國立台灣大學醫學院生物化學暨分子生物學研究所 博士論文
Graduate Institute of Biochemistry and Molecular Biology College of Medicine
National Taiwan University Doctoral Thesis
肝細胞癌之相關研究:
一、肝型脂肪酸結合蛋白促進肝細胞癌之血管新生及細胞移行 二、科羅索酸針對 VEGFR2/Src/FAK 路徑抑制肝細胞癌之細胞移行
Studies on Hepatocellular Carcinoma (HCC):
I. Liver Fatty Acid-Binding Protein (L-FABP) Promotes Cellular Angiogenesis and Migration in Hepatocellular Carcinoma
II. Corosolic Acid Inhibits Hepatocellular Carcinoma Cell Migration by Targeting the VEGFR2/Src/FAK Pathway
辜琮祐 Chung-Yu Ku
指導教授:呂紹俊 博士(Shao-Chun Lu, Ph.D.) 林榮耀 博士(Jung-Yaw Lin, Ph.D.)
中華民國 104 年 6 月
June, 2015
致 謝
首先,誠摯的感謝我的指導教授:林榮耀教授與呂紹俊教授,兩位老師悉心 的教導使我得以一窺癌症生物學及抗癌藥物機轉領域的深奧,並不時的討論指點 我正確的方向,使我在唸博士班的這些年中獲益匪淺,老師對於學問及實驗態度 上的嚴謹更是我學習的典範。感謝李明學教授及李德章教授在進度報告以及論文 修改上的建議;感謝方剛教授在口試前熱心幫忙讓口試能順利進行;感謝詹迺立 教授在結構生物學上提供幫助;感謝周綠蘋教授推薦我加入 R942 這個大家庭,
並且總是很關心我的博士生涯;感謝游偉絢教授樂於分享他研究的成果,還不嫌 棄我的排球技術;最後要感謝台大醫學院生化分生所上的每位老師,不論在專題 報告或研究中,都啟發了我在實驗設計及邏輯思考的能力。
七年的日子,不論是在 R942 或 D208,實驗室裡共同的生活點滴,學術上的 討論,當然還有不能讓老師知道的奇幻旅程以及每周固定的活動筋骨。感謝各位 學長姐、同學、學弟妹的陪伴,讓這些日子能過得多采多姿。
感謝郁蕙學姐給我許多實驗上的協助,以及讓我了解如何與老師溝通的技巧;
感謝玄原學長總是一肩扛起實驗室大大小小的事情,讓我能心無旁鶩的進行研究;
感謝賽文學長讓我見識到國境之南及蓬萊之巔的景色,學長爽朗又滄桑的笑聲道 盡了實驗生涯的一切;感謝子玲學姐總是在見面時給我一個溫暖的微笑,並一起 體驗了難得的總區助教經驗;感謝我的好同學:志強、俊榮、郁鈴、昭圻、雅姿 以及很少見面的曹甯與婕伶,在之後的人生中我相信大家一定都能在自己的領域 找到一片天;感謝台大與師大的學弟妹們:孟倫、詩瑾與銘得,雅月與柏均,綱 緒、銘亨與賀培,柏劭、瑀萱與蕍甄,還有喬偉與庭瑋,大家一起見識了 R942 到 D208 的演變,太多的回憶無法一一細數;感謝芬如學姐辛苦的報帳,並讓大 家每次都能一飽口福;感謝瓊瑛學姐與所辦的長輩們分享許多處事的經驗;秀儀、
慶桂、怡伶、嘉嬣、啟榮,喜甄與和惠,你們在實驗上的幫忙與協助讓我銘感在 心。
特別感謝我的老婆─佳純與她的老師─顧記華教授;佳純一路上陪著我,從 中國醫,北醫,再到台大,要同時兼顧老婆、媳婦、及母親的角色,蠟燭多頭燒 的辛苦我都看在眼裡;而顧老師除了在我投稿論文階段給予很大的幫助之外,也 讓我很放心佳純在實驗及工作上,能一路順遂;如果沒有你們,我不可能撐得過 這些日子。宗佑、丞斌與鄉民保護協會的大家,是與你們的歡樂時光讓我能在一 天辛苦的實驗結束後,至少還能笑著繼續明天的挑戰。
最後,謹以此文獻給我摯愛的父母,這段時間我沒能好好的陪伴你們,你們 是我能安心求學最大的後盾;我的岳父岳母,感謝你們肯將女兒嫁給當時還沒畢 業的我,而現在我也終於不用再拒絕你們釣遊四海的邀約了;我的妹妹─千芬,
希望我這個哥哥還能不讓妳覺得失望;還有在最後趕著來報到,讓我能沾上許多 好運的女兒─曼姿,是妳可愛的照片讓我在實驗室能度過許多寂寞的夜晚。
感謝一路上每一位幫助我、陪伴我的親人、朋友與同學,謝謝大家!
琮祐 謹上 中華民國一百零四年七月一日
Contents
Page
Abbreviations
1中文摘要
3Abstract
5Introduction
Hepatocellular carcinoma 7
Vascular endothelial growth factor and HCC 7
Liver fatty acid-binding protein (L-FABP) 8
Lipid rafts, receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases 9
Chinese herbal medicines and Actinidia chinensis 10
Corosolic acid (CA) 11
Materials and methods
Part IAntibodies used for western blot analysis and chemical inhibitors 12 Tissue microarray construction and immunohistochemistry 12
Cell culture 13
Creation and culture of L-FABP overexpressed stable clones 13
Western blot analysis and immunoprecipitation 14
Cell migration assay 14
Angiogenesis activity assay 15
Short interference RNA (siRNA) and short hairpin RNA (shRNA) 16
Lipid rafts isolation 17
Confocal microscopy analysis 18
Small GTPase binding assay 18
Construction of human VEGF-A promoter 19
Luciferase reporter assay 19
Animals 20
Cloning of L-FABP mutants 21
Statistical analysis 21
Part II
Plant extracts 22
HPLC analysis 22
Reagents 22
Cell culture 23
Cytotoxicity assay 23
Migration assay 24
Immunoprecipitation 24
Western blot analysis 25
Kinase activity assay 25
Rho GTPase activity assay 26
G-actin/F-actin activity assay 26
Confocal microscopy analysis 27
Animal model 27
Immunohistochemistry 29
Synergistic analysis 29
Molecular docking 29
SRB cell growth assay 30
Statistical analysis 30
Results
Part I1.Up-regulation of L-FABP expression in HCC tissues is correlated with VEGF-A
overexpression 31
2. L-FABP induces VEGF-A expression and angiogenic potential in immortalized
Hus and Huh7 cells 31
3. Association of L-FABP with VEGFR2 in membrane rafts 33 4. L-FABP increases VEGFR2/Src phosphorylation and cell migration by
FAK/cdc42 pathway 34
5. L-FABP induced VEGF-A expression by Akt/mTOR/P70S6K/4EBP1 in
translation level 35
6. L-FABP promoted tumor growth and metastasis in vivo 36 7. Cholesterol binding and membrane interacting properties are essential for
L-FABP induced cell migration and angiogenesis 37
Part II
8. Corosolic acid significantly decreases the migration activity of Huh7 cells 38
9. Corosolic acid inhibits VEGFR2 kinase activity 39
10. Corosolic acid decreases cell motility by inhibiting VEGFR2/Src/FAK/cdc42
activity and actin rearrangement 40
11. Corosolic acid exhibits anti-tumor effect in vivo 41 12. Synergistic effects of corosolic acid and sorafenib on HCC cells 42 13. Corosolic acid interacts with the ATP-binding site of VEGFR2 kinase domain
by molecular docking 43
14. Corosolic acid does not exhibit significant inhibitory effects on Huh7 cell 43
Discussion
Part IRole of L-FABP in hepatocellular carcinoma 45
Part II
Effects of corosolic acid on hepatocellular carcinoma 50
Summary
53Figures and Figure legend
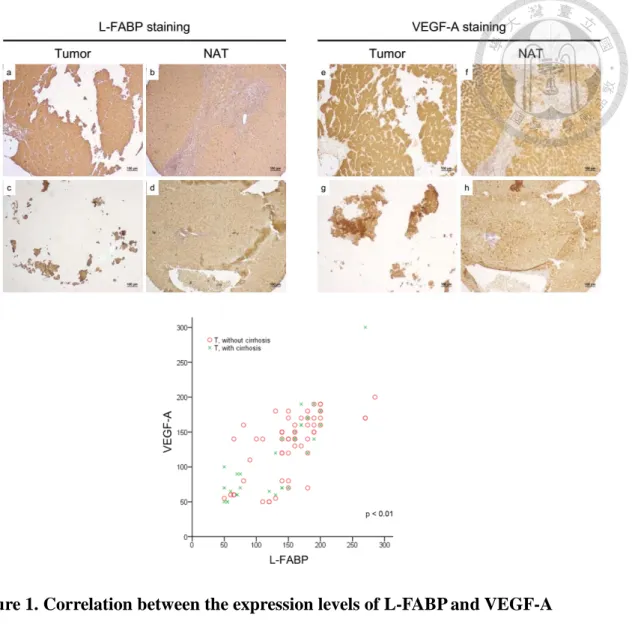
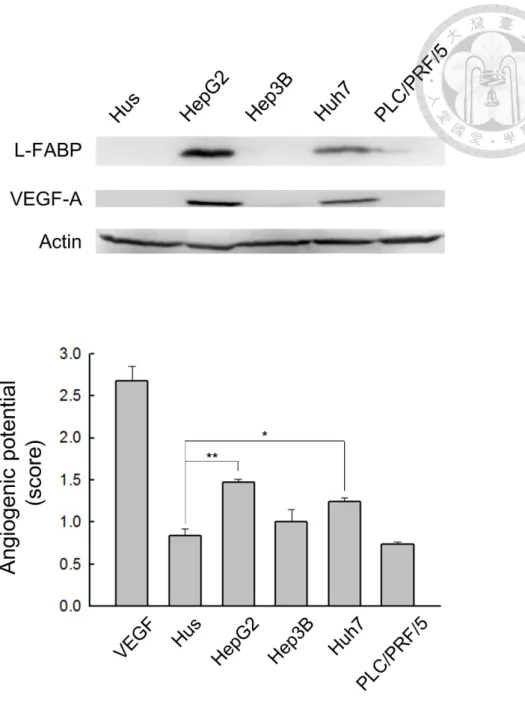
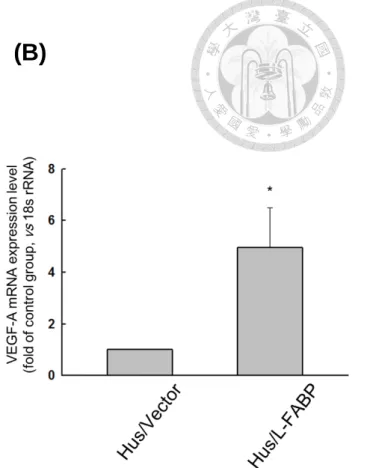
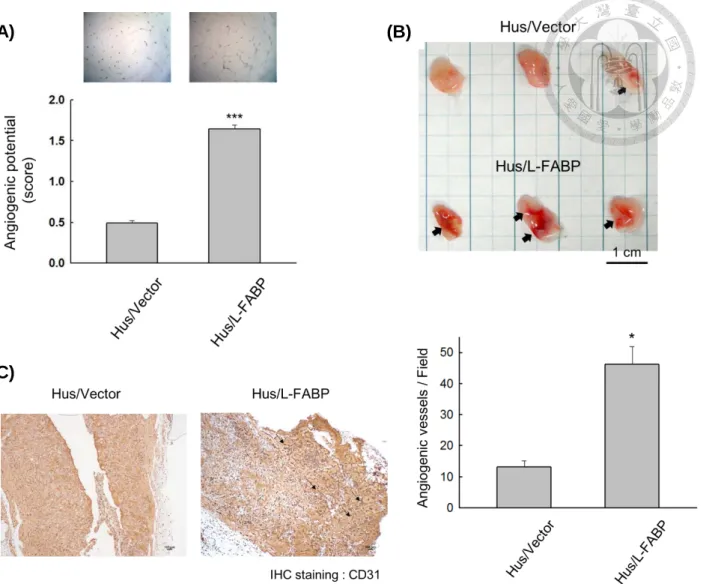
Part IFigure 1. Correlation between the expression levels of L-FABP and VEGF-A 54 Figure 2. L-FABP expression is associated with VEGF-A expression of HCC cells 55 Figure 3. Expression level of VEGF-A is up-regulated in L-FABP stably expressed
Hus cells 56
Figure 4. L-FABP promotes in vitro and in vivo angiogenic activity of Hus cells 57 Figure 5. Sequence aliment of L-FABP interacting domains 58 Figure 6. Co- immunoprecipitation of L-FABP and VEGFR2 in Hus/L-FABP cells 59 Figure 7. L-FABP associates with VEGFR2 in apical membrane of Hus/L-FABP
cells 60
Figure 8. Localization of L-FABP and signaling molecules in lipid rafts 61 Figure 9. L-FABP increases the phosphorylation level of VEGFR2 in Hus cells 62 Figure 10. L-FABP increases the phosphorylation level of Src and FAK kinases in
Hus cells 63
Figure 11. L-FABP promotes cdc42 activity of Hus cells 64 Figure 12. Analysis of migration activity of L-FABP stably expressed Hus cells 65 Figure 13. L-FABP up-regulates migration activity through VEGFR2/ Src pathway 66 Figure 14. L-FABP activates Akt/ mTOR/ P70S6K/ 4EBP1 signaling 67 Figure 15. HIF-1α significantly enriched in the nucleus of L-FABP overexpressed
cells 68
Figure 16. Role of HIF-1α in VEGF-A transcriptional activity of L-FABP
overexpressed cells 69
Figure 17. Post-transcriptional regulation of VEGF-A in L-FABP stably expressed
Hus cells 70
