Preparation of high-temperature stabilized
b-tricalcium phosphate
by heating deficient hydroxyapatite with Na4P2O7 ) 10H2O addition
Feng-Huei Lin
!, Chun-Jen Liao", Ko-Shao Chen", Jui-Sheng Sun#,*
! Center for Biomedical Engineering, College of Medicine, National Taiwan University, Taipei, Taiwan, ROC " Department of Material Engineering, Tatung Technology Institute, Taipei, Taiwan, ROC #Department of Orthopaedic Surgery, College of Medicine, National Taiwan University, Taipei, Taiwan, ROC
Received 28 April 1997; accepted 25 January 1998
Abstract
In this paper, the high-temperature stabilizedb-tricalcium phosphate (bTCP, b-Ca3(PO4)2) were prepared by heating the deficient HAP (d-HAP, Ca10~x(HPO4)x(PO4)6~x(OH)2~x) with tetra-sodium diphosphate decahydrate (NP, Na4P2O7 )10H2O) addition. The bTCP, d-HAP and d-HAP doped with 2.5, 5, 7.5 and 10 wt % NP were heated to different temperatures and were investigated by X-ray diffraction analysis (XRD) and Fourier-transformed infrared spectroscopy (FTIR). The results demonstrated that the HPO2~ 4 of d-HAP condensed into P2O4~7 occurred before 650°C. The P2O4~7 ions could be traced in the FTIR spectrum when the d-HAP was heated up to 750°C. The reaction of P2O4~7 with OH~ did not occur instantly but over a wide range of temperatures. The d-HAP doped with NP would decrease the decomposition temperature of d-HAP. NP doped into d-HAP not only induced the d-HAP decomposition at lower temperature but also stabilized thebTCP crystal structure at higher-temperature. It could also increase the conversion temperature ofbTCP to aTCP from 1180°C up to 1300°C. We could successfully prepare high-temperature (up to 1300°C) stabilizedbTCP by heating NP doped d-HAP. ( 1998 Published by Elsevier Science Ltd. All rights reserved
Keywords: Tricalcium phosphate; Bioresorbable; Phase transformation
1. Introduction
b-tricalcium phosphate (bTCP, b-Ca3(PO4)2) is cur-rently used as bone graft replacement material. It is well known that the bTCP have exceptionally good tissue compatibility, direct bonding to regenerated bone with-out intermediate connective tissue, faster bone regenera-tion, and resorbability as well [1—4]. However, bTCP ceramic is difficult to densify because high densification requires higher temperatures, but a sintering temperature beyond 1180°C induces the phase transformation ofb to a-tricalcium phosphate (aTCP). The density of aTCP has a lower density than that ofbTCP (aTCP: 2.77, bTCP: 3.07 g cm~3). The volume of sintered TCP will expand and the crack will generate inside the material while the phase transformation of bTCP to aTCP occurrs. It would lead to a doubtful mechanical stability under the physiological loading [4]. Therefore, it is important to
* Corresponding author. Fax: 886-2-23940049; e-mail: double@
ha.mc.ntu.edu.tw
stabilize the crystalline phase ofbTCP at higher temper-atures.
The Mg ions have been chosen to dope the TCP material, which could replace the Ca ion position in the lattice ofb-whitlockite to form compounds of Mg3(PO4)2 or Ca3~xMgx(PO4)2. These compounds could effectively stabilize theb-whitlockite structure at high temperatures [5—8]. However, the precursor of Mg ion, such as MgCl2, Mg(OH)2 or MgCO3, would form other stable inclusions such as chlorined-HAP (Ca10(PO4)6(Cl)2) or remain dif-ficult to remove magnesium oxide (MgO) in the TCP material during heat treatment. It would influence the mechanical strength of the TCP and the performance in the physiological environment. In addition, some investiga-tors have reported that the calcium phosphate doping with F~ ions or Mg2` ions leads to a decrease of the biode-gradation rate of the material in vivo [9]. Therefore, we search for another additive to stabilize thebTCP structure. Various ions can be incorporated into thebTCP and apatite crystal structure to replace the position of cal-cium or phosphorous ions but only biocompatible ones are considered here. Ca2`, PO3~4 , OH~, CO2~3 , and 0142-9612/98/$19.00( 1998 Published by Elsevier Science Ltd. All rights reserved.
