Production of Antibodies for Selective Detection of
Malachite Green and the Related Triphenylmethane
Dyes in Fish and Fishpond Water
M
EI-C
HUNY
ANG,
†J
IM-M
INF
ANG,*
,‡T
ZONG-F
UK
UO,
§D
A-M
INGW
ANG,
‡Y
I-L
INH
UANG,
‡L
IANG-Y
IRNL
IU,
†P
EN-H
ENGC
HEN,
† ANDT
ONG-H
SUANC
HANG†GlycoNex Inc., Taipei County, 221, Taiwan, Department of Chemistry, National Taiwan University, Taipei, 106, Taiwan, and Department of Veterinary Medicine, National Taiwan University, Taipei,
106, Taiwan
This study provides a practical method for production of the antibodies against malachite green (MG) and its primary metabolite leucomalachite green (LMG). Two ELISA kits are constructed with the MG and LMG antibodies for detection of the residual MG and LMG in fish muscle and fishpond water. The detection limit is established at the level of 0.05 µg/L for both MG and LMG. Our ELISA kits show the advantages of good specificity, high sensitivity, and convenience in rapid screening of MG and LMG residues. The sample of fishpond water, without extraction or prior preparation, is directly assayed by the ELISA kit. More then 80 fish samples can be simultaneously tested in a kit. The toxic crystal violet and its metabolite leucocrystal violet of illegal use in aquaculture are detected by our prepared MG and LMG antibodies, whereas the antibodies do not cross-react with common antibiotics, sulfonamides, and benzene derivatives.
KEYWORDS: Malachite green; leucomalachite green; antibody; ELISA; fish INTRODUCTION
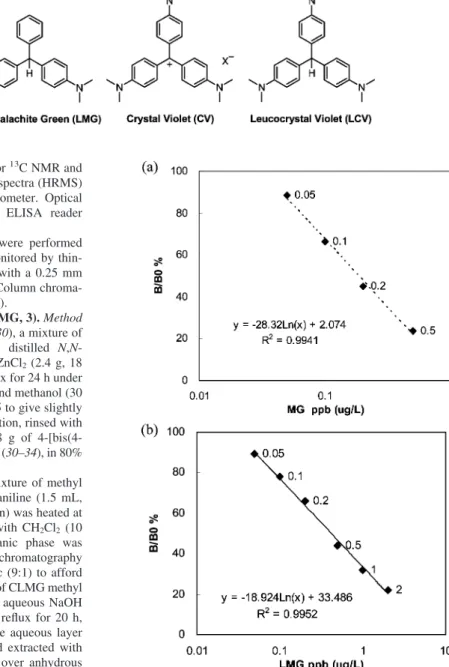
Malachite green (MG, see Figure 1) (1), a dye of
triphenyl-methane skeleton, has been extensively used in aquaculture for
prevention and treatment of external fungal and parasitic
infections in fish. MG is easily absorbed by fish during
waterborne exposure and is rapidly metabolized into
leuco-malachite green (LMG, see Figure 1). The reduction derivative
LMG is recognized as a major metabolite of MG in fish (2, 3)
and will store in fish muscle and tissues for months. Like other
triphenylmethane dyes, MG and LMG cause carcinogenesis,
mutagenesis, chromosomal fractures, teratogenesis, and
respira-tory toxicity in animals (4–7). Use of MG in aquatic food
animals is highly restricted or banned in several countries
because of toxicological considerations. However, illegal use
of MG continues worldwide in aquaculture due to its low cost
and ready availability (8, 9).
Surveillance of MG and LMG in aquaculture products is a
necessary means to protect human health. According to the
European Commission and U.S. Food & Drug Administration
(10), methods that can be used for the determination of MG
residues in fish muscles should meet a minimum required
performance limit of 2
µg/kg for the sum of MG and LMG.
High-performance liquid chromatography (HPLC) and liquid
chromatography–mass spectrometry (LC-MS) are two general
techniques for the quantitative analysis of MG and LMG in fish
tissues (11–29). These techniques rely on expensive instruments
operated by well-trained analysts, and prior preparation of
samples is time-consuming and is not ideal for screening large
number of samples. Alternatively, enzyme-linked
immunosor-bent assay (ELISA) is a rapid, specific, and sensitive method
that is applicable to the on-site examination of a large number
of samples. We thus generated two types of polyclonal
antibod-ies against MG and LMG, and further developed the indirect
competitive ELISA.
MATERIALS AND METHODS
All solvents and reagents were reagent-grade and were used without further purification. Crystal violet (CV, see Figure 1) chloride and malachite green oxalate were purchased from Merck (Rahway, NJ). Leucomalachite green, leucocrystal violet, Freund’s complete/incom-plete adjuvant, bovine serum albumin (BSA), ovalbumin (OVA), and goat anti-rabbit IgG-HRP were purchased from Sigma-Aldrich (St. Louis, MO).