Figure 18. L-FABP promotes tumor growth in vivo 71
Figure 19. L-FABP promotes in vivo metastasis by lung metastasis model 72 Figure 20. Effect of L-FABP mutants in VEGF-A expression 73 Figure 21. Effect of L-FABP mutants in migration activity 74 Figure 22. Cholesterol binding properties are essential for L-FABP induced cell
migration and angiogenesis 75
Figure 23. Knockdown of L-FABP in Hus/L-FABP cells reversely decreased
VEGF-A expression and migration activity 76
Figure 24. Knockdown of L-FABP in Huh7 cells down-regulates VEGF-A
expression and migration activity 77
Figure 25. Reduction of L-FABP and VEGFR2 co-localization on membrane is
observed in Huh7 L-FABP stably knockdown cells 79 Figure 26. Aberrant overexpression of L-FABP in HCC tissues (with cirrhosis) is
associated with worse outcome 80
Part II
Figure 27. Cytotoxicity and migration inhibitory effect of Actinidia chinensis on
Huh7 cells 81
Figure 28. HPLC analysis of Actinidia chinensis 82
Figure 29. Migration activity of Huh7 cells is inhibited by corosolic acid without
cytotoxicity 83
Figure 30. Corosolic acid reduces phosphorylation level of VEGFR2 84 Figure 31. Corosolic acid reduces VEGFR2 kinase activity 85 Figure 32. CA-induced inhibition of migration activity in Huh7 cells is VEGFR2
dependent 86
Figure 33. Corosolic acid down-regulates VEGFR2 downstream signals 87 Figure 34. Corosolic acid inhibits cdc42 activity 88 Figure 35. Effect of corosolic acid on actin rearrangement 89 Figure 36. Corosolic acid exhibits significant anti-tumor effects on Huh7 cells in
vivo 90
Figure 37. Combinatorial effects of corosolic acid and sorafenib on migration
activity of Huh7 cells 92
Figure 38. Combinatorial effects of corosolic acid and sorafenib on signaling
molecules of Huh7 cells 93
Figure 39. Combinatorial effects of corosolic acid and sorafenib on Huh7 cells by 94
in vivo xenograft model
Figure 40. Inhibitory effects of corosolic acid combined with sorafenib on Src and FAK kinases in vivo
95 Figure 41. Corosolic acid interacts with the ATP-binding site of VEGFR2 kinase
domain by molecular docking analysis
96 Figure 42. Analysis of relative distance and surface charge distribution between
corosolic acid and VEGFR2 ATP binding pocket
97 Figure 43. Corosolic acid inhibits growth of Huh7, HepG2, and Hep3B cells 98 Figure 44. Cytotoxicity and migration-inhibitory effects of corosolic acid on
HepG2 cells
99 Figure 45. Cytotoxicity and migration-inhibitory effects of corosolic acid on
Hep3B cells
100 Figure 46. Corosolic acid doesn’t exhibit significant inhibitory effect on invasion
activity of Huh7 cells
101 Figure 47. Corosolic acid shows no inhibitory effect on NFκB signaling 102
Tables
Table 1. Correlation between L-FABP and VEGF-A protein expression in tissue
pairs from 90 HCC patients 103
Table 2. Clinical characteristics of the cases included in analyses of L-FABP
protein expression evaluated by immunohistochemistry 103 Table 3. Association of L-FABP protein expression with clinical pathologic
characteristics in patients with HCC 104
Supplementary data
Table 1 105
Figure 1. Knockdown of VEGFR2 in Hus/L-FABP cells decreased the activation
of down-stream signaling molecules 107
Figure 2. Prediction of the interaction models of L-FABP and VEGFR2 kinase
domain 108
Figure 3. Amino acid substitution of L-FABP in present studies 109
References
110
Publications
1
List of Abbreviation
L-FABP: Liver fatty acid-binding protein HCC: Hepatocellular carcinoma
VEGF-A: Vascular endothelial growth factor A
VEGFR2: Vascular endothelial growth factor receptor 2 Akt: Protein kinase B
mTOR: Mammalian target of rapamycin P70S6K: 70 kDa ribosomal protein S6 kinase 1
4EBP1: Eukaryotic translation initiation factor 4E-binding protein 1 Src: Proto-oncogene tyrosine-protein kinase Src
FAK: Focal adhesion kinase 1
CDC42: CDC42 small effector protein 1 HIF-1α: Hypoxia-inducible factor 1-alpha PI3K: Phosphoinositide 3-kinase P.H. medium: Primary hepatocyte used medium
HEPES: 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid DMEM: Dulbecco's modified eagle medium
DMSO: Dimethyl sulfoxide G418: Geneticin
HUVEC: Human umbilical vein endothelial cells NOD/SCID mice: NOD.CB17-Prkdcscid/NcrCrl mice siRNA: Small interfering RNA
shRNA: Short hairpin RNA
O/N: Overnight
GTPase: Hydrolase enzymes that can bind and hydrolyze guanosine
2
triphosphate (GTP)
Rac1: Ras-related C3 botulinum toxin substrate 1 RhoA: Ras homolog gene family, member A
GST-PBD: Fusion protein of GST tag with The Rac/Cdc42 (p21) binding domain
GST-RBD: Fusion protein of GST tag with the Rho binding domain TNM stage: Tumor, lymph nodes, metastasis stage of cancer classification CD31: Platelet endothelial cell adhesion molecule
Sorafenib: Nexavar
PP1: Src inhibitor (CAS 172889-26-8)
MG132: Inhibitor of proteasome (CAS 133407-82-6) MβCD: Methyl-beta-cyclodextrin
MTT: 3- (4, 5-cimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide SRB: Sulforhodamine B
F-actin: Filamentous actin G-actin: Globular actin
3
中文摘要
國際上肝細胞癌在癌症發生率中排行第五,在癌症致死率中排行第三。肝細 胞癌的生長及進展仰賴於新生血管的形成,而血管內皮生長因子(VEGF)在此過程 中扮演非常重要的角色。
肝型脂肪酸結合蛋白(L-FABP)在肝細胞中大量表現,並已知可參與脂質代 謝。L-FABP 過度表現已在許多癌症中被發現,但它在肝細胞癌中扮演的角色仍不 清楚。本研究中,我們分析了 L-FABP 與 VEGF 在 90 個 HCC 患者中的關聯性。我們 發現,L-FABP 在肝癌組織中與 VEGF-A 呈現正相關性。此外,L-FABP 在異種移植 小鼠模式中可顯著促進腫瘤生長及轉移。我們亦討論 L-FABP 活性與腫瘤生成的關 係:L-FABP 可與細胞膜上脂筏中的 VEGFR2 結合,接著活化下游的
Akt/mTOR/P70S6K/4EBP1 與 Src/FAK/CDC42 路徑,這也使得 VEGF-A 表現量增加,
並促進血管新生與細胞移行之活性。我們的研究結果證實,L-FABP 可望成為治療 肝癌的新目標。
在臨床上,抑制第二型血管內皮生長因子受體(VEGFR2)之活性已被建議作為
治療 HCC 的重要策略。本研究中,我們發現獼猴桃根部之化合物,科羅索酸(CA),
對肝癌細胞表現出顯著的抗癌作用。研究指出, CA 可透過與 VEGFR2 上 ATP 結合 口袋的交互作用,抑制 VEGFR2 之活性。 CA 在 Huh7 細胞實驗中可抑制性調控 VEGFR2/Src/FAK/CDC42 路徑,減少絲狀肌動蛋白(F-actin)之形成,並降低細胞 移行能力。在動物實驗中,CA 對腫瘤生長的有效抑制劑量為每隻小鼠給予 5 毫克/
公斤/天。我們也證實, CA 與蕾莎瓦(Sorafenib)在廣範圍濃度下具有協同效應。
本研究闡明了 CA 抗肝癌的細胞分子機制,並建議 CA 可作為治療侵襲性肝癌之抗 癌藥或佐劑。
4
關鍵詞:
肝細胞癌,血管新生作用,肝型脂肪酸結合蛋白,血管內皮生長因子,科羅索酸,
細胞移行,第二型血管內皮生長因子受體
5
Abstract
Hepatocellular carcinoma (HCC) is the fifth most commonly occurring cancer and the third most common cause of cancer death worldwide. The progression of HCC relies on the formation of new blood vessels, and VEGF is critical in this process.