citrate are the main constituents for the inorganic com-position of the bone. Na`, Mg2`, Cl~ and F~ are the minor elements in the hard tissue. The sodium ions are the largest amount of metallic ions stored in bone min-eral after calcium and phosphorous ions. The difference of ionic radius between calcium and sodium ions is lower than 15%. Sodium ions could be expected to replace the calcium ions in the whitlokite and apatite structure at high temperatures [8]. Moreover, bTCP and apatite doped with sodium ions would have less remnant be-cause the sodium oxide or the compounds containing Na` ion have lower melting and boiling point, which could be volatilized or decomposed at higher temper-atures. Therefore, we try to use the precursor containing sodium ions to dope the TCP and/or apatite while preparing the high-temperature stabilizedbTCP sinter-ing body in the study.
In this experiment, we prepared a deficient HAP (d-HAP, Ca10~x(HPO4)x(PO4)6~x(OH)2~x, 0(X(2) with nanograde particle size as the starting material. The Ca/P ratio of the prepared d-HAP is similar to that of trical-cium phosphate, about 1.5. The crystalline structure of d-HAP are the same with stoichiometric HAP (s-HAP) at room temperature [10—12], however, the d-HAP could be decomposed into bTCP and s-HAP while being heated [12—15]. According to Fowler’s study on the pyrolysis reactions of octacalcium phosphate, the s-HAP could react with the pyrophosphate ions (P2O4~7 ) and turned into bTCP around 700°C [16]. Therefore, the tetra-sodium diphosphate decahydrate (NP, Na4P2O7 ) 10H2O) containing Na` and P2O4~7 ions was chosen as the additive while doping the d-HAP to develop high-temperature stabilizedbTCP. The phase transformation or crystal structure reconstruction of d-HAP with differ-ent NP additions while being heated are also described in the study. We would also like to know the role of the pyrophosphate ions on the decomposition of d-HAP.
In the present study, we used XRD (X-ray diffraction) analysis to examine the phase transformation of the ma-terials at different heating temperatures. The functional group ofbTCP, d-HAP and d-HAP with NP addition at different temperatures were observed by FTIR (Fourier-transformed infrared) spectroscopy.
2. Materials and methods
The d-HAP with an atomic ratio Ca/P"1.5 was pre-pared and is briefly described as follows. An aqueous solution of (NH4)2HPO4 was added dropwise to a Ca(NO3)2) 4H2O solution at 25°C, where the pH of both the solutions were maintained at about 11 by adding the NH4OH solution. After being mixed, the reaction solu-tion was heated up to its boiling point and refluxed for 20 min. It was then left standing for 20 h at room temper-ature without stirring. The d-HAP precipitates were
filtered and washed with deionized water. The products were dried at 70°C for 72 h. bTCP was prepared by heating a mixture of reagent-grade CaHPO4 and CaCO3 with molar ratio of 2 : 1 at 1000°C for 24 h. Na4P2O7) 10H2O was purchased from E. Merck, 64271 Darmstadt, Germany.
The d-HAP was mixed with different quantities of Na4P2O7) 10H2O in deionized water and dried at 70°C for 3 days. The well-mixed and dried cakes were ground and sieved. The samples were placed in covered platinum crucibles and heated to different temperatures at a heat-ing rate of 3°C min~1 in a conventional Ni—Cr coiled furnace, and then maintained for 6 h. The samples were then quenched to room temperature.