Melting points are uncorrected. Infrared (IR) spectra were recorded on a Nicolet Magna 550-II spectrometer. Proton NMR (1H NMR) and
carbon NMR (13C NMR and DEPT) spectra were recorded on Varian
Unity Plus-400 (400 MHz) and Bruker Avance-400 FT-NMR spec-trometers; chemical shifts are reported in unitδ relative to
tetrameth-ylsilane (TMS) with residual protons in the solvent as an internal * To whom correspondence should be addressed. Tel: (8862)3366
1663. Fax: (8862)2363 7812 . E-mail: jmfang@ntu.edu.tw.
†GlycoNex Inc. ‡
Department of Chemistry, National Taiwan University.
§Department of Veterinary Medicine, National Taiwan University.
10.1021/jf071195y CCC: $37.00 2007 American Chemical Society Published on Web 10/09/2007
Downloaded by NATIONAL TAIWAN UNIV on August 27, 2009 | http://pubs.acs.org
standard: CDCl3,δ 7.24 (for1H NMR) andδ 77.0 (for13C NMR and
DEPT). Mass spectra (MS) and high-resolution mass spectra (HRMS) were measured using a JEOL JMS-HX 110 spectrometer. Optical density (OD) of ELISA was measured using an ELISA reader (THERMOmax, Molecular Device, USA).
All experiments requiring anhydrous conditions were performed under an atmosphere of nitrogen. Reactions were monitored by thin-layer chromatography (TLC) using slides precoated with a 0.25 mm layer of silica gel containing a fluorescent indicator. Column chroma-tography was carried out on Kieselgel 60 (40–63µm).
Synthesis of Carboxyleucomalachite Green (CLMG, 3). Method
A. According to the previously described procedure (30), a mixture of
4-formylbenzoic acid (900 mg, 6 mmol), freshly distilled N,N-dimethylaniline (2.4 mL, 19 mmol), and anhydrous ZnCl2(2.4 g, 18
mmol) in absolute ethanol (60 mL) was heated at reflux for 24 h under an atmosphere of nitrogen. The mixture was cooled, and methanol (30 mL) and aqueous HCl (1 M) were added until pH ) 5 to give slightly greenish crystals. The crystals were collected by filtration, rinsed with water, and dried over KOH in vacuum to give 1.8 g of 4-[bis(4-dimethylaminophenyl)methyl] benzoic acid, CLMG (3) (30–34), in 80% yield.
Method B. Under an atmosphere of nitrogen, a mixture of methyl
4-formylbenzoate (0.55 g, 3.4 mmol), N,N-dimethylaniline (1.5 mL, 11.8 mmol) and conc H2SO4(0.25 mL of 96% solution) was heated at
120°C for 40 h. The mixture was cooled, diluted with CH2Cl2(10
mL), and washed with water (10 mL). The organic phase was concentrated, and the residue was purified by flash chromatography on a silica gel column with elution of hexane/EtOAc (9:1) to afford CLMG methyl ester (1.14 g, 88% yield). To a solution of CLMG methyl ester (1.03 g, 2.65 mmol) in THF (7 mL) was added aqueous NaOH (3 mL of 1 M solution). The mixture was heated at reflux for 20 h, cooled, and extracted with EtOAc (10 mL× 3). The aqueous layer was acidified to pH 5 with HCl solution (3 M), and extracted with CH2Cl2(50 mL × 3). The CH2Cl2 layer was dried over anhydrous
Mg2SO4, filtered, and concentrated under reduced pressure to give
CLMG (806 mg, 81% yield).
CLMG.
Light greenish solid, mp ) 250
°
C (decomposed); IR
(KBr) 3417, 2886, 1682, 1651, 1611, 1519, 1349, 1293, 1166
cm
-1;
1H NMR (CDCl
3, 400 MHz)
δ 7.98 (2 H, d, J ) 8.4
Hz), 7.22 (2 H, d, J ) 8.4 Hz), 6.95–6.93 (4 H, m), 6.66 (4 H,
d, J ) 8.8 Hz), 5.42 (1 H, s), 2.90 (12 H, s);
13C NMR (CDCl
3,
100 MHz)
δ 171.01, 151.25 (2
×), 148.44 (2×), 131.62, 129.68
(2
×), 129.51 (4×), 129.05 (2×), 126.61, 112.54 (4×), 55.23,
41.08 (2
×); HRMS (ESI) calculated for C
24H
27N
2O
2: 375.2073,
found: m/z 375.2022 [M
++ H].
CLMG Methyl Ester.