Liver fatty acid-binding protein (L-FABP) is abundant in hepatocytes and known to be involved in lipid metabolism. Overexpression of L-FABP has been reported in various cancers; however, its role in hepatocellular carcinoma (HCC) remains unclear.
In this study, we investigated L-FABP and its association with vascular endothelial growth factors (VEGFs) in 90 HCC patients. We found that L-FABP was highly expressed in their HCC tissues, and its expression level was positively correlated with that of VEGF-A. Additionally, L-FABP significantly promoted tumor growth and metastasis in a xenograft mouse model. We also studied the mechanisms of L-FABP activity in tumorigenesis: L-FABP was found to be associated with VEGFR2 on membrane rafts and subsequently activate the Akt/mTOR/P70S6K/4EBP1 and Src/FAK/cdc42 pathways. This resulted in up-regulation of VEGF-A expression accompanied by an increase in both angiogenic potential and migration activity. Taken together, our results suggest that L-FABP may be a potential target for HCC
chemotherapy.
Inhibition of VEGFR2 activity has been proposed as an important strategy for the
6
clinical treatment of hepatocellular carcinoma (HCC). In this study, we identified corosolic acid (CA), which exists in the root of Actinidia chinensis (藤梨), as having a significant anti-cancer effect on HCC cells. We found that CA inhibits VEGFR2 kinase activity by directly interacting with the ATP binding pocket. CA down-regulates the VEGFR2/Src/FAK/cdc42 axis, subsequently decreasing F-actin formation and
migratory activity of Huh7 cells in vitro. In an in vivo model, CA exhibites an effective dose (5 mg/kg/day) on tumor growth, and we further demonstrate that CA has a
synergistic effect with sorafenib within a wide range of concentrations. In conclusion, we elucidate the effects and molecular mechanism for CA on HCC cells and suggest that CA could serve as a therapeutic or adjuvant target for patients with aggressive HCC.
Keywords:
Hepatocellular carcinoma, angiogenesis, liver fatty acid-binding protein, vascular endothelial growth factor, corosolic acid, migration, vascular endothelial growth factor receptor-2 (VEGFR2)
7
Introduction
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC), the most common type of liver cancer, is
notoriously resistant to systematic therapies, and often accompanied by high recurrence.
Because of its poor prognosis, HCC causes more than 700,000 deaths annually and becomes the third leading cause of cancer-related death worldwide [1, 2]. Previous
studies have implicated several emerging pathways in HCC, such as HGF/MET,
Wnt/β-catenin, and VEGF/VEGFR, can serve as novel molecular targets for developing
anti-HCC therapies [3-5].
Vascular endothelial growth factor and HCC
Angiogenesis is known to play an important role in progression and metastasis of HCC. Vascular endothelial growth factor (VEGF) is a critical driver to stimulate new blood vessel formation to supply sufficient nutrients and oxygen for sustained tumor growth [1]. VEGF can bind to three similar receptor tyrosine kinases, including VEGFR1 (FLT1), VEGFR2 (KDR) and VEGFR3 (FLT4) by different affinities, yet VEGFR2 is the major receptor for VEGF-induced signaling, and serves as the main therapeutic target [6]. Previous studies also suggested a strong correlation of VEGFR2 expression with HCC malignance and liver cirrhosis [7, 8]. However, since HCC
8
patients are often diagnosed at an advanced stage accompanied with tumor angiogenesis and metastasis, VEGF-targeted therapies were seemed to have apparent therapeutic benefits [2, 9].
Liver fatty acid-binding protein (L-FABP)
Liver fatty acid-binding proteins (L-FABP) is a member of the FABP family, which expresses abundantly in cytoplasm and is capable of binding hydrophobic lipid ligands with a high specificity. The FABP family proteins (~15 kDa) show moderate amino acid
sequence homology, but highly similar tertiary structures, which are formed in a
β-barrel shape. L-FABP can uniquely bind two ligand molecules (long chain fatty acids),
or a various hydrophobic molecules, such as cholesterol and bile acids [10].
Furthermore, L-FABP can interact with plasma membrane to enhance cholesterol transfer or participate in membrane microdomains alteration [11], but its detailed mechanisms are less known.
Overexpression of L-FABP was observed in various cancer types, including liver [12], lung [13], gastric [14], pancreatic [15] and breast cancers [16, 17]. Although some studies have yielded contradictory findings that L-FABP expression is decreased in HCC [18], several reports showed that L-FABP expression was correlated with VEGF expression in HCC [12] and breast cancer [19], and the precise mechanisms remain to
9
be studied.
Lipid rafts, receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases Lipid rafts are ordered structures of membrane microdomains, characterized by high concentration of cholesterol and glycosphingolipids, and are involved in fundamental cellular functions such as endocytosis, protein trafficking, and signal transduction [20]. A prominent feature of lipid rafts is their insolubility in neutral detergents such as Triton X-100, a reason for which they are often referred to as detergent-insoluble membranes (DIMs). The ability of lipid rafts to enhance receptor signaling has led to the concept of a signalosome− a region where proteins are localized together to facilitate receptor signaling. For example, rafts may contain incomplete signaling pathways that are activated when a receptor and/or other required molecules are recruited into the raft [21].
Receptor tyrosine kinases are a prominent example of the proteins involved in cell signaling that are enriched in lipid rafts. The EGF receptor, the insulin receptor, the PDGF receptor, the VEGF receptor and the NGF receptor among others have been shown to be localized to low density, cholesterol-rich membrane domains [22]. In all cases, signaling by these receptors is modulated by changes in cellular cholesterol content. Thus, raft localization appears to be of functional importance to the receptors.
10
Non-receptor tyrosine kinases in lipid rafts
Lipid rafts are also thought to play a central role in facilitating signal transduction from non-receptor tyrosine kinases. Signaling molecules such as Src family protein tyrosine kinases and small GTP-binding proteins of the Ras superfamily can localize to rafts by virtue of lipid modification [23]. Other signaling enzymes such as PI3K also localize to rafts, but the mechanism of their recruitment to these microdomains is unclear. Disruption of lipid rafts by cholesterol depletion agent:
methyl-beta-cyclodextrin (MβCD), could inhibit multiple downstream signals of RTKs, including Src, FAK and Akt [24]. Mutation of the myristate or palmitate modification sites in Src kinases inhibits their partitioning into lipid rafts and blocks downstream signaling [25].
Chinese herbal medicines and Actinidia chinensis
Chinese herbal medicines (CHMs) have been used as potential therapies for a variety of human diseases, including hypertension, inflammation, and cancer [26].
Recent studies suggest that CHMs can be used to improve the efficiency of
conventional cancer therapies and relieve the side effects of chemotherapies [27].
Anti-cancer effects of A. chinensis on cell proliferation, apoptosis, and
angiogenesis have been noted in previous studies [28, 29]. In our study, A. chinensis
11
was found to exhibit a significant anti-migratory effect to Huh7 cells, and the IC50
migration of A. chinensis was identified as 0.2 mg/ ml. The cytotoxic effect of A.
chinensis to Huh7 cells was between 0.5–4 mg/ml (Figure 27, A and B).
