竹子生物炭碳化溫度對於玉米及小麥發芽與 生長速率的影響
歐蒂娣1 黃武章2,
*
黃盈賓31屏東科技大學熱帶農業研究所
2屏東科技大學環境工程與科學系
3工業研究院南分院奈米研究中心
摘 要
儘管有學者質疑生物炭的廣範應用會阻礙了森林砍伐的功能。本文主旨在 於利用廢棄生物質,並提出了一個在較高的範圍內或在一個工業化生產的方式 下,以免去廢棄物對於環境威脅和處理不當導致的毀林。我們認為對於地球,
應該有一個可持續的共生與環境的關係,採取雙重使用竹子是一個聰明的選 擇,特別是對於那些只要利潤,而不傷害任何生態系統的生物炭行業。本研究 的主要目的是調查竹子生物炭的潛在能力,及其影響食用作物 (玉米及小麥) 的發芽和生長。總之在這項研究中所使用的竹子生物炭四個碳化溫 度 (240ºC,300ºC,600ºC 和 700ºC),小麥種子發芽率以 50%的 240ºC 和 300ºC 的 應用較好,而 600ºC 和 700ºC 竹子生物炭測定條件下,則發現為抑制發芽率最 高 (100%)。
關鍵詞:生物炭,環境,竹子,竹炭,劣解溫度。
EFFECTS OF PYROLIZATION TEMPERATURE OF BAMBOO BIOCHARS ON THE GERMINATION AND GROWTH RATES OF ZEA
MAIZE L. AND BRASSICA RAPA
Odette Varela Milla1 Wu-Jang Huang2, * Yin-Ping Huang3
1Department of Tropical Agriculture and International Cooperation National Pingtung University of Science and Technology
Pingtung, Taiwan 912, R.O.C.
2Department of Environmental Science and Engineering National Pingtung University of Science and Technology
Pingtung, Taiwan 912, R.O.C.
3ITRI South Campus Industrial Technology Research Institute
Tainan City, Taiwan 734, R.O.C.
Key Words: biochar, environment, bamboo, bamboo biochar, pyrolyzation temperatures.
*通訊作者:黃武章,e-mail: wjhuang@mail.npust.edu.tw
Corresponding author: Wu-Jang Huang, e-mail: wjhuang@mail.npust.edu.tw
ABSTRACT
Even though biochar discourages deforestation and aims to use waste biomass, producing it at a high scale or in an industrialized manner pre- sents a significant threaten for the environment and can lead to deforesta- tion. There should be a sustainable and symbiotic relationship with the environment, giving to the earth double what we take from her. The use of bamboo wood is a smart option for those industries that want to trans- form biochar into a profit without harming the ecosystem. The main ob- jectives of this study are to investigate the potential capability of bamboo biochar to affect germination and growth of edible crops. In conclusion, the four temperatures (240°C, 300°C, 600°C and 700°C) of bamboo bio- chars used in this study generally increased wheat seed germination at rates of application of 50% for 240°C and 300°C biochars; while 600°C and 700°C biochars tended to inhibit germination at the highest rate of applica- tion (100%) under the bioassay conditions.
I. INTRODUCTION
Compared to timber forests in the same growing con- ditions, bamboo can yield up to 25 times the amount of timber because it is ready to harvest so quickly. Some studies have found that bamboo can sequester four times more carbon than timber forests alone and at the same time release 35% more oxygen than timber forests, so there are many ecological benefits to bamboo growth [1]. Since bamboo can be used as a substitute for timber, it will also help decrease deforestation. Moreover, bamboo is highly sustainable as it can be regenerated within two to three years while timber may take longer than 25 years [2].
Biochar may be considered as a potential alternative to bamboo products as a durable carbon stock. Through a process of pyrolysis, up to 50% of the carbon can be transferred from plant tissue to the biochar, with the re- maining 50% used to produce energy and fuels [3].
Bamboo charcoal (BC) is one kind of manufactured bio- char, a plentiful residual byproduct of the bamboo proc- essing industry. BC has a highly micro-porous physical structure. The porosity is about five times greater and the absorption efficiency ten times higher than that of wood charcoal [4]. Bamboo charcoal may be an ideal amend- ment for nutrient conservation and heavy metal stabiliza- tion due to its excellent adsorption capability. Recent research found that biochar could act as soil fertilizer or conditioner to increase crop yield and plant growth by supplying and retaining nutrients [5-7]. Hua et al. [8]
found that bamboo biochar is an effective fertilizer when incorporated with sludge composing thereby effectively reducing nitrogen loses in the soil. The positive effect
was related to the high adsorption capacity of biochar par- ticles during composting [9]. In a similar research made by Asada et al. [10], it was found that bamboo biochar was effective in absorbing ammonia in soils. This was attrib- uted to acidic functional groups (e.g. methylene (CH2) and methyl (CH3), carbonyls (C=O), alcohols (-OH), carbox- ylic acids (CO2H), and amines (NH2)), formed as a result of thermolysis of cellulose and lignin at temperatures of 400°C and 500°C [11]. Furthermore bamboo biochar has been used in studies in combination with municipal solid waste bottom ash as soil modifiers where the content of polyphenols released by the carbon matrix was measured, as well has been tested is combination with the same type of bottom ash as agronomic materials [12, 13].
However, there has been no research to date on the effects of pyrolyzation temperatures of bamboo biochar in seed germination and plant growth. In this study we pre- sent the results of a germination test and growth parame- ters made with four different biochars, produced under different pyrolysis temperatures (240°C, 300°C, 600°C and 700°C) and evaluated at two rates of applications (1-100 (10%) t/ha, calculated as soil volume to 10 cm soil depth, and 2- pure biochar without soil application).