White solid, mp ) 128–129
°
C; TLC
(EtOAc/hexane (1:9)) R
f) 0.10; IR (KBr) 2800, 1720, 1612,
1519, 1441, 1343, 1279, 1110, 1102 cm
-1;
1H NMR (CDCl
3,
400 MHz)
δ 7.90 (2 H, d, J ) 8.4 Hz), 7.19 (2 H, d, J ) 8.4
Hz), 6.95–6.93 (4 H, m), 6.64 (4 H, d, J ) 8.8 Hz), 5.40 (1 H,
s), 3.87 (3 H, s), 2.91 (12 H, s);
13C NMR (CDCl
3, 100 MHz)
δ 166.45, 150.35 (2
×), 148.37 (2×), 131.50, 129.46 (4×),
129.01 (2
×), 128.92 (2×), 127.28, 112.27 (4×), 55.11, 52.11,
40.95 (4
×); HRMS (ESI) calculated for C
25H
29N
2O
2: 389.2229,
found: m/z 389.2259 [M
++ H].
Synthesis of Carboxymalachite Green (CMG, 4). Method A. According to the previously described procedure (30–34), a mixture of CLMG (375 mg, 1 mmol), chloranil (295 mg, 1.2 mmol), and glacial
acetic acid (0.75 mL) in CHCl3(45 mL) was stirred at 25°C for 1.5 h.
The solids were collected by filtration, rinsed with CHCl3/CCl4(1:1),
and dried over KOH in vacuum to give crude CMG (360 mg), which was used without further purification for coupling with protein.
Method B. According to the previously described procedure (34), a
mixture of CLMG (150 mg, 0.60 mmol) and PbO2(0.63 mmol) in
aqueous HCl (1.5 mL of 2 M solution) was stirred at 25°C for 18 h. The mixture was diluted with MeOH (30 mL) and filtered through a Celite bed. The filtrate was concentrated in vacuum to give green solids of CMG (246 mg), which were used without further purification for coupling with protein. By a similar procedure, CLMG methyl ester was treated with PbO2in aqueous HCl to give CMG methyl ester.
Complete oxidation of CLMG using both methods was indicated by disappearance of the methine signal atδ 5.42 in the1H NMR spectrum
of the CMG product. The structure of CMG was supported by an exact mass measurement, giving m/z 373.1895 for the parent ion [C24H25N2O2]
+
. The prepared CMG was estimated at∼90% purity, and was good enough for the antibody production as shown in the inhibition assay without interference of any impurity (see Figure 3a).
CMG.1
H NMR (CD
3OD, 400 MHz)
δ 8.16 (2 H, d, J ) 7.8
Hz), 7.46 (2 H, d, J ) 7.8 Hz), 7.41 (2 H, d, J ) 8.0 Hz), 7.12
Figure 1. Structures of MG, LMG, CV, and LCV.
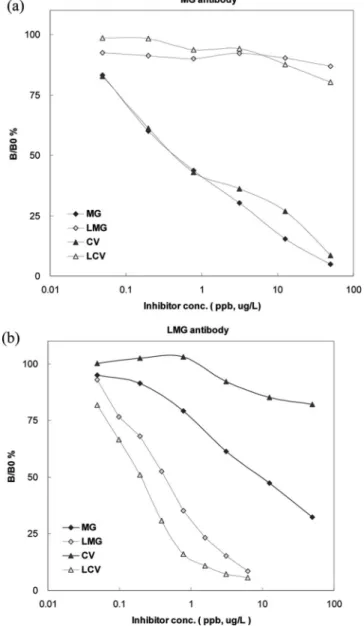
Figure 2. Standard titration curves of MG (a) and LMG (b) obtained by ELISA. B: Absorbance of MG or LMG standard. B0: Absorbance of blank solution.
Downloaded by NATIONAL TAIWAN UNIV on August 27, 2009 | http://pubs.acs.org
(2 H, d, J ) 8.0 Hz), 3.97 (3 H, s), 3.34 (12 H, s). HRMS
(ESI) calculated for C
24H
25N
2O
2: 373.1911, found: m/z 373.1895
[M
+].CMG Methyl Ester.
1H NMR (CD
3
OD, 400 MHz)
δ 8.18
(2 H, d, J ) 7.8 Hz), 7.40–7.35 (4 H, m), 7.05 (2 H, d, J ) 8.4
Hz), 3.35 (12 H, s). HRMS (ESI) calculated for C
25H
27N
2O
2:
387.2067, found: m/z 387.2197 [M
+].
Preparation of Immunogens by Coupling of CMG and CLMG with Proteins. In this study, OVA was used as a carrier for MG and LMG immunogens, and BSA was used as a carrier for the coated antigens in ELISA. To 0.85 mL of CMG or CLMG solution at 1 mg/ mL in 0.1 M MES (pH 6.0) with 50% Me2SO were added
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDCI) hydrochloride (0.44 mg) and N-hydroxysuccinimide (NHS, 0.26 mg, Pierce, USA). The mixture was stirred for 30 min at room temperature, and added dropwise to 5 mL of 1 mg/mL protein solution. The mixture was agitated for 2 h at room temperature. The conjugates were dialyzed against 0.1 M phosphate buffer (pH 7.4) to remove free reagents.