Corosolic acid (CA)
Corosolic acid (CA) is an ursane-type triterpenoid, and is known to be a STAT3 inhibitor in macrophages, myeloid cells, and ovarian cancer cells [30-32]. CA also has a significant inhibitory effect on endothelial angiogenic tube formation [29], and tumor growth in lung and ovarian cancer cells [31, 33]. In the above mentioned study, we observed that A. chinensis water extracts had an anti-migration effect in Huh7 cells.
Therefore, we performed HPLC analysis and identified the active component of A.
chinensis; corosolic acid (CA), which comprised about 8.4% of the dry weight of A.
chinensis (Figure 28), was suggested to be a novel anti-HCC compound in our studies.
12
Materials and methods
§Part I
Antibodies used for western blot analysis and chemical inhibitors
Antibodies specific to L-FABP, VEGF-A, Flotillin-2, Lamin A/C, α-tubulin and β-actin were purchased from Santa Cruz Biotechnology, USA. Antibodies specific to
VEGFR2, phospho-VEGFR2, Src, phospho-Src, FAK, phospho-FAK, PI3K (p85), Akt, phospho-Akt, mTOR, phospho-mTOR, phospho-4EBP1, 4EBP1 and HIF-1α were obtained from Cell Signaling Technology, USA. The chemical inhibitor Src inhibitor I was from Calbiochem, and Sorafenib was obtained from Selleckchem, USA.
Tissue microarray construction and immunohistochemistry
The tumor and adjacent normal tissues array (HLiv-HCC180Sur-02) were purchased from US Biomax, Inc. The microarray sections were immunestained with specific antibodies against L-FABP (1:100) and VEGF-A (1:100), respectively. The staining results were interpreted by pathologists of GenDiscovery Biotechnology, Taiwan. The staining results were emerged for intensity and percentage of staining area, respectively, and calculated by Quick-score analysis which scored by multiplying the percentage of positive cells (P) by the intensity (I). Formula: Q = P × I; Maximum = 300.
The results were then graded according to the following criteria: 1 for score 0-99, weak
13
staining; 2 for score 100-199, moderate staining; 3 for score 200-299, strong staining; 4 for score 300, very strong staining.
Cell culture
Huh7 cells were obtained from Japanese Collection of Research Bioresources (National Institute of Health Sciences; Japan, JCRB), and maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS. The immortalized cell line derived from human primary hepatocytes, Hus-E/2 (Hus), was cultured in P.H. medium (DMEM which contains 20 mM HEPES, 15 μg/ml L-proline, 0.25 μg/ml insulin, 50 nM
dexamethasone, 44 mM sodium bicarbonate, 10 mM nicotinamide, 5 ng/ml EGF, 0.1 mM ascorbic acid). All of these cell lines were incubated in 5% CO2 atmosphere at 37°C.
Creation and culture of L-FABP overexpressed stable clones
The pcDNA3.1/L-FABP was constructed by inserting full-length L-FABP cDNA fragment (1-121 aa) in the pcDNA3.1 Vector via TOPO PCR cloning system (Life technologies, USA), which was cloned by cDNA of Huh7 cells, and the construct was checked by nucleotide sequencing. Hus cells were transfected with pcDNA3.1/L-FABP using the Lipofectamine 2000 (Invitrogen, USA). Stable clones were selected by
14
medium containing 1 mg/ml G418 (Sigma-Aldrich, USA) for 2-4 weeks. Each of the clones was checked for L-FABP expression twice per month by western blot analysis.
Western blot analysis and immunoprecipitation
Purified proteins (50 μg) were resolved by 10% sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membrane was incubated with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies (Chemicon International, USA). Signals were visualized using enhanced chemiluminescence detection reagent from Millipore, and the images were obtained using a Luminescence/Fluorescence Imaging System (LAS-4000, Fuji).
For immunoprecipitation, cell lysates (500 μg protein) were pre-cleared by protein A/G Sepharose beads (Millipore), and then incubated with anti-L-FABP or
anti-VEGFR2 antibody overnight at 4°C. The immunoprecipitated complexes were washed three times by ice-cold PBS, and captured by protein A/G Sepharose beads, and then the immunoprecipitated proteins were subjected to western blot analysis.
Cell migration assay
Transwell Boyden chambers (Millipore) were applied to cell migration and invasion assays. For migration assay, cells were maintained in serum-free medium for
15
24 h and then seeded into the chambers, and followed by incubation in complete medium with 10% fetal bovine serum at 37°C for 16 h. The cells on the bottom side of the membrane were fixed with 1% formaldehyde/phosphate buffered saline for 15 min, stained with 0.1% crystal violet for 40 min and counted using an inverted contrast light microscope.
Angiogenesis activity assay 1. Cell culture
Primary HUVEC (Sciencell, California, USA) were grown in M199 medium containing with Endothelial Cell Growth Supplement (ECGS) (100 μg/ml), 10 ng/ml
heparin, and 5% fetal bovine serum (FBS) and cultured in 5% CO2 atmosphere at 37°C.
2. In vitro tube formation assay
A 24-well plate was coated with 100 μl of Matrigel (1 mg/ml; BD Biosciences), which was allowed to solidify at 37°C for 1 h. HUVEC (1×104 cells per well) were seeded on Matrigel and incubated with the conditioned medium collected from the indicated cultured cells (L-FABP overexpressed Hus cells or L-FABP stable knockdown Huh7 cells) for 8~12 h, whereas the VEGF group was used to check the angiogenic activity of HUVEC cells. Photographs from random fields were taken using a
microscope (Olympus, DP-50, Tokyo, Japan), and the quantification of each images was
16
followed by the following formula [34],
Angiogenic score= [(No. of sprouting cells) ×1 + (No. of connected cells) ×2 + (No. of polygons) ×3)] / Total number of cells + [0, 1 or 2]
The definition of cell types and parameters 0, 1 or 2 can be found in the above mentioned studies.
3. In vivo Matrigel plug assay and CD31 IHC staining
Matrigels (phenol red-free, BD Biosciences) were mixed with L-FABP overexpressed or L-FABP-knockdown stable clones (2 × 106 cells/ matrigel/ mouse). The Matrigel plugs were subcutaneously injected into 4-week-old male NOD/SCID mice, and then recovered on day 10 for following analysis. We performed CD31 IHC staining to determine the angiogenic activity of these tissues, since CD31 is used to serve as a superior marker for angiogenesis [35]. For detail, samples were fixed in 10%
paraformaldehyde, embedded in paraffin, sectioned, and then subjected to
immunohistochemical staining with the Novolink Polymer Detection System (Leica Biosystems). The sections were stained for CD31 (Santa Cruz Biotechnology), and the nuclei were counterstained with hematoxylin.
Short interference RNA (siRNA) and Short hairpin RNA (shRNA)
The modified oligonucleotides used as siRNA for L-FABP and the control siRNA
17
were obtained from Invitrogen. The shRNA clones were purchased from National RNAi Core Facility Platform, Taiwan. For transfection, 1 ×105 of Hus/L-FABP or Huh7 cells were plated in a six-well plate for 24 h, and siRNA or shRNA transfection was
performed using the Lipofectamine 2000 (Invireogen) to knockdown mRNA expression [36].
Lipid rafts isolation
Raft microdomains were purified by method described previously [37]. Briefly, cells were washed and applied to 700 μl 1% Triton X-100 lysis buffer, and the cell
membrane was disrupted by using a Teflon-coated dounce homogenizer (20-30 strokes).
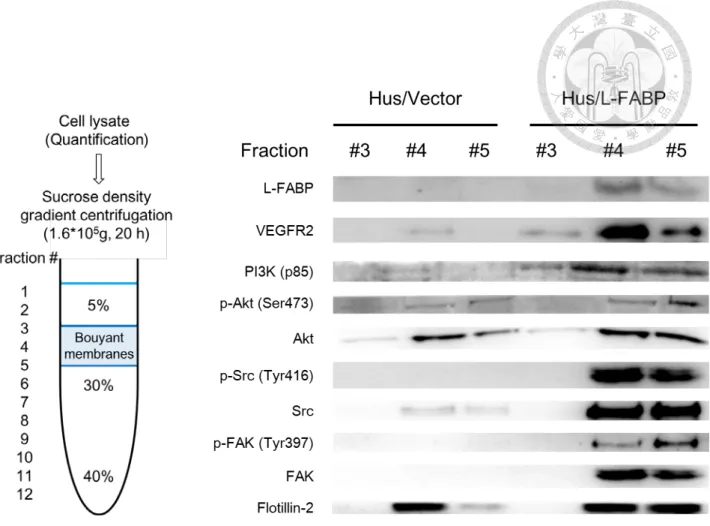
The lysate (4 mg) was then incubated at 4°C for 30 min, and mixed with the same volume of 80% sucrose solution to yield a mixture at a final of 40% sucrose gradient and then transferred into a 12 ml polyallomer ultracentrifuge tube (for an SW41 roter, Beckman Instruments). Then, 6.5 ml of 30% and 3.5 ml of 5% sucrose cushion was overlaid on the top of sample and applied to ultracentrifugation at 187,813 g, 20 h, 4°C using SW41 rotor. The floating opaque band corresponding to the detergent-resistant lipid rafts was collected and used for western blot analysis.