In a recent study presented by Solaiman et al. [14], biochars made from ‘Oil Mallee’, ‘Rice Husks’, ‘New Jarrah’, ‘Old Jarrah’ and ‘Wheat Chaff’ the authors con- cluded that biochar type and application rate influenced wheat seed germination and seedling growth in a similar manner in the soil-less Petri dish and soil-based bioassays that were performed. Germination and early root growth of mung bean and subterranean clover differed from that of wheat in response to the five biochars. According to
Rajkovich et al. [15], the effects of biochar properties on crop growth are little understood. For their study, biochar was produced from eight feedstocks and pyrolyzed at four temperatures (300°C, 400°C, 500°C, 600°C) using slow pyrolysis. In their results, animal manure biochars in- creased biomass by up to 43% and corn stover biochar by up to 30%, while food waste biochar decreased biomass by up to 92% in relation to similarly fertilized controls.
Increasing the pyrolysis temperature from 300°C to 600°C decreased the negative effect of food waste as well as pa- per sludge biochars. On average, plant growth was the highest with additions of biochar produced at a pyrolysis temperature of 500°C, but feedstock type caused eight times more variation in growth than pyrolysis temperature.
Biochar application rates above 2.0% (w/w) (equivalent to 26t ha-1) did generally not improve corn growth and rather decreased growth when biochars produced from dairy manure, paper sludge, or food waste were applied. In a similar study, Free et al. [16] used biochars made from biosolids, corn stover, eucalyptus, fresh pine or willow pyrolyzed at 550°C and incorporated into sandy loam at rates from 0 to 10 t/ha. The results showed that any of the biochars affected significantly the germination or early growth (root and coleoptile length, and dry weight) of maize seeds. There were no interactions between type and rate of biochar with soil type. Their results suggest that biochar incorporation prior to a maize crop should be investigated as a method of increasing stable soil carbon with the potential for mitigating carbon emissions. Pre- vious studies realized by the authors on water spinach growth where application of rice husk biochar and wood biochar at temperatures between 250°C and 350°C proved that the application of rice husk biochar improves biomass production. The wood biochar added soil increased the plant weight of water spinach by increasing the root size and leaf width; while the rice husk biochar supplemented soil increased the plant weight of water spinach by in- creasing the stem size and lead length. In addition, the stem size of water spinach is proportional to the water holding capacity/silt ratio, while the root size of water spinach is proportional to organic matter/organic carbon ratio of soil. We also proposed that the working mecha- nism of wood biochar and rice husk biochar in soil would be such that the decomposition of organic carbon in bio- char to soil organic matter resulted in the increased water holding capacity and decreased silt of biochar-added soil [17]. The main objectives of this study, therefore, are to investigate the potential capability of bamboo biochar to
affect germination and growth of edible crops. The type of bamboo used for biochar production was Phyllostachys heterocycla pubescens. We hypothesize that the results of this study may provide practical information about which temperatures are the best to use in biochar produc- tion for future agricultural applications.
II. MATERIALS AND METHODS
1. Biochar Production
Biochar made from bamboo was used to produce the biochars applied in this test. Bamboo biochar was gener- ated at different temperatures: 240°C, 300°C, 600°C and 700°C. All biochars were obtained by pyrolysis with a temperature raising rate of 5°C/min; biochars were sieved using a 4 mm sieve before use for the bioassays. Char- acterization of the material was made applying various test and analyses.
2. Biochar Characterization
Heavy metal analysis was carried out to identify the properties of the biochar used. The leaching extraction procedure followed the USA’s EPA method # 1311 with minor modifications [18]. Five grams of ground and weighed biochar were put in a volumetric flask together with 1000 ml of distilled water and 5.7 ml of acetic acid.
Samples were left for 18 h in a toxicity characteristics leaching procedure (TCLP) rotator. After this procedure, samples where filtered and analyzed through a Per- kin-Elmer 3000-XL inductively coupled plasma (ICP-AES) spectrometer.
Fourier Transform Infrared spectroscopy analysis (FT-IR) was used for the identification or fingerprint of a sample or solution to determine the organic functional groups. FT-IR determined the quality or consistency of the sample and its components. In this analysis of bio- char, a Bruker Vector 22 FT-IR spectrometer was used specifically to determine the functional groups present for each biomass, especially carbons and -OH groups. X-ray diffraction (XRD) analysis was carried out to identify any crystallographic structure in the four biochar samples us- ing a computer-controlled X-ray diffractometer (D8 Ad- vance, Bruker, Germany) equipped with a stepping motor and graphite crystal monochromator. Crystalline com- pounds in the samples were identified by comparing dif- fraction data against a database compiled by the Joint Committee on Powder Diffraction and Standards. The
scanned angles were 10° to 80° (2θ degree).
A HITACHI S-3000N scanning electron microscope equipped an energy dispersion X-ray (EDX) was used to examine the morphology of the biochar samples. The sample powder was sprinkled as a thin layer on an adhe- sive tape placed on the brass sample holder. Excess amount of the sample was removed using a small manual air blower. The adhered sample was then coated with gold powder using the sputtering device with the Ion Sputter E-1010 HITACHI, and then transferred into the JEOL sample chamber for the analysis. The accelerating voltage was set at 15-40 kV and 200°C, 300°C and 600°C time magnification was selected. The volatile matter in biochar was determined following the ASTM D 3175 -07 standard test method [19]. The water holding capacity (WHC) of biochars was measured regarding the following procedures of soil analysis manual [20]. Soil samples where oven dried for 24 hours under temperature of 105°C, then 5 g of each sample was poured on a 100mL beaker and distilled water was added to the sample until saturation point and the loss weight was counted for the water hold- ing capacity of the sample.
Electrical conductivity and total dissolved solid analy- sis are theoretically the best measure of salinity to indicate the actual salinity level experienced by the plant root [21].
Hence, electrical conductivity, total dissolved solids and pH were measured using a SUNTEX SC-110 portable conduc- tivity-meter. Samples were prepared at a ratio of 1:10 (sample: distilled water), mixed in a HMS-212 stirrer for 30 minutes, and then stood for 4 hours.