The crystalline phases of specimens were determined by Rigaku X-ray powder diffractometer with CuKa radi-ation and Ni filter. The scanning range of the samples was from 10 to 60° with a scanning speed of 4° min~1. The infrared spectra were recorded using KBr pellets (1 mg sample per 300 mg KBr) on a Jasco FTIR grating instrument with slow scan and normal silt width.
3. Results
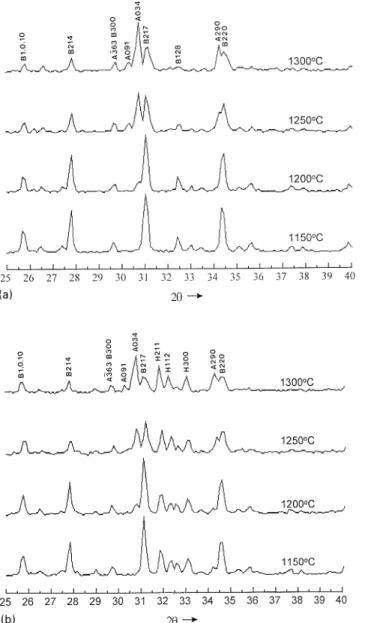
The XRD patterns ofbTCP and d-HAP without the addition of Na4P2O7 )10H2O at different heating tem-peratures are shown in Fig. 1. As seen in Fig. 1a and b, bTCP started transforming into aTCP at 1200°C. A trace ofaTCP with capital ‘A’ topped on the peaks of Fig. 1a was found in the XRD pattern after a temperature of 1200°C. The intensity of aTCP characterized peaks in-creased with heating temperature. bTCP characterized peaks were still observed even after the heating temper-ature of 1300 °C. d-HAP were decomposed into s-HAP andbTCP at the temperature of 1150°C as shown in Fig. 1b. s-HAP peaks could be traced in the XRD patterns and their intensities were not much changed in the tem-perature range 1150—1300 °C. Characterized peaks of aTCP appeared at 1200 °C and increased with heating temperature as well. At 1300 °C, the inversion ofbTCP to aTCP was still in progress and bTCP characterized peaks were still observed. By the way, s-HAP has the same XRD pattern as the d-HAP but is different in chemical composition. We will describe it later in Section 4.
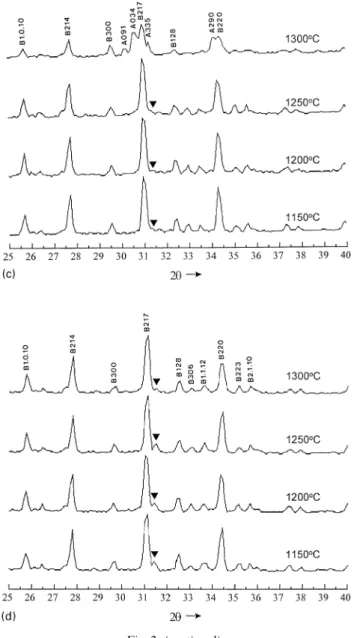
Fig. 2 summarizes the XRD patterns of d-HAP with different quantities of NP addition in the temperature range 1150—1300°C. All the d-HAP characterized peaks disappeared and turned into s-HAP and bTCP. Fig. 2a and b represent the XRD patterns of d-HAP with 2.5 and 5 wt% NP addition, respectively. The inversion temper-ature of bTCPPaTCP in the d-HAP with 5 wt% NP addition was higher than that of the 2.5 wt% NP addi-tion. As shown in Fig. 2a and b,bTCP turned into aTCP and nobTCP peaks remained once the temperature was
beyond 1250°C. The intensity of s-HAP characterized peaks gradually decreased as the quantity of NP addition increased. Comparing Fig. 2a with Fig. 1b, s-HAP char-acterized peaks of d-HAP with 2.5 wt% NP addition was lower than that of d-HAP without NP addition. For d-HAP with 5 wt% NP addition, only a few s-HAP peaks were still observed. Results of XRD analysis of the d-HAP with 7.5 and 10 wt% NP addition were shown in Fig. 2c and d, respectively. It showed that the more NP doped into the d-HAP, the higher phase transition tem-perature of bTCP to aTCP was observed. The d-HAP with 7.5 wt% NP addition could maintain the bTCP structure up to 1250°C, and d-HAP with 10 wt% NP addition could stabilize the bTCP structure beyond 1300°C. The s-HAP phase decomposed from d-HAP almost disappeared in the XRD patterns of d-HAP with
Fig. 1. X-ray diffraction patterns ofbTCP and d-HAP heated at 1150, 1200, 1250 and 1300°C: (a)bTCP; (b) d-HAP (B: bTCP phase; A: aTCP phase; H: s-HAP phase).