18
Confocal microscopy analysis
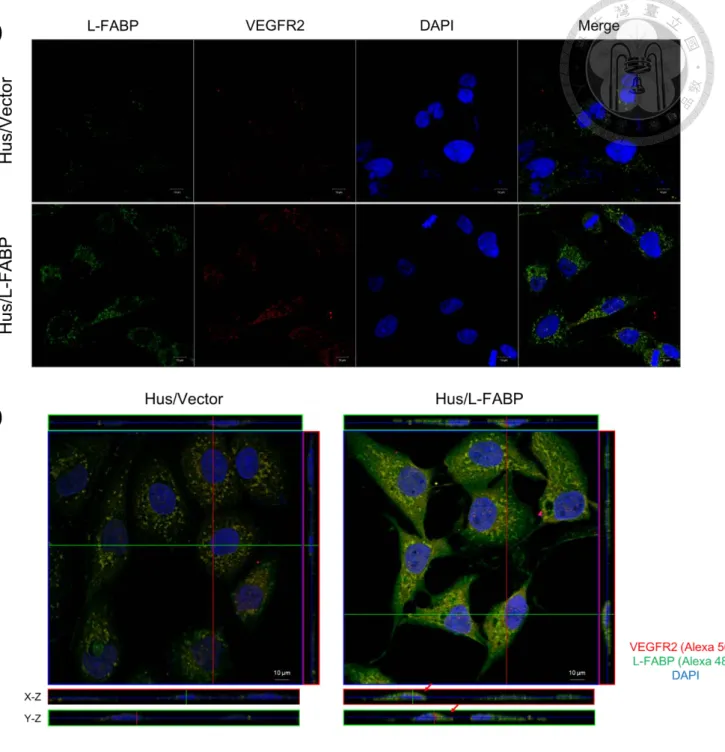
L-FABP stable expressed Hus cells were seeded on the 22 × 22 cover slide, washed, fixed, and permeabilized with 0.25% Triton X-100 for 10 min. For double staining, the slides were first incubated with L-FABP and VEGFR2 primary antibody O/N, and then stained with Alexa488 (anti-mouse) and Alexa568 (anti-rabbit) (20 mU/mL) for 1 h in darkness, followed by counter-staining for nuclei with DAPI (10 ng/mL) for 10 min. By using Leica TCS SP5 Spectral Confocal System, the images were captured and
analyzed.
Small GTPase binding assay
The small GTPase binding assay was referred to previous study [38]. For detail, 1×107 cells were seeded and collected in 0.4 ml of ice-cold lysis buffer (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 500 mM NaCl, 1% Triton X-100, and protease
inhibitor cocktail). After lysis for 20 min on ice, cell debris was removed by centrifugation at 300 g for 10 min at 4°C. Half of each lysate (100 μg protein) was mixed with 15 μl of GST-PBD or GST-RBD beads (50 μg of protein) and incubated for
1 h at 4°C with rotation. Samples were then centrifuged (5,000 rpm for 1 min at 4°C) and washed twice in ice-cold wash buffer (25 mM Tris-HCl, pH 7.5, 30 mM MgCl2, and 40 mM NaCl), finally resuspended in 30 μl SDS sample buffer and heated at 100°C
19
for 5 min, and then processed for western blot analysis.
Construction of human VEGF-A promoter
The VEGF-A promoter (full-length, bp -1127 to +73, total 1190 bps) was synthesized by ShineGene Molecular Biotech Inc, and constructed into puc57 vector.
By cutting with SacI and HindIII restriction enzymes, the full length promoter was cloned into pGL4.22 luciferase reporter vector. The 5’ serial deletion constructs of VEGF-A promoter were generated and named as follows: D1: bp -901 to +73; D2: bp -782 to +73; D3: bp -199 to +73. The primers used in the above cloning were listed in supplementary data, Table 1, and all constructs were checked by nucleotide sequencing.
Luciferase reporter assay
Luciferase activities were determined using Dual-Luciferase Reporter Assay System (Promega, USA). L-FABP overexpressed Hus cells were transfected with constructed pGL4.22/ VEGF-A promoter plasmids and pGL4-Renilla luciferase control reporter plasmid as an internal control. For 24 h incubation after transfection with lipofectamine 2000, the cells were lysed and the luciferase activities were examined by using the above assay system following the technical manual (Promega) and measured by SpectraMax L luminometer.
20
Animals
All animal experiments were carried out according to regulations approved by the Institutional Animal Care and Use Committee of College of Medicine, National Taiwan University. Male NOD-SCID mice (4 weeks old) were obtained from the LASCO Taiwan Co., Ltd. For xenograft experiments, Hus/L-FABP or Hus/Vector cell lines (2 × 106cells each) were suspended in 200 μl of OPTI-MEM (Invitrogen) and inoculated into the right hind limb of each mice (n=6 for each group). Tumor size was measured twice per week with calipers, and the tumor volume was estimated using the formula:
(width)2 × length/ 2. After 8 weeks, the mice were anesthetized by Zoletil 50 (Virbac Animal Health) and sacrificed by CO2 euthanasia, and the tumors were removed, measured, and processed for immunohistochemistry.
For metastasis assay, we used lung metastasis model according to previous studies
[39]. For detail, Hus/L-FABP or Hus/Vector cell lines (4 × 106 cells each) were suspended in 100 μl of OPTI-MEM, and inoculated i.v. into the tail vein of male
NOD/SCID mice (n=6 for each group). The experimental mice were anesthetized by Zoletil 50 (Virbac Animal Health) and sacrificed by CO2 euthanasia after 10 weeks; the metastatic colonies in lungs of each mice were counted and photographed, and all the lungs were removed, fixed, and embedded in paraffin for immunohistochemical analysis.
21
Cloning of L-FABP mutants
The amino acid substitution of wild-type L-FABP protein was carried out as follows: L-FABP point-mutation clones were generated by QuickChange
Site-Directed mutagenesis kit (Stratagene), including Phe3 to Trp (F3W), Lys31 to Glu (K31E), and Thr94 to Ala (T94A). The primers for PCR reaction and subsequent treatment with DpnI to eliminate the template DNA were listed in supplementary data, Table 1, and all constructs were checked by nucleotide sequencing.
Statistical analysis
Relationships between protein expression and categorical variables (sex, grade, invasion depth, lymph node metastasis and TNM stage) were compared using Chi-square tests. For multivariate analysis, independent prognostic factors were
determined using Cox’s proportional hazard model. Survival curves were calculated by the Kaplan-Meier method and compared by log-rank tests. The in vitro and in vivo experiments were analyzed by GraphPad Prism 5, with the data presented as the mean ± standard error of the mean (SEM). Statistical significance was defined as a p value <
0.05.
22
§Part II Plant extracts
Water extracts from A. chinensis were supplied by the Sun Ten Pharmaceutical Company (Taipei, Taiwan). The plant materials were boiled in water and concentrated to 1 g/ml with an evaporator, and the stock solutions were stored at −20°C until use.
HPLC analysis
We analyzed the constituent distribution and content in the water extracts of A.
chinensis by high-performance liquid chromatography-diode array
(HPLC-DAD)/evaporative light scattering detector (ELSD) chromatography under the following conditions: a linear gradient of ddH2O to methanol for 60 minutes, and 100%
methanol for another 10 minutes at a flow rate of 1mL/minute with DAD/ELSD.
Reagents
Corosolic acid (CA), ursolic acid, 3-(4,5-Dimethylthiazol-2-yl)-2,5-dipheny ltetrazoliumbromide (MTT), and sulphorhodamine (SRB) were obtained from Sigma-Aldrich. Sorafenib was purchased from Santa Cruz Biotechnology.
Lipofectamine 2000, VEGFR2 (KDR) siRNA, phalloidin, and Alexa Flour Dyes were obtained from Invitrogen Life Technologies. The primary antibodies against VEGFR2,
23
p-VEGFR2 (Tyr1054), p-VEGFR2 (Tyr951), Src, p-Src (Tyr416), FAK and p-FAK (Tyr397) were purchased from Cell Signaling Technology. The Matrigel Matrix was obtained from BD Biosciences.
Cell culture
The HCC cell lines: Huh7, HepG2 and Hep3B were obtained from Japanese Collection of Research Bioresources (National Institute of Health Sciences; Japan, JCRB) and maintained in Dulbecco’s Modified Eagle Medium-High Glucose
(Invitrogen) medium with 10% fetal bovine serum (FBS), 2mM L-glutamine
(Invitrogen), and 100 μg/mL penicillin-streptomycin (Invitrogen). Cells were cultured in
a humidified atmosphere in 5% CO2 at 37°C.
Cytotoxicity assay
To study the cytotoxicity of CA, the MTT assay was performed as described previously [40]. Huh7 cells were seeded at 5 × 103 cells/well in 96-well plates and treated with 0.1% DMSO (control) or various concentrations of CA for 24 h. The number of viable cells was estimated by measuring the conversion of tetrazolium salt MTT to formazan crystals. After incubation with MTT for 6 h, the formazan crystals were solubilized with an SDS solution (10% SDS and 0.01M HCl) and quantified by
24
measuring the absorbance at 590 nm with a reference wavelength of 650 nm.
Migration assay
In the upper chamber, Huh7 cells (5 × 104 cells) were starved overnight, and resuspended in 300 μL serum-free DMEM medium with 0.1% DMSO (control) or
various concentrations of CA, and seeded into Transwell inserts (8 μm pore; BD
Biosciences). The complete DMEM medium was added to the lower chamber, and then incubated for 16 h; the migrated cells were fixed, stained with crystal violet, and quantified in 3 random fields (40x magnification) per insert [40].
Immunoprecipitation
For immunoprecipitation, Huh7 cells were treated with 0.1% DMSO (control) or CA for 15 min and lysed in RIPA buffer. The lysates were then sonicated and
centrifuged, and the supernatant was incubated with anti-VEGFR1, R2, and R3 antibody (Santa Cruz Biotechnology, Inc.) overnight at 4°C. The immune-complexes were then incubated with PureProteome magnetic beads (Millipore) for 1 h at 4°C, washed and eluted with protein sample buffer, and analyzed by western blotting.