3. Plant Material
For this experiment, we used glutinous corn (Zea mays L.) and Chinese cabbage (Brassica Rapa) seeds. In Taiwan, corn is mainly grown for both human consump- tion and as feed for livestock. It can be harvested after 70-90 days. Germination takes place at around 7 days.
The temperature required for germination is between 20°C-25°C. Full sunlight and mild weather are also re- quired. Chinese cabbage of the mustard family, is eaten as a vegetable in eastern Asian cuisine. The seeds require 3-5 days to germinate. These crops where chosen due to their high demand and fast germination.
4. Germination and Plant Growth in Net House
For this assessment, a net house was adapted. Two different crops were evaluated, (glutinous corn and Chi- nese cabbage) addition of biochar at 100% (pure biochar
without soil - test 1) and 50% biochar (50-50 soil (pH 5.02) - biochar relation - test 2) were evaluated. Seed- beds where prepared in order to test the four different temperatures of bamboo biochars. Each treatment had 16 pots. Seeds were sown in 500 mL soil in a plastic con- tainer (16 cm × 10 .5 cm × 5 cm), the data of germination rate, started to be quantified on the 3rd day followed by measures on the 5th and 7th day of the trial. For corn a single seed was placed into the germination pots unlike cabbage, where 2-3 seeds where placed, due to their dif- ference in sizes. Generally, corn seeds start to germinate in 7 days and cabbage seeds will start to germinate after 7 or 10 days. Stem size, leaf number, leaf width and leaf length of each one of the emerged plants for corn and cab- bage was measured.
5. Statistical Analyses
Statistical analysis of variance (ANOVA) was per- formed using SAS (v.9.2) to separate the main effect or factors (biochar temperatures and rates of application) as well as their interactions. In addition, ANOVA was per- formed to find the effect of temperatures and rates of ap- plication in germination and growth parameters. The mean comparisons were made using Duncan’s Multiple Range test (DMRT) at p < 0.05 between treatments.
III. RESULTS AND DISCUSSION
1. Characteristics of Biochars
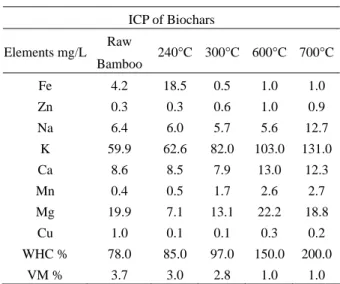
Bamboo biochar samples were ground in a commer- cial mix grinder and sieved to give a particle size of be- tween < 0.5 and >2.2 mm. To determine the total heavy metal content of the samples, biochar was analyzed using (ICP-AES). There are several factors controlling nutrient properties of biochar such as the nutrient composition and availability of biochars, nature of the feedstocks and the pyrolysis conditions under which they are produced [11].
In a study by Al-Wabel et al. [22], found that as pyrolysis temperature increased, ash content, pH, electrical conduc- tivity, basic functional groups, carbon stability, and total content of C, N, P, K, Ca, and Mg increased. In our study, the concentrations of the different elements (mg/L) are shown in Table 1. The contents determined by ICP showed that as pyrolysis temperature increased the pres- ence of elements in their majority also increased (Na, K, Ca, Mn, Mg). Another characteristic evaluated in the biochars was the water holding capacity; we found that
Table 1 Inductively coupled plasma analysis of bamboo biochars
ICP of Biochars Elements mg/L Raw
Bamboo 240°C 300°C 600°C 700°C Fe 4.2 18.5 0.5 1.0 1.0 Zn 0.3 0.3 0.6 1.0 0.9 Na 6.4 6.0 5.7 5.6 12.7
K 59.9 62.6 82.0 103.0 131.0 Ca 8.6 8.5 7.9 13.0 12.3 Mn 0.4 0.5 1.7 2.6 2.7 Mg 19.9 7.1 13.1 22.2 18.8
Cu 1.0 0.1 0.1 0.3 0.2 WHC % 78.0 85.0 97.0 150.0 200.0
VM % 3.7 3.0 2.8 1.0 1.0 WHC = water holding capacity, VM = volatile matter
WHC increased to a maximum value as pyrolyzation tem- perature increased (See Table 1). These findings match with the ones of Novak, et al. [23], who studied the water holding capacity of peanut hull & pecan shell biochar produced from different pyrolysis temperatures and found a higher water holding capacity in higher temperature biochar. Soil from NPUST campus field was found to be medium acidic with a pH of 5.02 (classified as very strong acid- medium acidic soil). Chan et al. [24] showed that biochar application had improved some physical soil prop- erties, such as increased soil aggregation, water holding capacity, and decreased soil strength. Raw bamboo has a percentage water holding capacity of 78.0% that is en- hanced by carbonization process, resulting in 200% water retention.