7.5 and 10 wt% NP addition. The characteristic peak of Na3Ca6(PO4)5 appearing at 1150°C was noteworthy. There seems some connections existing between the phase transformation temperature ofbTCP to aTCP and the decomposition of Na3Ca6(PO4)5. At the 7.5 wt% NP addition, the Na3Ca6(PO4)5 peaks disappeared at a temperature between 1250 and 1300°C. The phase transformation ofbTCP to aTCP occurred in the same temperature range as well. For the d-HAP with 10 wt% NP addition, the intensity of Na3Ca6(PO4)5 was stronger than that of d-HAP with 7.5 wt% NP addition. Though Na3Ca6(PO4)5 gradually disappeared with increased temperature in the d-HAP with 10 wt% NP addition, there was still some Na3Ca6(PO4)5 existing at the
Fig. 2. X-ray diffraction patterns of d-HAP with different additions of Na4P2O7 ) 10H2O heated at 1150, 1200, 1250 and 1300°C, respectively: (a) 2.5 wt% addition; (b) 5 wt% addition; (c) 7.5 wt% addition (charac-teristic peaks of Na3Ca6(PO4)5 phase did not appear in the patterns); (d) 10 wt% addition (B:bTCP phase; A: aTCP phase; H: s-HAP phase; .: Na3Ca6(PO4)5 phase).
Fig. 2. (continued ).
temperature 1300°C. There were noaTCP characteristic peaks observed at the same temperature.
To further understand the phase transformation in the system of d-HAP with NP addition, the d-HAP and d-HAP with 10 wt % NP was prepared and heated at a relative lower temperature. Fig. 3a and b showed the XRD patterns of d-HAP and d-HAP with 10 wt% NP addition from room temperature to 750°C. There was no significant difference in XRD patterns of d-HAP below 650°C, only s-HAP observed in the pattern as shown in Fig. 3a. In Fig. 3a,bTCP peaks could be traced on the XRD patterns at the temperature of 650°C. With temper-ature up to 750°C, the intensity of the bTCP peaks obviously increased. On the contrary, we could observe bTCP characteristic peaks such as B217 and B220 burg-eoned out on the XRD pattern of d-HAP with 10 wt% NP addition at 550°C (Fig. 3b). In addition, Na3Ca6 (PO4)5 peaks could be observed at 750°C in Fig. 3b).
Fig. 3. X-ray diffraction patterns of d-HAP and d-HAP with 10 wt% Na4P2O7) 10H2O addition at room temperature and heated at 550, 650, 750°C: (a) d-HAP; (b) 10 wt% addition (B:bTCP phase; H: s-HAP phase;.: Na3Ca6(PO4)5 phase).