25
Western blot analysis
Cells were collected at the indicated time points and protein was extracted with RIPA buffer. Proteins samples were analyzed by SDS-PAGE, transferred to PVDF membrane, and blocked with 5% milk in TBST. Membranes were then incubated with the following primary antibodies against VEGFR2, p-VEGFR2 (Tyr1054), p-VEGFR2 (Tyr951), Src, p-Src (Tyr416), FAK and p-FAK (Tyr397). After incubation with an HRP-conjugated secondary antibody (Santa Cruz Biotechnology), membranes were developed with ECL reagent (Millipore). Signals were captured with an LAS-3000 image capture system (Fuji) and quantified with ImageJ software [41].
Kinase activity assay
The experiment was performed with the ADP-Glo kinase assay kit (Promega, WI, USA). Briefly, CA was first diluted with kinase reaction buffer at a 1:2 dilution ratio in different tubes (starting from 1 mM). Three nanograms of KDR (#V2681, Promega)
were added to each tube and incubated for 10 min. Then, 0.1 μg/μL substrate and 10 μM ATP were added to each tube and incubated for 1h at room temperature. Next, 25 μL
ADP-Glo reagent was added to the mixture and incubated at room temperature for 40 min. Finally, 50 μL kinase detection reagent was added to introduce luciferase and
samples were measured with a SpectraMax L Microplate reader (Molecular Device, CA,
26
USA).
Rho GTPase activity assay
Huh7 cells were treated with 0.1% DMSO (control) or CA for 6 h and collected in RIPA buffer. Whole cell lysates (500 μg) were combined with purified GST fusion
protein conjugated with Rac1, RhoA, or cdc42 binding domain (PAK-PBD for Rac1 and cdc42, Raf-RBD for RhoA) and incubated with head-to-head rotation at 4°C overnight [42]. MagneGST beads (Promega, WI, USA) were then added to the mixture to pull down the immune-complex. Samples were centrifuged at 14,000 rpm for 30 min, washed with RIPA buffer 5 times, boiled with SDS sample buffer, and analyzed by western blot analysis.
G-actin/F-actin activity assay
The assay was performed as previously described [43]. To summarize, Huh7 cells were treated with 0.1% DMSO (control) or CA for 6 h and incubated in stabilizing buffer (1% Triton X-100, 1 μg phalloidin, and protease inhibitor cocktail) at room temperature for 5 min. Cell lysates were collected, followed by centrifugation at 100,000 g for 1 h at 37°C. The supernatant was removed and saved as the G-actin fraction. The pellets were washed twice with PBS and dissolved in 200 μL dissolving
27
buffer (1% Triton X-100, 2% SDS, and protease inhibitor cocktail) by sonication twice, put on ice for 1 h, and saved as the F-actin fraction. Both fractions were then analyzed by western blotting.
Confocal microscopy analysis
Huh7 cells were seeded on a 22 × 22 cover slide and treated with 0.1% DMSO (control) or CA for 6 h. At the indicated time, the cells were washed, fixed, and
permeabilized with 0.25% Triton X-100 for 10 min. For double staining, the slides were first incubated with p-FAK (Tyr397) primary antibody overnight, and then stained with Alexa488 (anti-rabbit) and Alexa568-phallodin (20 mU/mL) for 1 h in darkness [44].
Finally, the samples were counter-stained for nuclei with DAPI (10 ng/mL) for 10 min.
The images were captured and analyzed using the Leica TCS SP5 Spectral Confocal System. The actin filament intensity was measured by ImageJ (NIH) and calculated by the following formula [45]:
Corrected total cell fluorescence (CTCF) = Integrated Density – (Area of selected cell
× Mean fluorescence of background readings)
Animal model
All animal experiments were conducted according to the guidelines approved by
28
the Institutional Animal Care and Use Committee of the College of Medicine, National Taiwan University, and the study were approved by the Animal Care and Use
Committee at National Taiwan University. The male NOD/SCID mice (4–6 weeks old) were obtained from BioLASCO Taiwan Co., Ltd, and kept in Laboratory Animal Center of the College of Medicine, National Taiwan University. The experimental mice were housed into individually-ventilated cages (IVC), and free accessed to food and drinking
water. For studying the anti-tumor effect of CA alone, Huh7 cells (2 × 106 cells) were suspended in 200 μL of Opti-MEM (Invitrogen) and injected subcutaneously into the flanks of each mouse. After one week, the mice were treated with 50 μL DMSO (control)
or CA (5 mg/kg/day) by intraperitoneal injection (n = 5 for each group) for 21 days. To
study the combinatorial effect of CA and sorafenib, Huh7 cells (5 × 106 cells) were suspended in 100 μL of Opti-MEM with matrigel-matrix (1:1 mix ratio), and injected
subcutaneously into the flanks of each mouse. After one week, the mice were treated with 50 μL DMSO (control) and compounds by intraperitoneal injection (n = 5 for each
group) for 20 days. The tumor volume was calculated by the following formula: tumor volume [mm3] = (length [mm]) × (width [mm] 2) × 0.5. At the end of the experiment, the mice were anesthetized by Zoletil 50 (Virbac Animal Health) and sacrificed by CO2
euthanasia. The tumors were excised, weighed, and fixed for further studies.
29
Immunohistochemistry
Samples for these experiments were obtained from the xenograft experiment and fixed in 10% paraformaldehyde, embedded in paraffin, and sectioned. The tissue sections were then subjected to immunohistochemical staining with the Novolink Polymer Detection System (Leica Biosystems). The sections were stained for p-VEGFR2 (Tyr951, Cell Signaling Technology), Ki-67 and p-FAK (Tyr397) (Santa Cruz Biotechnology), and the nuclei were counterstained with hematoxylin.
Synergistic analysis
The synergistic analysis was analyzed by the Compusyn software, which was developed by Chou and Martin [46]. The software was used to estimate the combination index (CI) and fa (fraction affected by drugs) to study the combined effect of drugs. A CI < 1, CI = 1, and CI > 1 indicates synergistic, additive, and antagonistic effects, respectively.
Molecular docking
The interaction of CA and the ATP-binding site in VEGFR2 was studied by Discovery Studio Modeling 4.0 and displayed by PyMOL (ver. 1.6.0b1). The structure of CA was obtained from ZINC (code: 08829484), and the crystal structure of VEGFR2
30
was obtained from Protein Data Bank (PDB id: 1YWN).
SRB cell growth assay
Huh7, HepG2, and Hep3B cells were seeded into 96-well plates (5 × 103 cells/well) and treated with 0.1% DMSO (control) or various concentrations of CA and sorafenib.
After 24 hours, cells were fixed with 10% TCA and stained with SRB at 0.4% (w/v) in 1% acetic acid. The cells were then washed by 1% acetic acid, solubilized with 10 mM Tris base solution, and measured the absorbance by ELISA reader (515 nm wavelength).
Statistical analysis
Data were presented as means with standard errors (SE) and analyzed with Prism 6 (GraphPad Software, Inc.) and Sigmaplot version 10 (Systat Software Inc.). One-way ANOVA was used to compare results with more than one treatment, and the Student’s t-test was performed to compare differences between two groups. P < 0.05 was considered statistically significant.
31
Results
§Part I
1. Up-regulation of L-FABP expression in HCC tissues is correlated with VEGF-A overexpression
First, we performed IHC staining for the tissues from total 90 patients, including 12 females and 78 males, with average age 53.5 ± 10.0 years (Table 2). The expression level of L-FABP in 90 pairs of HCC tumor (T)/ normal adjacent tissue (NAT) were classified into different expression levels including weak, moderate and strong, and the related photographs were represented in Figure 1A. L-FABP showed a significantly higher expression level in tumor part compared with that of NAT part among all tissue types of HCC tissues (NAT, HCC with cirrhosis, HCC without cirrhosis) (Table 1, p=0.012). In addition, the level of VEGF-A revealed a strongly positive correlation to the level of L-FABP (r=0.737, p<0.01, n=90) (Figure 1B). Taken together, these clinical results indicate that L-FABP up-regulation is associated with VEGF-A expression in HCC.
2. L-FABP induces VEGF-A expression and angiogenic potential in immortalized Hus and Huh7 cells
The functional role of L-FABP in HCC was studied by analyzing the expression
32
level of L-FABP in various cell lines, including immortalized normal hepatocyte (Hus) and HCC (HepG2, Hep3B, Huh7 and PLC/PRF/5) cells. As shown in Figure 2A, L-FABP was highly expressed in HepG2 and Huh7 cells, and VEGF was also highly expressed in these cells; angiogenic potential was higher in these cells than in those with lower L-FABP expression level (Hus, Hep3B and PLC/PRF/5) (Figure 2B).
Accordingly, to examine the effects of L-FABP on VEGF-A expression, we generated Hus cells that stably express L-FABP, as well as Huh7 cells that L-FABP was
knockdown by shRNA. Figure 3 showing that Hus/L-FABP cells exhibited a higher VEGF-A expression level including mRNA, cytosolic protein, and protein secreted to cultured medium than that of control cells (Figure 3), whereas the expression levels of VEGF-A were decreased in Huh7/L-FABP shRNA cells (Figure 24). The Hus/L-FABP cells also exhibited higher angiogenesis activity than the control cells (Figure 4A), whereas angiogenic activity was down-regulated in Huh7/L-FABP shRNA cells (Figure 24B). To further examine whether L-FABP promotes angiogenesis in vivo, we
performed matrigel plug-in assay in NOD/SCID mice by using Hus/L-FABP (Figure 4, B and C) or Huh7/L-FABP shRNA cells (Figure 24C), and the results showed that L-FABP over-expressed cells promoted angiogenesis activity by inducing neovascular formation in matrigel as shown by anti-CD31 IHC staining.