Immunization. Two groups of New Zealand rabbits (in duplicate) were immunized by sc injection with CMG-OVA or CLMG-OVA. Primary immunizations were composed of 500µL of PBS containing
100 µg of immunogen emulsified in 500 µL of Freund’s complete
adjuvant. Subsequent immunizations, at 2–5 week intervals, were of the same volume, with complete adjuvant replaced by incomplete adjuvant. Rabbits were bled after each injection. The sera titer was detected by an indirect ELISA.
Characterization of MG and LMG Antibodies. The 96-well plates (Corning, NY) were coated with MG-BSA or LMG-BSA overnight at 4°C. The plates were washed 3 times with PBST, and then blocked with 0.1% skim milk at 37°C for 1 h. The plates were again washed with PBST, and incubated with animal sera (100µL/well) diluted in
0.1% BSA at 37°C for 1 h. After incubation, plates were washed with PBST three times. Goat anti-rabbit IgG antibody conjugated with HRP was diluted (1/2000) in 0.1% BSA, and 100µL of the resultant solution
was added to each well. The plates were incubated at 37°C for 1 h, and washed again with PBST three times. The substrate solution TMB (100µL) was added to each well and incubated at 37°C for 10 min. The reaction was stopped by addition of aqueous HCl (50µL of 2 M
solution). Optical density at a wavelength of 450 nm in each well was read using an ELISA reader.
The MG- and LMG-specific antibodies were purified from high-titer rabbit serum by affinity chromatography on a Protein A-Sepharose column (GE Healthcare, UK). The antibodies were concentrated using Centriprep (Amicon Ultra, 50000 MWCO; Millipore, USA). Antibody concentrations was measured with Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA).
The specificity of MG and LMG antibodies was determined by indirect competitive ELISA using MG, LMG, CV, and leucocrystal violet (LCV) as inhibitors. The inhibitors were diluted in 0.02 M HCl with 0.15 M NaCl. The indirect competitive ELISA was performed on MG-BSA or LMG-BSA coated 96-well plates. Serially diluted inhibitors were incubated with 0.5µg/mL of MG or 1.8 µg/mL of LMG antibodies in the antigen-coated
96-well plates at 37°C for 30 min. Antibodies bound to the MG-BSA or LMG-BSA were detected by goat anti-rabbit IgG-HRP, followed by treatment with TMB. The optical density at a wavelength of 450 nm was recorded using an ELISA reader. The cross-reactivity of MG and LMG antibodies was expressed as the ratio of MG or LMG concentration to the cross-reactant concentration that will show 50% of B/B0 %. For com-parison, 13 common antibiotics/sulfonamides and 3 benzene derivatives were also tested by a similar procedure.
Preparation of Fish Samples for ELISA. Fish samples were obtained from the local market including Oreochomis sp. (Tilapia and Taiwan Tilapia), Chanos chanos (milkfish), Epinephelus sp. (grouper),
Lateolabrax japonicus (Japanese sea perch), Micropterus salmoides
(California bass), and Pagrus major (Red sea bream). The fish was filleted, the skin and bones were removed, and the muscles were minced and frozen before being analyzed. Accurately weighed 1.0 g of homogenized fish muscle was put into a 15 mL centrifuge tube, which can be spiked with appropriate amounts of MG or LMG, and 1 mL of McIlvaine buffer (pH ) 3.0) and 6 mL of acetonitrile were added. The mixture was vigorously vortexed for 3 min, and centrifuged at 3500 rpm for 10 min. The supernatant was transferred into a centrifuge tube with 1.5 mL of CH2Cl2, and the sample was vortex-mixed followed
by centrifuging at 3500 rpm for 10 min. An aliquot of the upper organic phase (0.5 mL) was transferred into a tube (1.5 mL), and treated with strong anion exchanger AG 1 resin (0.1 mL, Bio-Rad Laboratories, Hercules, CA) at room temperature for 30 min to reduce interference from the extracts. The supernatant (0.4 mL) was dried by evaporation under a stream of nitrogen at 50 °C. The sample was reconstituted with 0.4 mL of sample diluent (0.01 M HCl) and detected by an MG or LMG ELISA.
MG ELISA of Fish Samples. MG-BSA was coated on a 96-well ELISA plate and reacted with fish samples and MG antibodies in equal volume at 37°C for 30 min. Unbound MG antibodies were removed by washing. Goat anti-rabbit IgG-HRP was added and treated with TMB. The quantity of MG antibodies bound on the MG ELISA plate was deduced from the OD at 450 nm. The percent binding (B/B0 %) for each standard or sample was calculated by the following equation, and the corresponding concentration of MG was interpolated from the standard curve. The sample concentration was corrected for dilution (7-fold).