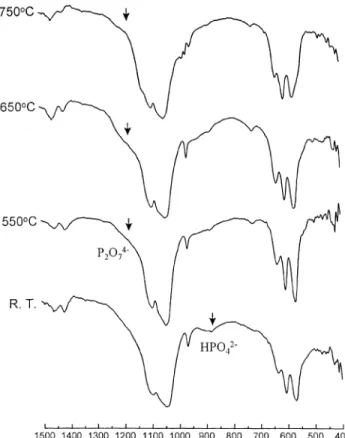
The FTIR spectrum of d-HAP heated at different temperatures were shown in Fig. 4a. The band of HPO2~4 at 875 cm~1 decreased with the heating temperature while the band of P2O4~7 at 1185 cm~1 slightly increased [16]. With the heating temperatures up to 750°C, the HPO2~
4 band almost disappeared but the P2O4~7 band was formed with decreased intensity. Bands of TCP grad-ually appeared at 1118, 973 and 945 cm~1. The spectrum of d-HAP with 10 wt% NP addition heated from room temperature to 750°C are shown in Fig. 4b. When com-pared to the spectrum at room temperature, the intensity of the HPO2~
4 band slightly decreased at 550°C. The P2O4~7 band was obviously raised and the bands of TCP were found at 973 cm~1 at 550°C. With temperatures up to 650°C, significant TCP bands appeared at 1118, 973,
Fig. 4. FTIR spectra of d-HAP and d-HAP with 10 wt% Na4P2O7 ) 10H2O addition at room temperature and heated at 550, 650, 750°C (a) d-HAP; (b) d-HAP with 10 wt% NP addition.
945, 603, 586, 649 and 540 cm~1. Only small HPO2~4 and P2O4~7 bands could be traced.
4. Discussion
The Ca/P ratio of d-HAP [deficient apatite Ca10~x(HPO4)x(PO4)6~x(OH)2~x(0(x(2)] is about 1.33—1.67 with apatite crystal structure. It is known that the HPO2~
4 ions in d-HAP will be condensed into P2O4~7 ions (2HPO2~4 P
P2O4~7 #
H2O) when heated to about 650°C. P2O4~7 ions will then react with OH~ as following formula [12]: P2O4~7 #2OH~P2PO3~
4 #
H2O. It will be accompanied with the d-HAP decom-posed intobTCP and s-HAP. The total reaction can be summarized as [12, 13]
Ca10~x(HPO4)x(PO4)6~x(OH)xP3XCa3(PO4)2 #
(1!X)Ca10(PO4)6(OH)2#H2O. (1)
Eq. (1) was supposed to have a slow reaction rate, which started at 650°C and still ongoing above 1000°C. Lee [15] suggested that the condensation of HPO2~
4 ions of d-HAP depend on their position in the crystal structure. The HPO2~
4 ions need greater energy to reach their condensation, which would lead to the condensation of HPO2~4 over a wide temperature range. He thought that P2O4~7 reacting with OH~ to form PO3~4 is an instant reaction. The reaction will occur once P2O4~7 ions are formed, therefore P2O4~7 ions are difficult to observe while heating.
From the results of FTIR analysis of this study, HPO2~
4 band of d-HAP was hardly detected at 550°C as shown in Fig. 4a. It means that HPO2~
4 has been trans-formed into P2O4~7 and did not exist at 550°C. The P2O4~7 band could be traced at 550°C and appeared at 650°C with an obvious slope on the FTIR curve (Fig. 4a). We could speculate that the condensation of HPO2~4 might occur before 650°C and the P2O4~7 ions would be formed simultaneously. A large amount ofbTCP should be formed at this temperature if P2O4~7 with OH~ was an instant reaction. However, only a small amount of bTCP could be observed at this temperature as shown in Fig. 3a. We thought that the P2O4~7 reacted with OH~ was not an instant reaction as described in the previous section. Fowler [16] has studied the pyrolysis reactions of octacalcium phosphate. The series reaction of octacal-cium phosphate pyrolysis are as follows:
Ca8H2(PO4)6 ) 5H2OP0.5Ca10(PO4)6(OH)2
(s-HAP)#1.5Ca2P2O7#5.5H2O, (2)
0.5Ca10(PO4)6(OH)2 (s-HAP)#1.5Ca2P2O7 #
5.5H2OP2Ca3(PO4)2#Ca2P2O7#6H2O. (3) It showed that the pyrolysis of CaHPO4 in the range of 325—450°C yielded Ca2P2O7 (Eq. (2)) and bTCP would
be obtained from the Ca2P2O7 and s-HAP reaction around 700°C. The study revealed that the P2O4~7 ions reaction with OH~ did not occur immediately. We speculated that the P2O4~7 ions should be accumulated to a critical concentration to react with OH~ and then induce d-HAP decomposition. The concentration of P2O4~7 might play an important role on the decomposi-tion of d-HAP. Since P2O4~7 could be traced even at temperatures up to 750°C, it seemed that the P2O4~7 reacting with OH~ to form PO3~
4 should occur over a wide temperature range. The rate of d-HAP con-version into bTCP and s-HAP should depend on the rate of P2O4~7 and OH~ reaction instead of HPO2~4 condensation.