33
3. Association of L-FABP with VEGFR2 in membrane rafts
Previous studies reported that some FABPs, such H-FABP or B-FABP, could interacts with membrane associated receptors, including integrin or dopamine D2 receptor [47-49]. It also suggested that L-FABP possibly associated with cell membrane or membrane proteins [10, 50]. Thus, we proposed that L-FABP could be also
associated with membrane receptors, and by the alignment of FABP interacted amino acid sequence in previous studies, we found that the consensus sequence-
WKIGFXKRLXXVXXXI (Figure 5) of membrane receptors is most likely as
interaction site with L-FABP. By comparing the consensus sequence to other membrane receptors, we observed that the kinase domain of VEGFR2 showed a possibility of interacting to L-FABP. Thus, we performed co-immunoprecipitation by using primary antibodies against VEGFR2 or L-FABP, followed by western blotting with L-FABP, or VEGFR2. Both experiments showed that L-FABP could interact with VEGFR2 (Figure 6). Furthermore, we used confocal microscopy analysis revealed that L-FABP located in both membrane and cytosol, whereas VEGFR2 was located mainly on membrane.
Notably, the co-localization of L-FABP and VEGFR2 in apical membrane was demonstrated in Hus/L-FABP cells (Figure 7, indicated by arrows). Furthermore, isolation of membrane by sucrose gradient ultra-centrifugation also showed
co-localization of L-FABP and VEGFR2 in membrane. As shown in Figure 8, fractions
34
with lipid rafts of Hus/L-FABP cells were identified by lipid raft marker, flotillin-2;
interestingly, not only L-FABP and VEGFR2, membrane associated signal transduction proteins including PI3K (p85), p-Akt/Akt, p-Src/Src, p-FAK/FAK were also detected the increasing distribution levels in membrane rafts. Taken together, these results
indicated that overexpressed L-FABP not only associated with membrane VEGFR2, but may also activate its downstream signal transduction signals including PI3K/Akt and Src/FAK.
4. L-FABP increases VEGFR2/ Src phosphorylation and cell migration by FAK/cdc42 pathway
Previous reports indicated that VEGFR2/Src pathway is associated with cancer cell migration by activating FAK and Rho-GTPase [51-53]. In Hus/L-FABP cells, the
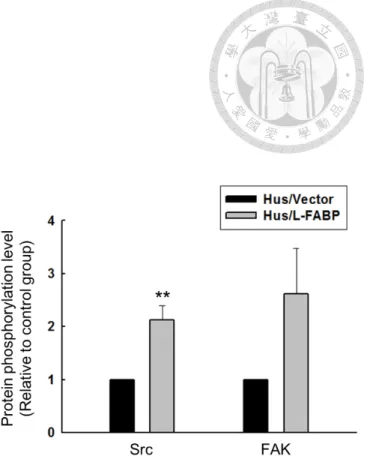
phosphorylation of VEGFR2, Src and FAK was increased significantly (Figure 9 and 10), and by small GTPase binding assay, the activity of cdc42 was significantly
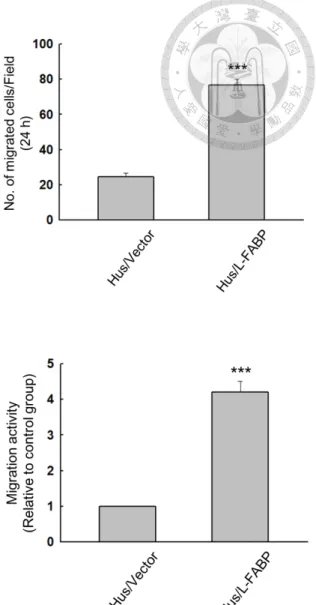
up-regulated in Hus/L-FABP cells (Figure 11). By performing wound-healing assay for studying 2D migration activity (Figure 12A), and Boyden chamber based migration assay for studying 3D migration activity (Figure 12B), Hus/L-FABP cells had higher migration activity than that the control cells. Furthermore, L-FABP knockdown resulted in a significant decrease in 3D migration activity in Huh7 cells. (Figure 24D).
35
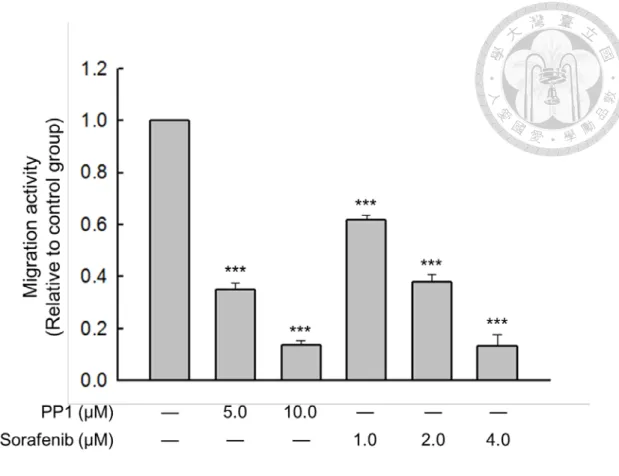
Additionally, by treating Hus/L-FABP cells with Sorafenib (VEGFR2 inhibitor) or PP1 (Src inhibitor), significant inhibitory effects on migration activity were found (Figure 13). Moreover, knockdown of L-FABP in Hus/L-FABP cells reversely down-regulated its 3D migration activity (Figure 23C). These results suggest that VEGFR2/
Src/FAK/cdc42 signaling is participated in L-FABP induced migration activity.
5. L-FABP induced VEGF-A expression by Akt/mTOR/P70S6K/4EBP1 in translation level
According to our above-mentioned results in Figure 8, we proposed that the signal transduction of L-FABP mediated VEGF-A expression was activated through Akt pathway. Since Akt signaling has been reported to be the major pathway to increase VEGF-A expression level in previous reports [54, 55]. Therefore, we performed western blot analysis, and the results showed that the L-FABP activated Akt/mTOR/
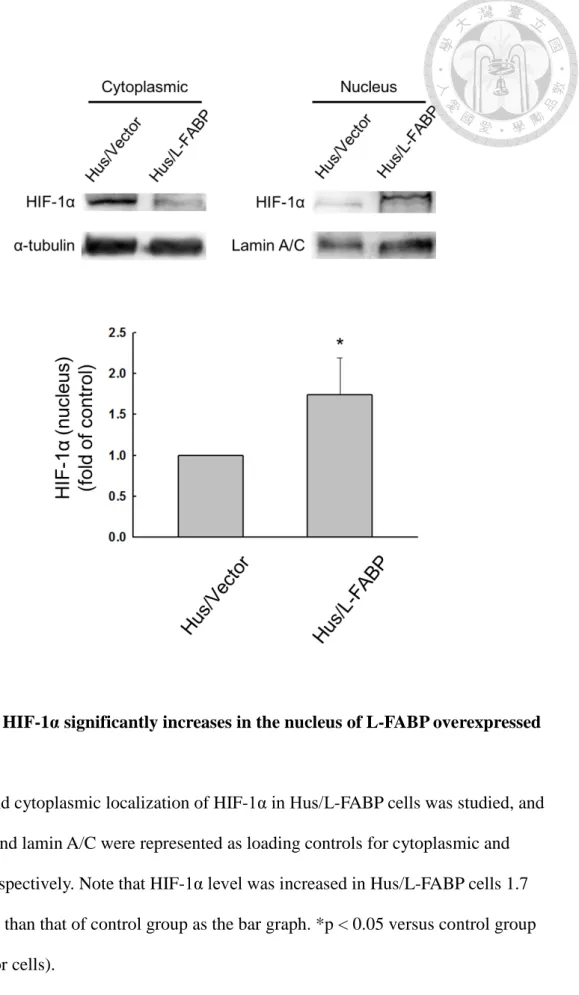
P70S6K/4EBP1 pathway in Hus/L-FABP cells (Figure 14). Previous results have suggested that VEGF-A mRNA expression level could be regulated by HIF-1α dependent or independent manner [54, 56]. In our studies, we found that the mRNA expression level of VEGF-A was significantly up-regulated in L-FABP overexpressed cells as showed in Figure 3, and HIF-1α, which serves as the major transcription factor to regulate VEGF-A expression, was also shown an increased level in the nucleus
36
fraction of Hus/L-FABP cells (Figure 15). To further confirm this observation, the full-length construct and a serial of successive 5’ deletions (D1-D3 constructs) of VEGF-A gene promoter were cloned into pGL4.22 luciferase reporter vector, and the luciferase reporter assay was performed to measure the transcriptional activity of VEGF-A promoter and its deletion mutants in L-FABP overexpressed Hus cells. The results revealed that the VEGF-A transcriptional activity was elevated ~16.5-fold as compared with that of control cells, whereas the deletion of HIF-1α binding site (D1-D3) clearly abolished its activity to ~2.5 fold of control group (Figure. 16).
To further discuss the regulation of VEGF-A expression in post-transcription level, Hus/L-FABP cells were treated with Rapamycin (mTOR inhibitor) or Cyclohexamide (translation inhibitor), and a dose-dependent decreased of VEGF-A expression level or its angiogenic potential was found (Figure 17, A and C). The effects of proteasome inhibitor, MG132, on Hus/Vector cells were investigated, and the results indicated that L-FABP induced VEGF-A expression was not via the inhibition of protein degradation (Figure 17B). Taken together, these data suggested that the induction of VEGF-A expression by L-FABP was regulated both in transcription and translation levels.