[OD (standard or sample)/OD (blank)]× 100% ) B/B0 % LMG ELISA of Fish Samples. For LMG ELISA, an ELISA plate was coated with LMG-BSA. The fish samples and LMG antibodies in equal volume were subjected to indirect competitive ELISA, and the concentration of LMG was calculated by a procedure similar to that for MG ELISA.
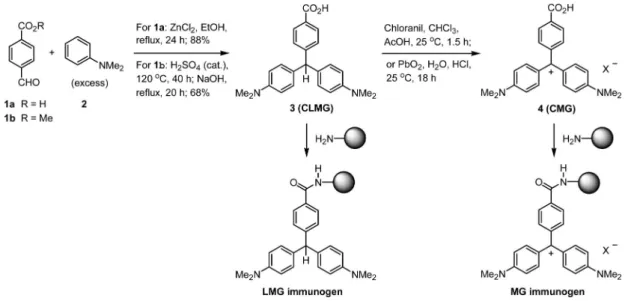
Figure 3. (a) Cross-reactivity of MG antibody against CV, and (b) cross-reactivity of LMG antibody against MG and LCV.
Downloaded by NATIONAL TAIWAN UNIV on August 27, 2009 | http://pubs.acs.org
MG ELISA of Fishpond Water. The water sample collected from fishponds was diluted with 3 volumes of 0.02 M HCl, with or without spiking of MG at 0.5 or 2µg/L. The sample was centrifuged at 3500
rpm for 10 min, and an aliquot of supernatant (100µL) was taken for
the MG ELISA.
RESULTS AND DISCUSSION
The compounds CLMG (3) and CMG (4) bearing a carboxyl
group on the phenyl ring were designed as the appropriate
analogues of MG and LMG for linkage with carrier proteins
(Scheme 1). The synthesis of CLMG and CMG is
straightfor-ward by using known methods with minor modification (30–34).
LCMG and CMG were attached to the carrier proteins BSA
and OVA via amide bond formation using NHS and EDCI as
the condensation agents. Two groups of New Zealand rabbits
(in duplicate) were immunized by subcutaneous injection with
the MG- and LMG-OVA immunogens prepared as such to
generate the specific antibodies, respectively, using the standard
procedures. The results of the MG- and LMG-ELISA produced
linear ranges from 0.05 to 0.5
µg/L of MG (Figure 2a) and
from 0.05 to 2
µg/L of LMG (Figure 2b). The detection limit
of this assay was 0.05
µg/L for both MG and LMG, lower than
the regulatory limit by the European Commission and U.S. Food
& Drug Administration (10).
Both our MG- and LMG-ELISA kits use a cutoff level of 1
µg/kg to distinguish positive from negative samples. A total of
53 fish samples were detected by MG ELISA. The MG
concentrations in fish muscle were spiked with 0.5 or 2
µg/kg
of MG, followed by 7-fold dilution, in appropriate cases for
the ELISA experiments. There was 98% agreement between
the results obtained by ELISA and theoretical concentration.
The result shows 100% sensitivity and 96% specificity (Table
1). The recovery of MG-spiked samples was varied from 71%
to 108%. A total of 115 fish samples were detected by LMG
ELISA. The LMG concentrations in fish muscle were spiked
with 0.5, 0.75, 1.5, or 2
µg/kg of LMG in appropriate cases for
analysis. There was 90% agreement between the results obtained
by ELISA and those by theoretical concentration. The result
shows 83% sensitivity and 98% specificity (Table 1). The
recovery of LMG-spiked samples was varied from 62% to
105%. It was noted that fatty samples might cause lower
recovery of LMG, and thus inferior sensitivity in ELISA.
Presumably due to the lipophilic nature of LMG, the extraction
from fatty samples might become less efficient.
MG ELISA was also used for fishpond water detection. The
samples were diluted and directly analyzed by MG ELISA
without the need of extraction or prior preparation. A total of
27 water samples were tested. The result showed that sensitivity
and specificity are both 100% (Table 2).
The MG and LMG antibodies were insensitive to common
antibiotics, sulfonamides, and benzene derivatives. No
cross-reactivity (<0.005%) in the ELISA was observed in the 16 test
compounds, including sulfadiazine, sulfamonomethoxine,
famethazine, sulfamethoxypyridazine, sulfadimethoxine,
sul-fathiazole, sulfaquinoxaline, Sulfamethoxazole, penicillin G,
gentamicin, oxytetracycline, tetracycline, chloramphenicol, aniline,
dimethylaniline and benzaldehyde, up to a concentration of 1000
µg/L.