The HPO2~4 band of d-HAP with 10 wt% NP showed no significant difference from room temperature to 550°C (Fig. 4b). Comparing Fig. 4b with Fig. 4a, the P2O4~7 band of d-HAP with 10 wt% NP addition was more obvious than that of d-HAP without NP addition from room temperature to 550°C. Most of the P2O4~7 ions bands appearing in d-HAP with 10 wt% NP addi-tion at 550°C (Fig. 4b) was coming from the Na4P2O7) 10H2O addition. The foreign P2O4~7 ions coming from NP addition would raise the concentration of P2O4~7 ions in the system and lead to the d-HAP decomposition at a lower temperature around 550°C (Fig. 1b). According to Eq. (1), d-HAP should be con-verted intobTCP and s-HAP at 650°C. However, s-HAP peaks almost disappeared andbTCP progressively pro-duced peaks in the XRD pattern of d-HAP with 10 wt% NP addition at the temperature of 650°C (Fig. 3b). The reaction might occur in the system as follows:
3Ca10(PO4)6(OH)2#2Na4P2O7P10Ca3(PO4)2 #
3Na2O#2NaPO3#3H2O. (4) It reflects that the NP doped into d-HAP not only induced the d-HAP decomposition at a lower temper-ature but also reacted with s-HAP to produce more bTCP in the system.
It is known that the transformation ofbTCP to aTCP started at 1180°C. From XRD analysis,bTCP still exis-ted while bTCP or d-HAP without NP addition was heated up to 1300°C (Fig. 1a and b). The results reveal that the transformation rate of bTCP to aTCP takes place at a slow pace. We observe that the intensity of bTCP characteristic peaks in Fig. 2a and b are higher than that of d-HAP at 1250°C (Fig. 1a). The authors speculated that NP might stabilizebTCP at higher tem-peratures or might increase the conversion temperature ofbTCP to aTCP, once it was doped into d-HAP.
In Fig. 2c and d, Na3Ca6(PO4)5 could be observed in the XRD patterns of the d-HAP with 7.5 and 10 wt% NP addition. The Na3Ca6(PO4)5 with chemical composition of 2.4CaO ) 0.6Na2O ) P2O5 was found by Ando [19] in the study of the binary phase diagram of Ca3(PO4)2—
NaCaPO4(rhenanite). The phase could be easily formed in the range of 2.6CaO ) 0.4Na2O) P2O5 to 2.4CaO ) 0.6Na2O ) P2O5 [19,20]. Lin [21], studied the sintering behavior of bTCP bioceramics with NP addition and observed Na3Ca6(PO4)5 in XRD patterns with bTCP doped with NP up to 5 wt%. The intensity of Na3Ca6(PO4)5 characteristic peaks increased with the quantity of NP added to the system. Suchanek [22] doped Na4P2O7 into hydroxyapatite to promote the sintering process, where Na3Ca6(PO4)5 could be ob-served in the system.