6. L-FABP promotes tumor growth and metastasis in vivo
The role of L-FABP in tumorigenesis was examined in immune-deficient
37
NOD/SCID mice, and the results indicated that tumor weight was significantly
enhanced in the group injected with Hus/L-FABP cells as measured on day-50. (Figure 18A). The levels of VEGF-A in mice serum were also up-regulated 2.8-fold in
Hus/L-FABP group than that of control group (Figure 18B), and the
immunohistochemistry staining of CD31 also indicated that L-FABP induced
angiogenesis in vivo (Figure 18C). We further investigated the role of L-FABP in tumor metastasis in vivo, and the Hus/L-FABP cells or control cells were injected i.v. into the tail vein of NOD/SCID mice. After 60 days, the number of metastatic nodules formed in lung was 3.9-fold higher in Hus/L-FABP group than that of control group (Figure 19A), the increase of angiogenic vessel formation in these nodules was also demonstrated (Figure 19B). These in vivo experiments further supported the correlation of L-FABP and VEGF-A expression in present clinical tissue analysis.
7. Cholesterol associating and membrane interacting activities are essential for L-FABP induced cell migration and angiogenesis
Previous studies suggested that L-FABP mutations result in the ablation of fatty acid or cholesterol uptake, even the membrane structure [57-61]. Thus, to examine how L-FABP interacts with membrane in overexpressed cells, we used site-directed
mutagenesis to generate L-FABP mutant stable clones with the substitution of different
38
functional amino acids expressed in Hus cells. As showed in Figure 20A, three mutants including F3W, K31E, and T94A showed a reduced VEGF-A expression level and a significantly decreased angiogenic activity than that of wild type group (Figure 20B).
However, the migration level down-regulated significantly only in K31E and T94A mutants, but not in F3W mutant which exhibited minor effect (Figure 21). T94A is the most common mutation occurred in Europeans and has been found to affect fatty acid and cholesterol uptake as a loss-of-function mutation [61]. Thus, to verify this result, we reduced membrane cholesterol content with MβCD (cholesterol depletion reagent) in Hus/L-FABP cells, and the result suggested that the VEGF expression, migration activity, and their related signals in Hus/L-FABP cells were all down-regulated significantly (Figure 22, A and B). Taken together, the oncogenic activity of L-FABP showed a certain degree of correlation to its membrane-binding property.
§Part II
8. Corosolic acid significantly decreases the migration activity of Huh7 cells To study anti-migration effects of corosolic acid (CA) on Huh7 cells in vitro, we first treated Huh7 cells with various concentrations of CA for 24 h. Cell viability was then measured with an MTT assay, and as shown in Figure 29A, CA decreased the survival rate of Huh7 cells; the IC50 of cytotoxicity was determined to be 50 μM. Then,
39
we performed a transwell assay with Huh7 cells, CA inhibited Huh7 cell migration in a dose-dependent manner, and the IC50for migration was found to be 2.5 μM (Figure 29B). The results indicate that CA has a relatively higher inhibitory effect on Huh7 cell migration than cell viability. (IC50 cytotoxicity/IC50 migration = 20).
9. Corosolic acid inhibits VEGFR2 kinase activity
Previous studies suggest that VEGF/VEGFR signaling can facilitate cancer cell metastasis [62], and inhibition of VEGFR can reduce HCC cell migration [63]. Thus, to investigate whether CA inhibits VEGFR activation, we performed immunoprecipitation to pull down three key VEGFRs in Huh7 cells, including VEGFR1, R2, and R3,
followed by blotting with phospho-tyrosine antibody. The results suggest that CA significantly reduced phosphorylation of VEGFR2 by 70% without affecting total
VEGFR2 expression, while CA exhibited weaker effect to VEGFR1 & R3 (Figure 30).
With a VEGFR2 kinase activity assay, 0.95 μM CA was also found to inhibit VEGFR2
kinase activity by 50% (Figure 31). To examine whether the anti-migration effect of CA is mediated by VEGFR2, we attenuated endogenous VEGFR2 of Huh7 cells by siRNA.
The knockdown cells lost sensitivity to CA-induced inhibition of migration (Figure 32).
Taken together, these results suggest that CA inhibits Huh7 cell migration by inhibiting VEGFR2 activation.
40
10. Corosolic acid decreases cell motility by inhibiting VEGFR2/Src/FAK/cdc42 activity and actin rearrangement
To further elucidate the mechanism underlying the anti-migration effect of CA, we performed western blot analysis. Treatment with CA decreased the phosphorylation level of VEGFR2 (Tyr1058), and the phosphorylation level of non-receptor tyrosine kinase, Src (Tyr416), and focal adhesion kinase, FAK (Tyr397), were also
down-regulated by CA (Figure 33). It was reported previously that focal adhesion kinase (FAK) is activated by membrane receptors such as RTKs or integrins, then the Src/FAK complex modulates cell migration and actin rearrangement via Rho-GTPase pathways. Therefore, using a Rho-GTPase activity assay, we found that active cdc42, but not active Rac1, or active RhoA, is significantly down-regulated by CA treatment (Figure 34). Recent studies have revealed that cdc42 may play an important role in the dynamic change of actin and the formation of filopodia during cell migration. To study whether CA disrupts actin rearrangement in Huh7 cells, we performed a G-actin/F-actin assay. The results demonstrated that CA treatment reduces the ratio of F-actin/G-actin (polymer/monomer) by about 50% compared to that of control group (Figure 35A). By confocal microscopy analysis, we also found that CA decreases the co-localization of phospho-FAK (Tyr397) and F-actin on the filopodium (leading edge) in Huh7 cells
41
(Figure 35B). Taken together, these results indicate that CA inhibits Huh7 cell migration by suppressing the VEGFR2/Src/FAK/cdc42 pathway and actin rearrangement.
11. Corosolic acid exhibits anti-tumor effects in vivo
The effects of CA on tumor growth were investigated in vivo using a xenograft model. Mice were given daily i.p. injection of CA (5 mg/kg/day). CA had significant inhibitory effects on tumor growth in NOD/SCID mice injected with Huh7 cells (2 × 106 cells/mice) (Figure 36A). After 21 days of treatment, the mice were sacrificed and the volume of tumors in CA-treated group (63 ±19 mm3) were much smaller than that of control group (669 ±67 mm3). In addition, the CA-treated group (5 mg/kg/day) showed 85% reduction in tumor mass compared to that of the control group (Figure 36B). Body weight of mice treated with CA were similar to that of control group (Figure 36C), suggesting that the dosage of CA administered had no significant toxic effects to the mice. The levels of Ki-67, phospho-VEGFR2 and phospho-FAK in tumor lesions were examined by immunohistochemistry; CA reduced the expression level of Ki-67, and the phosphorylation of both VEGFR2 and FAK significantly in HCC xenograft mice (Figure 36D).
42
12. Synergistic effects of corosolic acid and sorafenib on HCC cells
Sorafenib (Nexavar), a multi-kinase inhibitor including VEGFR2/3, PDGFRβ, and Flt-3, has been used to treat HCC patients and has a significant migration-inhibitory effect on HCC cells [64]. We performed a transwell assay with both CA and sorafenib
treatment; CA exhibited a migration inhibitory activity comparable with that of
sorafenib (Figure 37A). Ursolic acid (3β-hydroxyurs-12-ursen-28-ic acid) (UA) shares a
similar chemical structure with CA and has been implicated in cancer prevention [65].
However, in the transwell assay, UA exhibited no significant anti-migration activity on Huh7 cells compared to that of CA. We demonstrated that CA has an inhibitory effect on migration comparable to sorafenib in HCC.
Then, to explore the effects of CA when used in combination with
chemotherapeutic agents for HCC, we studied the combinatorial effects of CA and sorafenib on migration activity of Huh7 cells. The results of transwell assay
demonstrated that CA has a synergistic effect with sorafenib on cell migration at a wide range of doses (Figure 37B). Moreover, to verify this, we performed a western blot analysis, and found that CA enhances sorafenib-mediated inhibition of phosphorylation of VEGFR2, Src, and FAK (Figure 38). Finally, the xenograft model indicated that combined treatment with CA and sorafenib showed a synergistic effect on tumor growth (CA 2.5 mg/kg/day with sorafenib 10 or 20 mg/kg/day) (Figure 39). These results
43
demonstrate a synergistic interaction between CA and sorafenib in the treatment of HCC cells.
13. Corosolic acid interacts with the ATP-binding site of VEGFR2 kinase domain by molecular docking
To further study whether CA decreases phosphorylation of VEGFR2, we used molecular docking software to analyze the interaction between CA and the kinase domain of VEGFR2. This analysis suggests that CA may bind to the ATP-binding cavity of the VEGFR2 kinase domain (Figure 41A). Previous studies suggested that Gln883, Cys917, and Asp1044 of VEGFR2 are involved in ligand binding through H-bond interactions [66]. As shown in Figure 41B, CA potentially interacts with Gln883 at a distance of 2.67Å. It also interacts with Val846, Lys866, Val897, Val914, and Cys1043.
These interactions between CA and the VEGFR2 kinase domain could result in inhibition of VEGFR2 and subsequent downstream intracellular signaling.
14. Corosolic acid does not exhibit significant inhibitory effects on Huh7 cell invasion
The matrix metalloproteinases (MMPs) are very important factors on cancer migration or metastasis [67]. To examine whether corosolic acid (CA) could inhibit
44
invasion activity of Huh7 cells, studies on the effects of CA for MMPs and NF- kappa B pathway, which is an important event to regulate the MMPs activity were evaluated.
However, in our model, corosolic acid (CA) had no significant inhibitory effect on Huh7 cell invasion (Figure 46A). The expression level of MMP2 and MMP9 and the
activity of MMP1, MMP2, and MMP9 were not affected by CA treatment (Figure 46B).
The level of phosphorylated IκB, IκB, and NFκB was maintained at a stable level (Figure 47) which suggested that NFκB pathway may not participate in CA effect.