The specificity of MG and LMG antibodies for the
triph-enylmethane dyes MG and CV as well as their metabolites LMG
and LCV were investigated by indirect competitive ELISA. The
MG antibody showed 100% cross-reactivity to CV, but was
Scheme 1. Synthesis of Carboxymalachite Green (CMG) and Carboxyleucomalachite Green (CLMG)
Table 1. Comparison of MG and LMG Concentrations Determined by ELISA and Theoretical Concentrations in Fish Muscle (n ) 53 for MG and
n ) 115 for LMG) MG LMG ELISA +a -b +c -b +d 26 1 50 1 -e 0 26 10 54 sensitivityf 100% 83% specificityg 96% 98%
aMG at concentration of 0.5 or 2 µg/L was spiked into the samples.bNo MG or LMG was spiked into the samples.cLMG at concentration of 0.5, 0.75, 1.5, or 2 µg/L was spiked into the samples. dPositive result of the relevant ELISA. eNegative result of the relevant ELISA.f% sensitivity ) (True positives – False negatives)/(True positives)× 100%g% specificity ) (True negatives – False positives)/(True negatives)× 100%
Table 2. Comparison of MG Concentrations Determined by ELISA and Theoretical Concentrations in Water Samples (n ) 27)
MG ELISA + -+ 9 0 - 0 18 sensitivity 100% specificity 100%
Downloaded by NATIONAL TAIWAN UNIV on August 27, 2009 | http://pubs.acs.org
insensitive to LMG or LCV (Figure 33a). This result might be
attributable to the similar structures between MG and CV having
4-(dimethylamino)phenyl groups surround the methylium center
of planar shape (Figure 11). Because LCV exhibited a methine
center of tetrahedral shape, as that of LMG, the LMG antibody
also reacted strongly with LCV (200% cross-reactivity), but not
with CV (Figure 3b). A slight cross-reactivity of the LMG
antibody with MG (
∼3 %) was also observed. The reason is
unclear, though we speculate that a small amount of MG may
be derived from air oxidation of LMG during the process of
immunogen preparation and immunization.
To our knowledge, using the commercially available ELISA
kits, e.g., MaxSignal Malachite Green ELISA Test Kit of Bioo
Scientific Co. (Texas, USA), for detection of LMG in fish
samples requires a prior oxidation of LMG to MG. On the other
hand, our LMG ELISA is directly applied to detection of LMG
in fish samples. Though a side-by-side comparison experiment
was not carried out, our MG ELISA shows a detection limit of
0.05
µg/L, better than the BIOO specification (0.5 µg/L).
In conclusion, we have successfully utilized CMG-OVA and
CLMG-OVA as immunogens to produce, respectively, a high
titer of rabbit polyclonal antibodies against MG and LMG.
Detection of MG and LMG residues in spiked fish muscle with
the ELISA kits showed 96% specificity and 100 % sensitivity
for the MG antibody, as well as 98 % specificity and 83 %
sensitivity for the LMG antibody. The diluted fishpond water,
without extraction or prior preparation, was directly subjected
to the MG ELISA analysis to show 100% specificity and 100
% sensitivity. MG and LMG were readily detected in a
concentration as low as 0.05
µg/L by our ELISA kits in a short
period of time (<2 h), including the time needed for fish sample
preparation. The described ELISA method allows a direct
analysis of MG and LMG in fish and water samples without an
additional step for oxidation of LMG to MG, which is often
required in the previously reported methods using HPLC and
LC-MS. While HPLC methods are used for quantitative and
confirmatory determination of MG and LMG in aquaculture
products, the ELISA method is good for rapid on-site screening
in a semiquantitative sense.
ABBREVIATIONS USED
BSA, bovine serum albumin; CLMG, carboxyleucomalachite
green; CMG, carboxymalachite green; CV, crystal violet; DEPT,
distortionless enhancement by polarization transfer; EDCI,
N-(3-dimethylaminopropyl)-N
′
-ethylcarbodiimide; ELISA,
enzyme-linked immunosorbent assay; ESI, electrospray ionization;
FT-NMR spectrometer, Fourier transform nuclear magnetic resonance
spectrometer; HPLC, high performance liquid chromatography;
HRMS, high-resolution mass spectra; HRP, horseradish
per-oxidase; IgG, immunoglobulin G; IR spectra, infrared spectra;
LC-MS, liquid chromatography–mass spectrometry; LCV,
leu-cocrystal violet; LMG, leucomalachite green; MG, malachite
green; MES, 2-(N-morpholino)ethanesulfonic acid; MS, mass
spectrometry or mass spectra; NHS, N-hydroxysuccinimide;
NMR spectra, nuclear magnetic resonance spectra; OD, optical
density; OVA, ovalbumin; PBS, phosphate-buffered saline;
PBST, phosphate-buffered saline with 0.05% Tween 20; TMB,
3,3
′
,5,5
′
-tetramethylbenzidine; TLC, thin-layer chromatography;
TMS, tetramethylsilane.