Schaeken [8] heated mixtures of CaHPO4, CaCO3 and Na2CO3 at temperatures between 700°C and 1000°C and found that sodium was incorporated in the crys-tal structure of whitlockite and apatite. It is known that the mixture of CaHPO4 and CaCO3 could produce bTCP [10], and the Na2CO3 would be decomposed into Na2O and CO2 in the same temperature range 700—1000°C. According to the results of Schaeken, Na2O could be assumed to react with bTCP and form the sodium-containing whitlockite in the temperature range 700—1000°C. In the present study, sodium-containing whitlockite is quite possible to be formed in the system of d-HAP with NP addition as expressed in eq. (4). In the system, bTCP would react with Na2O and then form sodium-containing whitlockite once d-HAP de-composed. Na3Ca6(PO4)5 was formed around 750°C (Fig. 3b), that might be the reaction product of thebTCP and Na2O. Comparing Fig. 2c with d, we observed that the intensity of Na3Ca6(PO4)5 characteristic peaks in-creased with the quantity of NP in the system. The intensity had a negative tendency with temperature and would finally disappear around the temperature of 1250—1300°C.
From the phase diagram of Mg3(PO4)2—Ca3(PO4)2 system, Mg2` ions incorporating into the TCP structure are effective in stabilizing thebTCP structure and raising thebTCP H aTCP transforming temperature from 1180 up to 1500°C [18, 19]. Kreidler and Hummel [23] inves-tigated the system of Ca3(PO4)2—Zn3(PO4)2 and found that the Zn2` ion incorporating into bTCP structure has the same effect as that of Mg2` ions in the bTCP crystal structure. Ando [24] also found that magnesia, alumina and ferric oxides could effectively stabilize the bTCP phase and prevent the inversion ofbTCPPaTCP while heating. However, the effect of Na` ions incorporating into the TCP structure depended on its concentration in the structure because Na2O was easily sublimble. In this study, the transformation temperature ofbTCPPaTCP increased with the NP addition as shown in Fig. 2a—d. It proved that the Na` ion incorporating into the TCP crystal structure could effectively stabilize the bTCP structure and keepbTCPPaTCP from transformation. Though Na` ions would stabilize the bTCP structure only up to 1300°C, the temperature is enough to obtain high densifiedbTCP ceramic after sintering.
5. Conclusion
The decomposition of d-HAP as Eq. (1) was supposed to have a slow reaction which started at 650°C and still ongoing above 1000°C. It is known that the HPO2~
4 ions in d-HAP will condense into P2O4~7 ions (2HPO2~4 P P2O4~7 #
H2O) when heated to about 650°C. P2O4~7 ions will then react with OH~ as P2O4~
7 #2OH~P
2PO3~
4 #
H2O. From the study, we could speculate that the condensation of HPO2~
4 might occur before 650°C and the P2O4~7 ions would be formed simultaneously. Since P2O4~7 could be traced even at temperatures up to 750°C, it seemed that the P2O4~7 reacting with OH~ forming PO3~4 should occur over a wide temperature range. The rate of d-HAP conversion into bTCP and s-HAP should depend on the rate of the P2O4~7 and OH~ reaction instead of the HPO2~
4 condensation. NP doped into d-HAP not only induced the d-HAP decomposition at lower temperature but also stabilized the bTCP crystal structure at higher temperatures or increased the conversion temperature of bTCP to aTCP. In this study, the transformation temperature of bTCP PaTCP increased with the NP addition. It pro-ved that the Na` ions incorporating into the TCP crystal structure could effectively stabilize the bTCP structure and keepbTCPPaTCP from transformation. By mix-ing NP with d-HAP, we could obtain a pure bTCP sintering body at the temperature up to 1300°C without aTCP transformation.
References
[1] Klein CPAT. Interaction of biodegradableb-whitlockite ceramics with bone tissue: an in vivo study. J Biomed Mater Res 1984;18:845—59.
[2] Kotani S, Fujita Y, Kitsugi T, Nakamura T, Yamamuro T, Ohtsuki C, Kokubo T. Bone bonding mechanism ofb-tricalcium phosphate. J Biomed Mater Res 1991;25:1303—15.
[3] Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Rel Res 1981;157:259—78.
[4] Katthagen BD. Bone regeneration with bone substitutes. Berlin, Heidelberg: Springer, 1987.