Generally, water retention is increased as the rate of soil organic matter increases. Sohi et al. [25] hypothe- sized that additions of biochar may lead to increased levels of soil organic matter which may partially explain im- proved water retention, while Glaser et al. [26] found that Terra Preta soils rich in charcoal had 18% greater water retention than neighboring soils that did not have signifi- cant charcoal deposits. The functional groups of biochar (bamboo) was examined by FT-IR spectroscopy (shown in Fig. 1). This analysis tool has frequently been used in investigations of surface chemistry of chars and activated carbons [27], as it provides valuable information on the chemical nature and the concentration of the surface func- tional groups. In this analysis of biochar, the FTIR was
4000 3500 3000 2500 2000 1500 1000 00
0.6 0.7 0.8 0.9 1.0
(e) (d)
(b)(c) a - Raw bamboo
b - Bamboo 240°C c - Bamboo 300°C d - Bamboo 600°C e - Bamboo 700°C Amine
CO2 -CH3
C-O Aromatic
-CH2- C=O -OH
Intencity (%)
Wave number (cm-1) (a)
Fig. 1 FTIR Spectra of tested samples, each letter (a, b, c, d, e) represents raw bamboo biomass and dif- ferent pyrolysis temperature: (a) Raw bamboo, (b) 240°C, (c) 300°C, (d) 600°C, and (e) 700°C
used specifically to determine the functional groups pre- sent for each temperature and biomass, especially carbons and aromatics. Various bonds in the spectra (at 3441 cm-1) corresponds to a -OH stretching vibrations and this may be caused by acid and/or alcohol structures. Increasing T from 300°C to 700°C resulted in a decrease of O-H stretch- ing (3350 cm-1) and aliphatic C-H stretching (2910 cm-1), indicating the process of dehydration, CH2 (2922 cm-1), all of the samples also have a C≡N bonding in the same posi- tion of the CO2 peak (2348 cm-1), this is expected due to the possible presence of nitrogen in the biochar. C=O stretching (1614 cm-1) (the transformation products for lignin) was intensified from 300°C to 500°C, and gradu- ally disappeared with the increasing temperature. These groups indicates the existence of ester and carboxylic acid structure, carboxylic acid CO-OH (1473 cm-1), and aro- matics (788 cm-1) in all temperatures of bamboo biochar.
An increasing degree of condensation at pyrolyzation temperatures of 600°C and higher (loss of intensity at 1575 cm-1 relative to 819 cm-1) were also noticed. The results of FT-IR and elemental analysis shows regardless of the similarity in temperatures in some biochars, the intensity and the concentration of the surface functional groups would vary. According to Nakanishi and Solomon [28], -OH, C=O, and C-O, belong to acid functional groups. Infor- mation taken from various studies [29] points out that the relative concentration of each of the functional groups depends on the biomass initial composition, final reaction temperature, composition of the gas surrounding the char- ring particle (final reaction temperature), heating rate and post-treatment. The C functional group chemistry and molecular form of biochar may be expected to significantly differ between biochars, given the differences in feedstock
10 20 30 40 50 60 70 80 (e)
(d) (c) (b) (a)
101100
002
Graphite crystal
Cellulose a - Raw bamboo
b - Bamboo 240°C c - Bamboo 300°C d - Bamboo 600°C e - Bamboo 700°C
Intensity (a. u.)
2 Theta (degree)
Fig. 2 X-ray diffraction patterns of biochar samples, each letter (a, b, c, d, e) represents raw bamboo biomass and different pyrolysis temperature: (a) Raw bamboo °C, (b) 240°C, (c) 3000°C, (d) 600°C, and (e) 700°C.
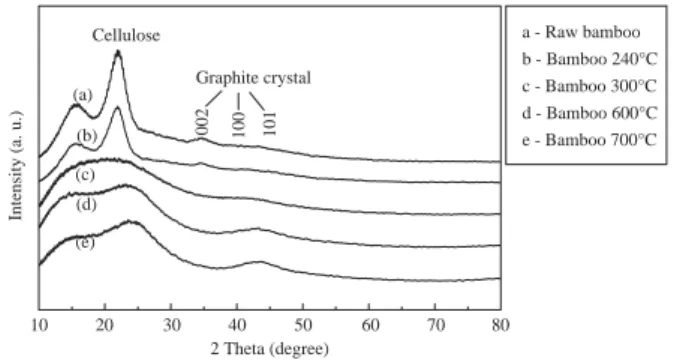
types and production conditions. Whereas charge proper- ties and, consequently, oxidized functional groups such as carboxyl groups differed significantly depending upon the production temperature [3].The importance of these differ- ences from a soil fertility point of view is that surface area and porosity of the biochar play a significant role in soil fertility. In contrast to the optimum conditions for the for- mation of the acid functional groups, more intense charring conditions (higher temperatures and longer charring times) are required for the formation of porosity and surface area in the biochar [30]. The X-ray diffraction patterns of bamboo biochars are represented in Fig. 2, showing intensity of the diffracted beam as a function of the Bragg angle (2θ).
Sharp, non-labeled peaks in grass chars indicate miscella- neous inorganic components. Peaks in bamboo biochars at higher T indicate the decrease of cellulose crystallinity.
Peaks situated at approximately at 23 theta degree corre- spond to the diffuse graphite (002) bands. As the charring T increases from 240°C to 700°C the (002) band shifts from 21.5 to 23. According to Bragg’s law, the d002 value (the spacing between graphitic sheets) decreases as temperature increased, indicating a more condensed char structure.
According to Kercher and Nagle [31] and Paris et al. [32], at advanced carbonization stages (i.e., temperatures > 400°C), X-ray scattering has revealed progressive stacking of gra- phene sheets.
Recent studies suggest that the types and rates of interac- tions (e.g., adsorption-desorption, precipitation-dissolution, redox reactions) that take place in the soil depend on the following factors: (i) feedstock composition, in particular the total percentage and specific composition of the min- eral fraction (especially Fe, Mn, Na and Ca); (ii) pyroly-
sis process conditions; (iii) biochar particle size; and (iv) soil properties and local environmental conditions [33].
There is an increasing awareness of the importance of understanding the chemistry of biochar to optimize its role as a soil additive. The addition of SEM–EDX pro- vides a comprehensive picture of the organic and inorganic chemistry on a biochar [33]. The pores at higher tem- perature were observed to be bigger compared to those at lower temperatures. As the temperature and severity of pyrolysis process elevated, enlargement of pores could be observed, Fig. 3 (a, b, c, d). The formation of particle size is showed for the four temperatures applied to obtain biochar. Is observed how porosity is developed, higher temperatures – the porosity number increases and the size of the pores narrows, giving as a result better water hold- ing capacity.
