricted T-cell epitope, i.e. HBsAg amino acids 41-49. On the contrary, only 25% had mutations at the “a” determinant region of the surface gene.
After the launch of universal HBV vaccination program in Taiwan, the infection rate and the chronic carrier rate are effectively reduced. However, problems against eradication of HBV infection are still present. The main underlying causes are two folds : one is immunoprophylaxis failure, the other is no vaccination due to limited resources or ignorance. In Taiwan, the coverage rate of HBV vaccination is good ( around 87 to 97% in the general children population). Immunoprophylaxis failure is an important problem to be explored. Approximately 0.7 to 1.7% of children in the general population failed to respond to immunoprophylaxis and became chronic HBsAg carriers (16). We have performed sequential seroepidemiologic studies in children of Taipei city in 1984, 1989, 1994 and 1999 (17). The HBsAg seropositive rates decreased from 10% in 1984 before the vaccination program to <1% after the vaccination program.
We have studied the prevalence of HBV surface mutants at the “a’ determinant region in carrier children before and after the vaccination era( 18). The prevalence of surface gene mutants in carrier children increased from 7.8% in 1984, just prior to the
universal vaccination program, to 28.1% in 1994, 10 years after the launch of the program. But the role of mutation at the T-cell epitopes of surface gene in vaccine failure remains unknown and needs further exploration.
The aims of this study are (I) to investigate the role of mutation at the HLA- class I restricted T cell epitope and B cell epitope of hepatitis B surface antigen (HBsAg) gene in children with chronic hepatitis B virus ( HBV) infection with or without hepatocellular carcinoma (HCC), and (II) to compare the mutation rates at the T cell epitope and the B cell epitope in children with chronic HBV infection and HCC with and without hepatitis B immunoprophylaxis in infancy.
SUBJ ECTS AND METHODS
I. SUBJ ECTS :
Totally 112 children , 97 HBsAg carrier children and 25 HCC children, under parental consents, were enrolled to this study. Among the 97 HBsAg carrier children, 53 children had received HBV immunoprophylaxis; another 44 HBsAg carrier children who did not received immunoprophylaxis were randomly selected from the 415 long term followed HBsAg carrier children for comparison. At each visit, physical examination, blood test for liver function profiles, and HBV markers were conducted. The genetic changes at the mutation hot spots in the HLA class I-restricted T cell epitope and the B cell epitope on HBV surface antigen gene were studied in these children.
II. METHODS :
The clinical course and severity of hepatic inflammation ( ALT and AST levels) during long-term follow-up, the immunoporphylaxis records, and age of HBeAg seroconversion were correlated with the genetic changes at the T cell and B cell epitopes of the HBsAg gene of HBV.
Careful vaccination history including hepatitis B immunoglobulin and HBV vaccine were studied in each of the 112 children. They were divided into vaccinated group and unvaccinated group. Mutatios of the HLA class I restricted T cell epitope and B cell epitope on HBsAg gene were studied and compared in those 112 children with o without HCC, and also in those who did and those who did not receive HBV immunoprophylaxis.
1. HBV Mar ker s (including HBsAg, anti-HBs, anti-HBc, HBeAg, anti-HBe) : by radioimmunoassay using the commercially available kits manufactured by
Abbott Company, U.S.A.
2. Liver Function Profiles : (Including ALT, AST, bilir ubin, albumin/ Globulin)
Using Hitachi 7450 Autoanalyzer ( Tokyo, Japan).
3. Detection of Mutation in the Gene of HLA Class I-Restr icted T Cell Epitope on HBsAg.
DNA was extracted from 20 ul of serum and was resuspended in 10 ul of RNAase – free water. The first polymerase chain reaction (PCR) was carried out in 40 ul of reaction solution, which contained 1 ul of extracted DNA from the serum, 50ng each of the sense and antisense primers, 200uM of dNTP, 1 U of DNA polymerase
(Biotolls, B & M Lab, S.A., Spain) and 4ul of 10X DNA polymerase reaction buffer. The first PCR primers corresponding to HBV genomic regions of nucleotide 67-86 (P9, sense, 5’GGCTC, MAGTT,CMGGA, ACA GT 3’) and 1,136-1,154 (P6, antisense, 5’GCA AC, GGGG T , AAAGG , TTCA3’) were used. Amplification was
performed in 40 cycles, with denaturation at 95℃ (10 min), annealing at 55 ℃ (20 sec.) and extension at 72℃ (1 min) using a thermocycler (PTC-200, MJ Research, Waltahm, MA, U.S.A.). Nested PCR was conducted in those with negative result for first PCR using primers P5 (nucleotide 167-186, sense, 5’ACATC, AGGAT,TCCTA, GGACC 3’) and P6. Direct sequencing of the PCR products was carried out as described below.