[5] Dickens B, Schroeder LW, Brown WE. Crystallographic studies of the role of Mg as a stabilizing impurity inb-Ca3(PO4)2. I. The crystal structure of pure b-Ca3(PO4)2. J Sol State Chem 1974; 10:232—48.
[6] Schroeder LW, Dickens D, Brown WE. Crystallographic studies of the role of Mg as a stabilizing impurity inb-Ca3(PO4)2. II. Refinement of Mg-containingb-Ca3(PO4)2. J Sol State Chem 1977;22:253—62.
[7] Bigi A, Foresti E, Gregorini R, Ripamonti A, Roveri N, Shah JS. The role of magnesium on the structure of biological apatites. Calcif Tissue Int 1992;50:439—44.
[8] de Groot K. Bioceramics of calcium phosphate. Boca Raton, FL: CRC Press, 1983.
[9] Klein CPAT, Driession AA, de Groot K. Biodegradation of calciumphosphate ceramics-ultrastructural geometry and dissolu-bility of different calcium phosphate ceramics. In Grote JJ, edi-tors. Biomaterials in Otology: The Hague: Nijhoff, Martinus, 1984.
[10] Aoki H. Medical application of hydroxyapatite. Ishiyaku Euro America, Inc. Tokyo, St. Louis Takayama Press, 1994. [11] Asada M, Miura Y, Osaka A, Oukami K, Nakamura S.
Hy-droxyapatite crystal growth on calcium hyHy-droxyapatite ceramics: J Mater Sci 1988;23:3202—5.
[12] Mortier A, Lemaitre J, Rodrique L, Rouxhet PG, Synthesis and thermal behavior of well-crystallized calcium-deficient phosphate apatite. J Solid State Chem 1989;78:215—19.
[13] Ishikawa K, Ducheyne P, Radin S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J Mater Sci Mater Med 1993;4:165—8.
[14] Yubao L, Klein CPAT, de Wijn J, van De Meer S. Preparation and characterization of nanograde osteoapatite-like rod crystals. J Mater Sci Mater Med 1994;5:252—5.
[15] Yubao L, Klein CPAT, de Wijn J, van De Meer S. Morphology and composition of nanograde calcium phosphate need-like crys-tals formed by simple hydrothermal treatment. J Mater Sci Mater Med 1994;5:326—331.
[16] Fowler BO, Moreno EC, Brown WE. Infra-red spectra of hy-droxyapatite, octacalcium phosphate and pyrolysed octacalcium phosphate. Arch Oral Biol 1996;11:477—92.
[17] Reser MK. Phase diagrams for ceramists. Ceramic Drive, 65 Ohio Columbus: The American Ceramic Society, 1964.
[18] Mathew M, Schroeder LW, Dickens B, Brown WE. The crystal structure ofa-Ca3(PO4)2. Acta Crystallogr B33:1325—33. [19] Ando J. Phase diagrams of Ca3(PO4)2—Mg3(PO4)2 and
Ca3(PO4)2—CaNaPO4 systems. Bull Chem Soc Jpn 1958;31: 201—5.
[20] Ando J, Matsuno S Ca3(PO4)2—CaNaPO4 system. Bull Chem Soc Jpn 1968;41:342—7.
[21] Lin FH, Hon MH. Sinteringb-tricalciumphosphate bioceramics with Na4P2O7 )10H2O. J Mater Sci Lett 1987;6:501—3. [22] Suchanek W, Yashima M, Kakihana M, Yoshimura M.
Hy-droxyapatite ceramics with selected sintering additives. Bio-materials 1997;18:923—33.
[23] Kreidler ER, Hummel FA. Phase relationships in the system SrO—P2O5 and the influence of water vapor on the formation of Sr4P2O9. Inorg Chem 1967;884—91.
[24] Ando J. Tricalcium phosphate and its variation. Bull Chem Soc Jpn 1958;31:196—201.