If the development of pores in biochar samples is enhanced with increasing temperature (especially at 600°C and 800°C), it may result in significant improvement in the pore properties of biochars [22]. In Table 2, the re- sults of the energy-dispersive X-ray spectroscopy are pre- sented; it can be observed how the different elements are formed in the materials depending on the pyrolysis tem- peratures. Some elements are not present in biochar with higher temperatures (e.g., Boron is not detected in raw bamboo, 240°C, 300°C, 600°C, but is available at 700°C).
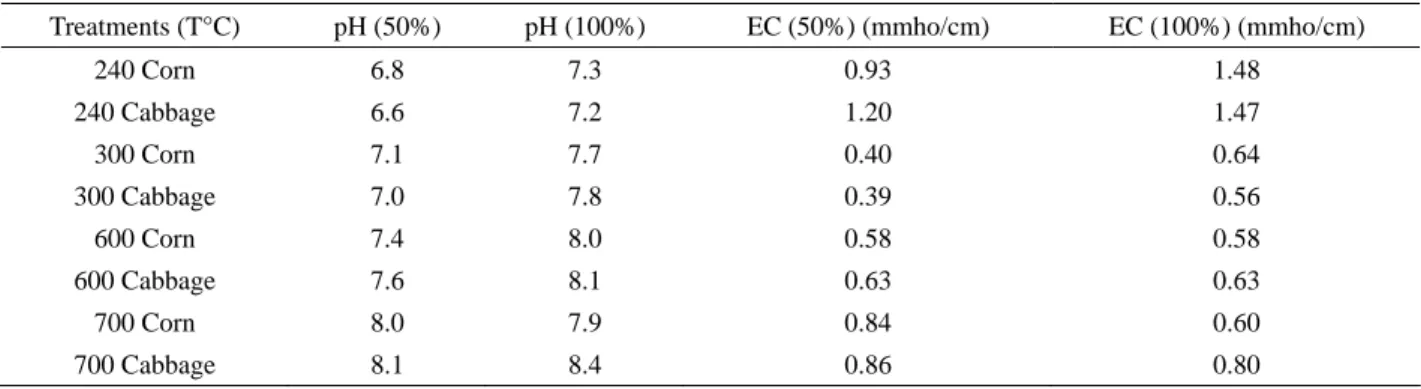
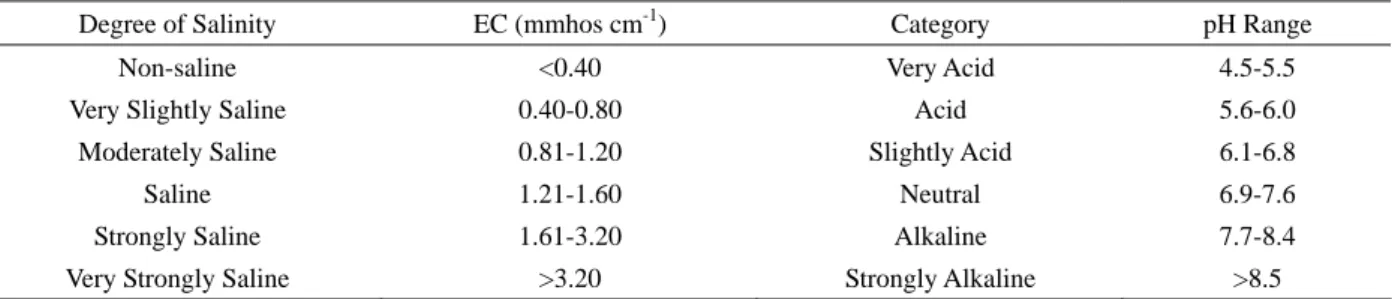
From Table 3, the applications of pure bamboo biochar are shown (100%) and in combination with soil (50-50% rela- tion) showed the differences in terms of pH, electrical conductivity and total dissolved solids, showing that when different pyrolysis temperatures are applied to produce biochar the characteristics of the material increase in its content also demonstrates that when biochar is combined with soil the pH can be controlled. Therefore applying high quantities of biochar is not recommended due to its high pH content (no more than 10 ton per hectare). Bio- char at 240°C applied in a 50% showed to be moderately saline unlike biochar applied in a 100% that showed to be saline. Table 4, explains the degrees of salinity in the electrical conductivity and the range for pH results given in Table 3.
2. Germination and Plant Growth Net House Bioassay for Corn and Cabbage
ANOVA mean comparisons were made using Duncan’s Multiple Range test (DMRT) at p < 0.05 between treat- ments. Bamboo biochar at 50% rates applied at 240°C,
Table 2 Energy-dispersive X-ray spectroscopy of biochars
Elements Temperatures (°C) Weight (%) Raw bamboo 240 300 600 700
B - - - - 66.46
C 51.2 58.16 75.88 85.7 33.16 O 48.79 40.99 21.04 13.56 0.14
Cl - - - - 0.02
Mg - - - 0.34 -
K - 0.86 3.8 0.78 0.22
Ca - - - 0.12 -
Mn - - - 0.13 0.4
Table 3 pH and Electrical conductivity evaluated from biochar applications at 50 and 100% rates in corn and cabbage
Treatments (T°C) pH (50%) pH (100%) EC (50%) (mmho/cm) EC (100%) (mmho/cm)
240 Corn 6.8 7.3 0.93 1.48
240 Cabbage 6.6 7.2 1.20 1.47
300 Corn 7.1 7.7 0.40 0.64
300 Cabbage 7.0 7.8 0.39 0.56
600 Corn 7.4 8.0 0.58 0.58
600 Cabbage 7.6 8.1 0.63 0.63
700 Corn 8.0 7.9 0.84 0.60
700 Cabbage 8.1 8.4 0.86 0.80
(a) 240 ± 30°C/12~14 (µm) (b) 300 ± 30°C/20~30 (µm)
(c) 600 ± 30°C/20~30 (µm) (d) 700 ± 30°C/8~14 (µm)
Fig. 3 SEM−EDX analysis of biochar (a, b, c, d). The formation of particle size is shown for the four temperatures ap- plied to obtain biochar. It is observed how porosity is developed, higher temperatures - the porosity number in- creases and the size of the pores narrows down, giving as a result better water holding capacity.