LITERATURE CITED
(1) Alderman, D. J. Malachite green: a review. J. Fish Dis. 1985, 8, 289–298.
(2) Plakas, S. M.; El Said, K. R.; Stehly, G. R.; Gingerich, W. H.; Allen, J. H. Uptake, tissue distribution, and metabolism of malachite green in the channel catfish (Ictalurus punctatus). Can.
J. Fish Aquat. Sci. 1996, 53, 1427–1433.
(3) Plakas, S. M.; Doerge, D. R.; Turnipseed, S. B. Disposition and metabolism of malachite green and other therapeutic dyes in fish. In Xenobiotics in Fish; Beconi-Barker, M., Gingerich, W. H., Smith, D. J., Eds.; Plenum Press: New York, 1999; pp 149–166. (4) Culp, S. J.; Beland, F. A. Malachite green: A toxicological review.
J. Am. Coll. Toxicol. 1996, 15, 219–238.
(5) Fernandes, C.; Lalitha, V. S.; Rao, K. V. K. Enhancing effect of malachite green on the development of hepatic pre-neoplastic lesions induced by N-nitrosodiethylamine in rats. Carcinogenesis 1991, 12, 839–845.
(6) Culp, S. J.; Blankenship, L. R.; Kusewitt, D. F.; Doerge, D. R.; Mulligan, L. T.; Beland, F. A. Toxicity and metabolism of malachite green and leucomalachite green during short-term feeding to Fischer 344 rats and B6C3F1 mice. Chem. Biol.
Interact. 1999, 122, 153–170.
(7) Mittelstaedt, R. A.; Mei, N.; Webb, P. J.; Shaddock, J. G.; Dobrovolsky, V. N.; McGarrity, L. J.; Morris, S. M.; Chen, T.; Beland, F. A.; Greenlees, K. J.; Heflich, R. H. Genotoxicity of malachite green and leucomalachite green in female Big Blue B6C3F1 mice. Mutat. Res. 2004, 561, 127–138.
(8) Schnick, R. A. An overview of current programs on drug development and regulation for aquaculture: US Fish and Wildlife Service perspective. Vet. Hum. Toxicol. 1991, 33 (Suppl. 1), 4– 5.
(9) Veterinary Residues Committee. Annual Report on Surveillance for Veterinary Residues in Food in the UK for 2001, 2002, and 2003, available at http://www.vet-residues-committee.gov.uk/. (10) Commission Decision 2004/25/EC as regards the setting of
minimum required performance limits (MRPLs) for certain residues in food of animal origin. Official Journal of the European
Union 2004, L6, 38–39.
(11) Allen, J. L.; Gofus, J. E.; Meinertz, J. R. Determination of malachite green residues in the eggs, fry, and adult muscle tissue of rainbow trout (Oncorhynchus mykiss). J. AOAC Int. 1994, 77, 553–557.
(12) Roybal, J. E.; Pfenning, A. P.; Munns, R. K.; Holland, D. C.; Hurlbut, J. A.; Long, A. R. Determination of malachite green and its metabolite, leucomalachite green, in catfish (Ictalurus punctatus) tissue by liquid chromatography with visible detection. J. AOAC
Int. 1995, 78, 453–457.
(13) Turnipseed, S. B.; Roybal, J. E.; Hurlbut, J. A.; Long, A. R. Gas chromatographic/mass spectrometric confirmation of leucomala-chite green in catfish (Ictalurus punctatus) tissue. J. AOAC Int. 1995, 78, 971–977.
(14) Plakas, S. M.; el Said, K. R.; Stehly, G. R.; Roybal, J. E. Optimization of a liquid chromatographic method for determina-tion of malachite green and its metabolites in fish tissues. J. AOAC
Int. 1995, 78, 1388–1394.
(15) Doerge, D. R.; Churchwell, M. I.; Gehring, T. A.; Pu, Y. M.; Plakas, S. M. Analysis of malachite green and matabolites in fish using liquid chromatography atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1998, 12, 1625–1634.
(16) Tarbin, J. A.; Barnes, K. A.; Bygrave, J.; Farrington, W. H. H. Screening and confirmation of triphenylmethane dyes and their leuco metabolites in trout muscle using HPLC-vis and ESP-LCMS. Analyst 1998, 123, 2567–2571.
(17) Bergwerff, A. A.; Scherpenisse, P. Determination of residues of malachite green in aquatic animals. J. Chromatogr., B 2003, 788, 351–359.
(18) U.S. Food and Drug Administration. Guideline for Industry: Mass spectrometry for confirmation of the identity of animal drug residues. Fed. Regist. 2003, 68, 25617–25618.
(19) Brandt, A.-M.; Laerke, S. Determination of malachite green and leucomalachite green by HPLC with postcolumn oxidation. In
Proceedings of the EuroResidue V Conference;Noordwijkerhout,
The Netherlands, 2004; pp 358–362.