4. DNA Sequencing
Automatic sequencing was conducted using the ABI prism dye terminator cycle sequencing ready reaction kit according to the manufacturer’s instruction and read
by the ABI 373 DNA sequencer. Sequencing primers using the above mentioned P5 and P8 (nucleotide 842-822, antisense, 5’TTAGG, GTTTA, AATGT,ATGCC,C3’).
RESULTS
Totally 112 children with chronic HBV infection , 15 with HCC and the
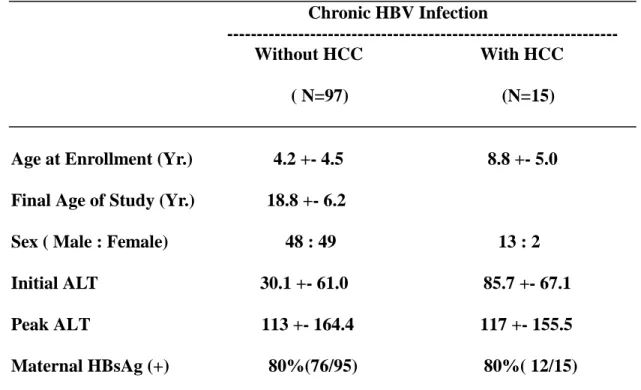
remaining 97 without HCC, were studied for T cell epitope and B cell epitopes at the HBV surface antigen gene region. Totally 54 children ( 5 of them with HCC) had received three or four doses of HBV vaccination, and four children had received three doses of hepatitis B immunoglobulin (HBIG) prophylaxis at 0,1 and 6 month old. They account for 53 of the 97 children with chronic HBV infection, and 5 of the 15 HCC children. Their basic characteristics are listed in table 1.
Sixteen children ( two with HCC) were infected with adr type of HBV, while 96 children ( thirteen with HCC) had adw type of HBV. One (9.1%) of the 16 children with adr type of HBV and 11 (11.4%)of those 96 with adw type of HBV had T cell epitope mutation. None of the 16 children with adr type HBV had mutation at the B-cell epitope, no matter HBV immunized or not. On the contrary, sixteen ( 16.7%) of the 96 children with adw typed HBV had B cell epitope mutation.
As sown in Table 2, HCC children had a significantly higher rate of mutation rate at the T cell epitope of HBsAg gene than that in children chronically infected by HBV but without HCC (46.7% versus 6.2%, 7/15 versus 6/97; p=0.0013). Amino acids 40, 44, ad 47 are the most common site of mutation for T cell epitope. They occurs in 5 (38.5%), 6(46.1%) and 3(23.1%) children of the 13 children with T cell epitope mutation respectively. No difference in the rate of mutation at the B-cell epitope was found between children with and without HCC (20% versus 15.5%, 3/15 versus 15/97; p=0.70).
A trend of higher mutation rate in the B-cell epitope was found in children who received HBV immunoprophylaxis than that in children without (22.4% versus 9.3%, 13/58 versus 5/54; p=0.063 ). Amino acids 145 and 126 are the most common sites of surface gene mutation. They occurred in nine (50%) and six (33.3%) of the 18
children with B-cell epitope mutation, respectively. No difference in the rate of T cell epitope mutation was found between the vaccinated and unvaccinated children (8.6% versus 14.8% , 5/58 versus 8/54; p=0.30).
Six patients lost their HBsAg during follow–up : three unvaccinated and three vaccinated children with chronic HBV infection. The peak ALT levels during long term follow-up in children with chronic HBV infection did not differ significantly between those with and those without T cell epitope mutation. In those children without HCC, the peak ALT levels n those with cell epitope mutation were below 70 IU/L in four (80%) and >200 IU/L (251 IU/L) in one (20%). In contrast, the peak ALT levels in children without T cell epitope mutation were >200 IU/L in 14 ( 16.7%), 70-200 IU/L in 18 ( 21.4%) children, and < 70 IU/L in 52 (61.9%) children.