Table 4 Definition of Soil Classification of Salinity and pH Range
Degree of Salinity EC (mmhos cm-1) Category pH Range
Non-saline <0.40 Very Acid 4.5-5.5
Very Slightly Saline 0.40-0.80 Acid 5.6-6.0 Moderately Saline 0.81-1.20 Slightly Acid 6.1-6.8
Saline 1.21-1.60 Neutral 6.9-7.6
Strongly Saline 1.61-3.20 Alkaline 7.7-8.4 Very Strongly Saline >3.20 Strongly Alkaline >8.5
30 40 50 60 70 80 90 100 110
b a
b aab b ab a a a a a a
4 3 2
1 1234 1234
4 3 2 1
Seed germination mean (%)
Biochar treatments
Pyrolysis temperatures 1) 240°C 2) 300°C 3) 600°C 4) 700°C
Pyrolysis temperatures 1) 240°C 2) 300°C 3) 600°C 4) 700°C
Pyrolysis temperatures 1) 240°C 2) 300°C 3) 600°C 4) 700°C
Corn 50% Cabbage 50% Corn 100% Cabbage 100%
a
(a)
0 1 2 3 4 5 6
Stem size mean (cm)
Biochar treatments b
a
cc a
a bb
bc a
ab
c b
a
(b)
0 1 2 3 4 5 6
Leaf number mean (cm)
Biochar treatments a a a a ab
aab b
aa
b b
a
(c) 4
3 2
1 1234 1234
4 3 2
Corn 50% Cabbage 50% Corn 100% Cabbage 100%1 1234 1234 1234 1234 Corn 50% Cabbage 50% Corn 100% Cabbage 100%
Fig. 4 Effects of different temperatures and rates of bamboo biochar applied for corn and cabbage. (a) Seed germination percentage, (b) stem size (cm), and (c) leaf number were tested at rates of 50 and 100%. Error bars show standard errors of the mean. Mean data followed by a similar letter are not statistically significant within each biochar tem- perature
300°C, 600°C and 700°C, did not alter seed germination of corn (p < 0.05); it showed a complete germination, which was not the case in biochar application were at 100% rates germination was only 70~100%, showing best results at 240°C and 600°C temperatures (Fig. 4(a)). Applications of pure biochar (100%), significantly affected the germi- nation of corn and cabbage (p < 0.001), it decreased sig- nificantly as biochar temperatures increased. Results were more sensitive for cabbage seeds were germination was inhibited by temperatures at 600°C and 700°C.
Results for stem size means from corn and cabbage treated with 50% bamboo biochar showed very similar patterns in response to temperature applications. Higher size in stems was observed at 300°C temperatures as it was for biochar applied at 100% in corn and cabbage (Fig.
4(b)). In terms of biomass production measured by leaf number, similar results were observed for corn and cab- bage, 50% biochar applications showed a slight difference between pyrolyzation temperatures were 300°C had the higher mean. For 100% application in treatments there were significant differences between temperatures for corn and cabbage. Leaf number significantly increased at 300°C
bamboo biochar applications (Fig. 4(c)). In a study pre- sented by Solaiman et al. [14], the inhibition of seed ger- mination and/or root growth was observed at the highest rate of biochar application they argument that this effect might be due to trace levels of compounds that occur only above economic agronomic rates of application of biochar.
Not all biochars produced from green waste are suitable as soil amendment, particularly if they contain un-desirable compounds that could inhibit seed germination and root growth and development [34, 35]. Depending from the agricultural biomass resource used to produce biochar; it could contain traces to elevated nutrient concentrations of that can have an effect on seed germination [36]. Given this fact we based this evaluation on Zea Maize L. and Brassica Rapa germination and growth as a test for pre- liminary screening of biochars prior to their application as a soil amendment. Fast biological tests, as well as seed germination tests, are considered practical and positive in evaluating the existence of toxic compounds in biochars [37].
The effect of biochars on seed germination and seed- ling growth showed different behavior with biochar ap-
plication percentages and pyrolyzation temperatures. In our study, there was less inhibitory effect of biochars on seed germination and seedling growth with biochars in combination with soil (50%) and with lower pyrolyzation temperatures (240°C~300°C). Novak et al. [38] noticed that biochar incorporation to soil enhanced fertility by increasing soil pH, soil organic carbon, Ca, K, Mn, and P and this might help to overcome inhibitory effects of higher rates of biochar application on seed germination.
The biochars used in our study were generally alka- line after 600°C. Both high alkalinity and acidity may inhibit seed germination [39, 40] for some biochars ap- plied at high rates as in our study. In this investigation, the influence of biochar temperature and rate on seed ger- mination and seedling growth varied among the two agri- cultural plant species. Germination percentage decreased with the higher rate of biochar application and inhibited seed development in a 100% where biochar pyrolyzed at higher temperatures was applied. This investigation supports the proposal of Major [37] that a germination test could be a useful screening process for evaluating bio- chars.
IV. CONCLUSIONS
In conclusion, the four temperatures (240°C, 300°C, 600°C and 700°C) of bamboo biochars used in this study generally increased corn seed germination at rates of ap- plication of 50% for 240°C, 300°C biochars. While 600°C and 700°C biochars tended to inhibit germination at the highest rate of application (100%) under the bioassay con- ditions. 600°C and 700°C biochar at 100% had the greatest inhibitory effect on seed germination among the biochars compared when applied at higher rates. As pyrolysis tem- perature increased, total content of Fe, Na, K, Ca, Mn, and Mg increased, which also increased pH, electrical conduc- tivity, water holding capacity and basic functional groups.