Downloaded by NATIONAL TAIWAN UNIV on August 27, 2009 | http://pubs.acs.org
(20) Van Rhijn, J. A.; Mulder, P. P. J.; van Baardewijk, F.; te Brinke, E. M.; Lasaroms, J. J. P. Confirmatory analysis of traces of malachite green and its main metabolite leucomalachite green in muscle tissue of Atlantic salmon. In Proceedings of the
EuroResi-due V Conference;Noordwijkerhout, The Netherlands, 2004; pp
808–813.
(21) Turnipseed, S. B.; Andersen, W. C.; Roybal, J. E. Determination and confirmation of leucomalachite green in salmon using no-discharge atmospheric pressure chemical ionization LC-MS. US
Food Drug Admin. Lab. Inf. Bull. 2004, 20, LIB No. 4333.
(22) Valle, L.; Diaz, C.; Zanocco, A. L.; Richter, P. Determination of the sum of malachite green and leucomalachite green in salmon muscle by liquid chromatography-atmospheric pressure chemical ionisation-mass spectrometry. J. Chromatogr., A 2005, 1067, 101– 105.
(23) Scherpenisse, P.; Bergwerff, A. A. Determination of residues of malachite green in finfish by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2005, 529, 173–177.
(24) Mitrowska, K.; Posyniak, A.; Zmudzki, J. Determination of malachite green and leucomalachite green in carp muscle by liquid chromatography with visible and fluorescence detection. J.
Chro-mat. A 2005, 1089, 187–192.
(25) Van de Riet, J. M.; Murphy, C. J.; Pearce, J. N.; Potter, R. A.; Burns, B. G. Determination of malachite green and leucomalachite green in a variety of aquacultured products by liquid chromatog-raphy with tandem mass spectrometry detection. J. AOAC Int. 2005, 88, 744–749.
(26) Andersen, W. C.; Roybal, J. E.; Turnipseed, S. B. Liquid chromatographic determination of malachite green and leuco-malachite green (LMG) residues in salmon with in situ LMG oxidation. J. AOAC Int. 2005, 88, 1292–1298.
(27) Turnipseed, S. B.; Andersen, W. C.; Roybal, J. E. Determination and confirmation of malachite green and leucomalachite green residues in salmon using liquid chromatography with no-discharge
atmospheric pressure chemical ionization. J. AOAC Int. 2005, 88, 1312–1317.
(28) Turnipseed, S. B.; Andersen, W. C.; Karbiwnyk, C. M.; Roybal, J. E.; Miller, K. E. No-discharge atmospheric pressure chemical ionization: Evaluation and application to the analysis of animal drug residues in complex matrices. Rapid Commun. Mass
Spec-trom. 2006, 20, 1231–1239.
(29) Andersen, W. C.; Turnipseed, S. B.; Roybal, J. E. Quantitative and confirmatory analyses of malachite green and leucomalachite green residues in fish and shrimp. J. Agric. Food Chem. 2006,
54, 4517–4523.
(30) Müller, W.; Hattesohl, I.; Schuetz, H. J.; Meyer, G. Polyethylene glycol derivatives of base and sequence specific DNA ligands: DNA interaction and application for base specific separation of DNA fragments by gel electrophoresis. Nucleic Acids Res. 1981,
9, 95–119.
(31) Inoue, T.; Kikuchi, K.; Hirose, K.; Iino, M.; Nagano, T. Small molecule-based laser inactivation of inositol 1,4,5-trisphosphate receptor. Chem. Biol. 2001, 8, 9–15.
(32) Finer, J. T.; Chabala, J. C.; Lewis, E. Preparation of triphenyl-methanes as kinesin KSP inhibitors. PCT Int. Appl. 2002, 50. (33) Nakanishi, W.; Kikuchi, K.; Inoue, T.; Hirose, K.; Iino, M.;
Nagano, T. Hydrophobic modifications at 1-phosphate of inositol 1,4,5-Trisphosphate analogues enhance receptor binding. Bioorg.
Med. Chem. Lett. 2002, 12, 911–913.
(34) Cho, B. P.; Yang, T.; Blankenship, L. R.; Moody, J. D.; Churchwell, M.; Beland, F. A.; Culp, S. J. Synthesis and characterization of N-demethylated metabolites of malachite green and leucomalachite green. Chem. Res. Toxicol. 2003, 16, 285– 294.
Received for review April 23, 2007. Revised manuscript received July 27, 2007. Accepted July 30, 2007. We thank the Council of Agriculture, Taiwan, for financial support [95-Rescure and Regulate-Fishery-01(2)]. JF071195Y
Downloaded by NATIONAL TAIWAN UNIV on August 27, 2009 | http://pubs.acs.org