DISCUSSION
The pathogenesis of HCC remains largely unknown. The early carcinogenesis in childhood HCC is particularly a mystery. The HCC in children in Taiwan is nearly 100% related to HBV infection. Although HCV has been closely related to HCC in adults, HCV did not play a role in the childhood HCC in Taiwan and world wide. According to our previous study, HCC in children occurs mainly in children of 6 years and old. It has a shorter incubation period after HBV infection than adult HBV related HCC, and the chance of exposure to the confounding factors, such environmental factors or hormonal factors, are also much less. It is therefore a good model to study the role of HBV in the carcinogenesis of HCC.
Our study revealed that the rate of mutation at the T-cell epitope region amino acid residues 40-49 of the HBV surface gene is much higher in HCC children than that in the chronic HBV carrier children without HCC (6.2% v.s. 46.7%,p<0.002). This trend has not been observed in the HBV surface gene B cell epitope tope and other sites of HBsAg gene in HCC children. A high mutation rate at amino acid 28-51 of the surface antigen was also observed in HCC adults. Tai et al. reported a mutation rate of 83% at this hot spot area in adult HCC and surrounding non-tumor liver tissues.
A similar trend of difference was found between adult HCC patients and asymptomatic HBV carriers by Liu et al. Virus strains isolated from adult HCC patients in Taiwan had an approximately two times higher frequency of amino acid variation at the T cell epitope region of the surface gene of HBV than that of the asymptomatic carrier adult patients. Although older adult patients usually have a higher rate of mutation for HBV, a lower frequency of amino acid variation at the surface gene region of adw serotype HBV among was observed in those HCC patients > 50 years old than that in > 50 years old (relative frequency 0.44, p=0.04). Taken together, the higher frequency of T cell epitope mutations at aminoacid residues 40-49 in children and possibly also in younger adults with HCC implicates the close
relationship of T cell epitope mutation with the early carcinogenesis. This trend has not been found in the B cell epitope, nor in other reported T cell epitopes of the surface gene such as amino acids 88-106, 158-172, and 172-191).
The total mutation rate at the T-cell epitope was significantly higher in children with HBV related HCC than HBsAg carrier children without HCC ( R.R. =7.5). In comparison to the results in adults, children with HCC had a higher risk ratio of T-cell epitope mutation than adults with HCC. As reported by Liu et al. , and Chen et al. Liu et al. Although adults with chronic HBV infection were reported to have a high rate of amino acid 40-47 mutation, the risk ratio between chronic carriers and HCC was around 2.0. While older patients have a higher rate of mutation of the HBV geneome during chronic HBV infection, HCC patients younger than 50 years of age were reported to have a higher risk ratio ( R.R.=2.3) of T cell epitope mutation than those older than 50 years of age. Our HCC children are in the youngest age group in HBV related HCC patients. This T cell epitope mutation at amino acid 40-47 suggests some relationship with early hepatocarcinogenesis.
The B –cell epitope mutations were mainly detected at amino acids 126, 127, and 145, in both immunized and unimmunized children. The mutations at amino acids 126 and 145 were particularly prevalent in immunized children. In our previous report, hepatitis B surface antigen variants were found both before and after the universal hepatitis B vaccination program in Taiwan, 7.8% in 1984, 19.6% in 1989, and 28.1%
in 1994 . the most prevalent site of mutation were reported to locate at the “a” determinant hydrophilic loops at residues 125-129, and 140-149.
In conclusion, during chronic HBV infection, mutation at the T-cell epitope of the HBV surface gene amino acid residue 40-49 might be associated with early hepatocarcinogenesis.
REFERENCES
1. Beasley RP, Stevens CE. Epidemiology of hepatitis B virus infection in Taiwan. In Sung JL, Yu JY, Wang TH, eds. “Proceedings of the International Symposium on Hepatitis in Taipei”. Taipei, Gastroenterologic Society of the Republic of China, pp.
1-10.
2. Hsu HY, Chang MH, Chen DS, et al. Baseline seroepidemiology of hepatitis B virus in children in Taipei, 1984 : A study just before mass hepatitis B vaccination program in Taiwan. J Med Virol 1986; 18: 301-7.
3. Sung JL, Chen DS, Lai MY, et al. Epidemiological study on hepatitis B virus infection in Taiwan. Chinese J Gastroenterol 1984; 1: 1-9.
4. Stevens CE, Beasley RP, Tsui J, et al. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 1975; 292:771-4.
5. Chang MH, Hwang LY, Hsu HC, Lee CY, Beasley RP. Prospective study of
asymptomatic HBsAg carrier children infected in the perinatal period : clinical and liver histologic studies. Hepatology 1988; 8: 373-7.