The data of Fourier transformation infrared shows an in- crease in aromaticity and a decrease in the polarity of bio- char produced at a high temperatures. With pyrolysis tem- perature, crystalline mineral components and cellulose loss increased, as shown by X-ray diffraction analysis and scanning electron microscope images. Results propose that biochar pyrolyzed at high temperatures could possess an elevated potential of carbon sequestration when added to the soil compared to the one obtained at lower tempera- tures. We hope that the results of this study may provide practical information about which pyrolyzation tempera-
tures are the best to use in biochar production and future agricultural applications of bamboo biochar.
REFERENCES
1. Brenner. K., “Mapping Materials” (2008). From:
http://www.metrofieldguide.com/portfolio/Bamboo.pdf 2. FAO Non-Wood Forest Products NWFP-Digest-L.
“Bamboo in Nepal: bamboo houses could help allevi- ate poverty,” Himalayan News Service (2010). From:
http://www.fao.org/forestry/63579/en/#one
3. Lehmann, J., “Bioenergy in the Black,” Frontiers in Ecology and the Environment, Vol. 5, No. 7, pp. 381- 387 (2007).
4. Zhang Q. S., Prospect and Utilization of Bamboo Re- sources in China, Mechanism and Science of Bamboo Charcoal and Bamboo vinegar (in Chinese), China Forestry, Beijing, pp. 1-25 (2001).
5. Glaser, B., Balashov, E., Haumaier, L., Guggenberger, G., and Zech, W., “Black Carbon in Density Fractions of Anthropogenic Soils of the Brazilian Amazon Re- gion,” Organic Geochemistry, Vol. 31, No. 7-8, pp.
669-678 (2000).
6. Major, J., Steiner, C., Ditommaso, A., Falcǎo, N. P. S., and Lehmann, J., “Weed Composition and Cover After Three Years of Soil Fertility Management in the Cen- tral Brazilian Amazon: Compost, Fertilizer, Manure and Charcoal Applications,” Weed Biology and Man- agement, Vol. 5, No. 2, pp. 69-76 (2005).
7. Steiner, C., Teixeira, W. G., Lehmann, J., Nehls, T., De Macêdo, J. L. V., Blum, W. E. H., and Zech, W., “Long Term Effects of Manure, Charcoal and Mineral Fertili- zation on Crop Production and Fertility on a Highly Weathered Central Amazonian Upland Soil,” Plant and Soil, Vol. 291, No. 1-2, pp. 275-290 (2007).
8. Hua, L., Wu, W., Liu, Y., McBride, M. B., and Chen, Y.,
“Reduction of Nitrogen Loss and Cu and Zn Mobility during Sludge Composting with Bamboo Charcoal Amendment,” Environmental Science and Pollution Research, Vol. 16, No. 1, pp. 1-9 (2009).
9. Dias, J. M., Alvim-Ferraz, M. C. M., Almeida, M. F., Rivera-Utrilla, J., and Sánchez-Polo, M., “Waste Mate- rials for Activated Carbon Preparation and Its Use in Aqueous-Phase Treatment: A Review,” Journal of En- vironmental Management, Vol. 85, No. 4, pp. 833-846 (2007).
10. Asada, T., Ishihara, S., Yamane, T., Toba, A., Yamada, A., and Oikawa, K., “Science of Bamboo Charcoal:
Study on Carbonizing Temperature of Bamboo Char- coal and Removal Capacity of Harmful Gases,” Jour- nal of Health Science, Vol. 48, No. 6, pp. 473-479 (2002).
11. Lehmann, J. and Joseph, S., Biochar for Environmental Management: Science and Technology, Earthscan Ltd, London, UK (2009).
12. Milla Varela, O. and Huang, W. J., “Identifying the Advantages of Using MSW Bottom Ash in Combina- tion with Rice Husk and Bamboo Biochar Mixtures as Soil Modifiers: Enhancement of the Release of Poly- phenols from a Carbon Matrix,” Journal of Hazardous, Toxic, and Radioactive Waste Management, Vol.17, No.
3, pp. 204-210 (2013).
13. Milla Varella, O., Wang, H. H., and Huang, W. J.,
“Feasibility Study using Municipal Solid Waste Incin- eration Bottom Ash and Biochar from Binary Mixtures of Organic Waste as Agronomic Materials,” Journal of Hazardous and Toxic Radioactive Wastes, Vol. 17, No.
3, pp. 187-195 (2013).
14. Solaiman, Z. M., Murphy D. V., and Abbott, L. K.,
“Biochars Influence Seed Germination and Early Growth of Seedlings,” Plant and Soil, Vol. 353, No.
1-2, pp. 273-287 (2012).
15. Rajkovich, S., Enders, A., Hanley, K., Hyland, C., Zimmerman, A. R., and Lehmann, J., “Corn Growth and Nitrogen Nutrition after Additions of Biochars with Varying Properties to a Temperate Soil,” Biology and Fertility of Soils, Vol. 48, No. 3, pp. 271-284 (2012).
16. Free, H. F., McGill, C. R., Rowarth, J. S., and Hedley, M. J., “The Effect of Biochars on Maize (Zea Mays) Germination,” New Zealand Journal of Agricultural Research, Vol. 53, No. 1, pp. 1-4 (2010).
17. Milla Varela, O., Rivera E. B., Huang, W. J., Chien, C.
C., and Wang, Y. M., “Agronomic Properties and Characterization of Rice Husk and Wood Biochars and their Effect on the Growth of Water Spinach in a Field Test,” Journal of Soil Science and Plant Nutrition, Vol.
13, No. 2, pp. 251-266 (2013).
18. EPA, “Toxicity Characterization Leaching Procedure (TCLP),” US Government Printing Office, Washington, DC (1990).
19. Annual Book of ASTM Standards, “Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke,” ASTM D3175-11 (2007).
20. Lee, F. Y., Laboratory Manual for Soil Analysis (in Chinese), New Wun Ching Development Publishing Co., New Taipei City, pp. 70-75 (2007).