6. Hsu HM, Chen DS, Chuan CH, et al. Efficacy of a mass hepatitis B vaccination program in Taiwan : studies on 3464 infants of hepatitis B surface antigen-carrier mothers. JAMA 1988; 260: 2231-5.
7. Hsu HY, Chang MH, Hsieh KH, et al. Cellular immune response to hepatitis B core antigen in maternal-infant transmission of hepatitis B virus. Hepatology 1992; 15 : 770-6.
8. Chang MH, Hsu HY, Hsu HC, Ni YH, Chen JS, Chen DS. The significance of spontaneous HBeAg seroconversion in childhood : with special emphasis on the clearance of HBeAg before three years of age. Hepatology 1995; 22 : 1387-92. 9. Hsu HY, Chang MH, Ni YH, Lee CY, Chen JS, Hsu HC, Chen DS. Spontaneous
loss of hepatitis B surface antigen in children with chronic hepatitis B virus infection. Hepatology 1992; 15: 382-6.
10.Chang MH, Hsu HY, Ni YH, et al. Preocore stop codon mutant in chronic hepatitis B virus infection in children : Its relation to hepatitis B seroconversion and
maternal hepatitis B surface antigen. J Hepatol 1998; 28: 915-22.
11.Ni YH, Chang MH, Hsu HY, Chen HL. Long-term follow-up study of core gene deletion mutants in children with chronic hepatitis B virus infection. Hepatology 2000; 32 : 124-8.
12. Waters JA, Kennedy M, Voet P, et al. Loss of common “a” determinant of
hepatitis B virus correlate with fulminant and severe hepatitis . J Clin Invest 1993; 91: 1206-13.
13. Pircher H, Moskophidis D, Rohrer U, et al. Viral escape by selection of cytotoxic T cell- resistant virus variants in vivo. Nature 1990; 346: 629-33.
14.Tai PC, Banik D, Lin GI, et al. Novel and frequent mutation of HBV coincide with a major histocompatibility complex Class I-restricted T-cell epitope of the surface antigen. J Virol 1997; 71: 4852-6.
15. Chen WN, Oon CJ. Mutational ‘hot spot” in HLA class I-restricted T cell epitope on HBsAg in chronic carriers and hepatocellular carcinoma. Biochemical and
Biophysical Research Communication 1999; 262: 757-61.
16. Hsu HM, Lu CF, Lee SC, Lin SR, Chen DS. Seroepidemiologic survey for hepatitis B virus infection in Taiwan : the effect of hepatitis B mass immunization. J Infect Dis 1999 ; 179 : 367-70.
17. Ni YH, Chang MH, Huang LM et al. Hepatitis B virus infection in children and adolescents in an hyperendemic area : 15 years after universal hepatitis B vaccination. Ann Intern Med 2001; 126: 84-91.
18. Hsu HY, Chang MH, Liaw SH, Ni YH, Chen HL. Changes of hepatitis B surface variants in carrier children before and after universal vaccination in Taiwan. Hepatology 1999; 30 : 1312-7.
Table 1. Basic Char acter istics of the 112 Childr en with chr onic HBV infection
with or without HCC.
Chronic HBV Infection
Without HCC With HCC
( N=97) (N=15)
Age at Enrollment (Yr.) 4.2 +- 4.5 8.8 +- 5.0
Final Age of Study (Yr.) 18.8 +- 6.2
Sex ( Male : Female) 48 : 49 13 : 2
Initial ALT 30.1 +- 61.0 85.7 +- 67.1
Peak ALT 113 +- 164.4 117 +- 155.5
Mater nal HBsAg (+) 80% (76/95) 80% ( 12/15)
---Table 2. Mutations of the HBV Sur face Gene T-Cell Epitope and B Cell Epitopes
in Children with Chronic HBV Infection ver sus Those with HCC
---Chr onic HBV Infection HCC
Vaccinated Unvaccinated Vaccinated Unvaccinated
(n=53) (n=44) (n=8) (n=17)
---T Cell Epitope
aa40 N->S(2) N->S(1)* N->S (2)
aa44 G->E(1)※ G->E (1) G->E (2)*# G->E (2)@
aa45 T->I(1)※
aa47 V->E(1) T->K(1)# V->E(1)
aa49 L->P(1)@
---B Cell Epitope
aa126 T->I(2)**,T->A(2) T->A(1) T->A(1)
aa127 P->T(1) P->T(1),P->S(1)
aa143 T->S(1)**
aa145 G->R(6) G->R(1) G->R(1) G->R(1)
Number in the parenthesis = number of patients with the mutation ; ※,*,**,#,@