21. Corwin, D. L and Lesch, S. M., “Application of Soil Electrical Conductivity to Precision Agriculture: The- ory, Principles, and Guidelines,” Agronomy Journal, Vol. 95, No. 3, pp. 455-471 (2003).
22. Al-Wabel, M. I., Al-Omran, A., El-Naggar, A. H., Nadeem, M., and Usman, A. R. A., “Pyrolysis Tem- perature Induced Changes in Characteristics and Chemical Composition of Biochar Produced from Conocarpus Wastes,” Bioresource Technology, Vol.
131, No. 3, pp. 374-379 (2013).
23. Novak, J. M., Lima, I., Xing, B., Gaskin, J. W., Steiner, C., Das, K. C., Ahmedna, M., Rehrah, D., Watts, D. W., Busscher, W. J. and Schomberg, H., “Characterization of Designer Biochar Produced at Different Tempera- tures and Their Effects on a Loamy Sand,” Annals of Environmental Science, Vol. 3, pp. 195-206 (2009).
24. Chan, K. Y., van Zwieten, L., Meszaros, I., Downie, A., and Joseph, S., “Agronomic Values of Green Waste Biochar as a Soil Amendment,” Australia Journnal of Soil Reserch, Vol. 45, No. 8, pp. 629-634 (2007).
25. Sohi, S., Loez-Capel, E., Krull, E., and Bol, R., “Bio- char’s Roles in Soil and Climate Change: A Review of Research Needs,” CSIRO Land and Water Science Report 05/09, Australia (2009).
26. Glaser, B., Lehmann, J., Steiner, C., Nehls, T., Yousaf, M., and Zech, W., “Potential of Pyrolyzed Organic Matter in Soil Amelioration,” Proceedings of the 12th International Soil Conservation Conference, Beijing, China (2002).
27. Purevsuren, B., Avid, B., Tesche, B., and Davaajav, Y.,
“A Biochar from Casein and Its Properties,” Journal of Materials Science, Vol. 38, No. 11, pp. 2347-2351 (2003).
28. Nakanishi, K. and Solomon, P. H., Infrared Absorption Spectroscopy (2nd Ed.). Holden-Day, San Francisco (1977).
29. Elizalde-Gonzalez, M. P., Mattusch, J., Pelaez-Cid, A.
A., and Wennrich, R., “Characterization of Adsorbent Materials Prepared from Avocado Kernel Seeds: Natu- ral, Activated and Carbonized forms,” Journal of Ana- lytical and Applied Pyrolysis, Vol. 78, No. 1, pp. 185- 193 (2007).
30. Rutherford, D. W., Wershaw, R. L., and Cox, L. G.,
“Changes in Composition and Porosity Occurring Dur- ing the Thermal Degradation of Wood and Wood Components,” USGS Scientific Investigations Report, Vol. 2004-5292, pp. 78-79 (2004).
31. Kercher, A. K. and Nagle, D. C., “Microstructural
Evolution during Charcoal Carbonization by X-Ray Diffraction Analysis,” Carbon, Vol. 41, No. 1, pp. 15- 27 (2003).
32. Paris, O., Zollfrank, C., and Zickler, G. A., “Decompo- sition and Carbonisation of Wood Biopolymers - A Microstructural Study of Softwood Pyrolysis,” Carbon, Vol., 43, No. 1, pp. 53-66 (2005).
33. Chia, C. H., Gong, B., Joseph, S. D., Marjo, C. E., Munroe, P., and Rich, A. M., “Imaging of Mineral- Enriched Biochar by FTIR, Raman and SEM-EDX,”
Vibrational Spectroscopy, Vol. 62, pp. 248-257 (2012) 34. Garnett, E., Jonsson L. M., Dighton, J., and Murnen,
K., “Control of Pitch Pine Seed Germination and Initial Growth Exerted by Leaf Litters and Polyphenolic Compounds,” Biology and Fertility of Soils, Vol. 40, No. 6, pp. 421-426 (2004).
35. Hille. M. and Ouden, J. D., “Charcoal and Activated Carbon as Adsorbate to Phytotoxic Compounds - A Comparative Study,” Oikos, Vol. 108, No. 1, pp. 202- 207 (2005).
36. Gaskin, J. W., Steiner, C., Harris, K., Das, K. C., and Bibens, B., “Effect of Low-Temperature Pyrolysis Con- ditions on Biochar for Agricultural Use,” Transactions of the ASABE, Vol. 51, No. 6, pp. 2061-2069 (2008).
37. Major J., “A Guide to Conducting Biochar Trials - International Biochar Initiative,” http://www.biochar- interntional.org/sites/default/files/IBI%20Biochar%20 Trial%20Guide%20final.pdf, pp. 1-30 (2009).
38.Novak, J. M., Busscher W. J., Laird D. L., Ahmedna M, Watts D. W., and Niandou M. A. S., “Impact of Bio- char Amendment on Fertility of a Southeastern Coastal Plain Soil,” Soil Science, Vol. 174, No. 2, pp.105-112 (2009).
39. Pierce G. L., Warren S. L., Mikkelsen R. L., and Linker H. M., “Effects of Soil Calcium and pH on Seed Ger- mination and Subsequent Growth of Large Crabgrass (Digitaria Sanguinalis),” Weed Technology, Vol. 13, No.
2, pp. 421-424 (1999).
40. Horne, J. E., Kalevitch, A. E., and Filimonova, M. V.,
“Soil Acidity Effect on Initial Wheat Growth and De- velopment,” Journal of Sustainable Agriculture, Vol. 7, No. 2-3, pp. 5-13 (1996).
Manuscript Received: Mar. 19, 2013 First Revision Received: Mar. 27, 2013 Second Revision Received: Jul. 08, 2013 and Accepted: Aug. 09, 2013