Research Express@NCKU - Articles Digest
Research Express@NCKU Volume 21 Issue 4 - February 3, 2012 [ http://research.ncku.edu.tw/re/articles/e/20120203/3.html ]
Characterization of aqueous dispersions of Fe
3
O
4
nanoparticles and their biomedical applications
Fong-Yu Cheng
1, Chio-Hao Su
1, Chen-Sheng Yeh
1,*Chiau-Yuang Tsai
2, Chao-Liang Wu
2,
Dar-Bin Shieh
31 Department of Chemistry, National Cheng Kung University
2 Department of Biochemistry, National Cheng Kung University, Tainan 701, Taiwan, ROC
3 Department of Radiology, Kaohsiung Veterans General Hospital, Kaohsiung 813, Taiwan, ROC
4 Institute of Oral Medicine, National Taiwan University
csyeh@mail.ncku.edu.tw
Biomaterials, 2005, 26, 729-738 Times Cited: 205
S
tudies of nanoscale materials have captured significant scientific and industrial interest in recent years. Magnetite (Fe3O4) nanoparticles have been extensively exploited as ferrofluids. Various approaches were developed to synthesize iron oxides. In recent advanced nanoparticles synthesis, Sun and co-workers manipulated mono-dispersed Fe3O4 nanoparticles. Following high-temperature decomposition of an iron precursor in an organic solution phase, they were able to produce magnetite withseveral controllable particle diameters. Conversely, well-dispersed aqueous Fe3O4 colloids fabrication has met with very limited success. Although the Fe(II) acetate sonication method has been reported by Gedanken et al., most of the common protocols involve ferrous and ferric ions coprecipitation with aqueous NH4OH or NaOH. In any case, severe particles aggregation accompanied with facile precipitation are the inevitable outcomes. The synthesis of biocompatible superparamagnetic materials has long been of interest in biomedical applications including magnetic resonance imaging for clinical diagnosis, magnetic drug targeting, hyperthermia anti-cancer strategy, andenzyme immobilization.
The efficacy of many medical applications may strongly rely upon generating narrow size distribution and well-dispersed nanoparticles in an aqueous solution. Iron-oxide of nanometer size presents superparamagnetic property and is ideal for MR contrast enhancement by alterations of proton relaxation in the tissue microenvironment. However, magnetic nanoparticles without polymer coating often suffered from the aggregation problem in water or tissue fluid, which may limit in vitro magnetic-based isolation and detection strategies, as well as clinical MRI applications. Polymer coated iron-oxide particles (SPIO and USPIO) reduced aggregation problems and has been developed for various fields of clinical MR imaging. In fact, all SPIO or USPIO MR contrast agents already approved for clinical usage nowadays, as well as most of the currently developing contrast agents, were stabilized by dextran or its derivatives. The polymer coating significantly increases their overall size and therefore may limit their tissue distribution, penetration, and metabolic clearance. Polymer-coated particles are often uptaken rapidly by the reticuloendothelial system, such as Kupffer cells of the liver. In general, the biodistribution of these polymer-based nanoparticles was mainly influenced by their size and surface chemistry. In the surface chemistry aspect, hydrophobic surface may enhance the uptake of the nanoparticles by the liver, while hydrophilic coating and surface charges may influence their retention period in the circulation and the chance to penetrate into interstitial spaces. Although these polymer coatings are generally considered to be biocompatible, adverse reactions have also been reported. On the other hand, only limited recent reports have investigated non-polymer
Research Express@NCKU - Articles Digest
coated superparamagnetic nanoparticles in MR imaging. These particles were stabilized by citrate monomer and presented adequate performance in coronary MR angiography. In this study, we have prepared another type of non-polymer dispersive superparamagnetic iron oxide nanoparticle and demonstrated their potential as a new class of MR contrast agent for the future development.
In the current study, a modified Fe(II) and Fe(III) salt coprecipitation synthesis using tetramethylammonium hydroxide (N(CH3) 4OH) was used to produce well dispersed Fe3O4 colloidal solutions. The physical properties and the crystalline structure of the newly formed magnetite nanoparticles were characterized by Transmission electron microscopy (TEM) and ray diffraction (XRD). Fourier transform infrared spectrometer (FT-IR) and X-ray photoelectron spectrometer (XPS) were performed to analyze the surface characteristics of the nanoparticles. The magnetization was determined from superconducting quantum interference measurement device (SQUID) measurements. Furthermore, the in vitro cytotoxicity test and in vitro hemolysis assay were performed to evaluate the biocompatibility of the prepared Fe3O4 nanoparticles in vitro. To further investigate the potential usage of the nanoparticles in MR imaging, the T1 longitudinal and T2 transverse relaxation times were measuredusing the NMR spectrometer at 9.4 T. Although the ability of a given contrast agent to enhance the longitudinal and transverse relaxation rates is usually specified in terms of the in vitro dipolar relaxivity values (r1 and r2) of the agent, the observed T1 and T2_ effects in MR imaging may not be directly deduced from these values alone since r1 and r2 only describe the agent’s ability to enhance the respective relaxation rates in a perfectly homogeneous medium, typically water without interactions with their local micro-environment.
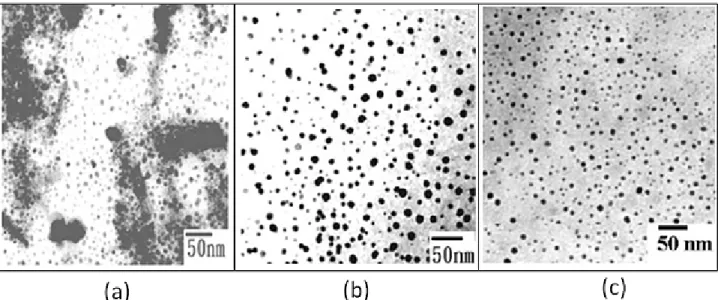
Fig. 1 shows TEM images of the iron oxide suspensions. In addition to the dispersed and well-separated features, the prepared colloids also exhibited some degree of aggregated morphology, as shown in Fig. 1a. The particle diameter was calculated as 9.1±2.1 nm. Following centrifugation at 10000 rpm for 10 min, the particles in
suspensions exhibited mostly the well-dispersed appearance. The average size decreased to 6.2±1.4 nm, as seen in Fig. 1b.
Fig. 1. TEM imaging showing Fe3O4 nanoparticles (a) from the as-synthesized colloidal suspensions and (b) after following centrifugation at 10000 rpm.
The XRD indicates that magnetite (Fe3O4) was the resulting material and FT-IR and XPS analysis were performed to characterize the surface nature of the resulting magnetite nanoparticles SQUID shows the magnetization loop of the Fe3O4 nanoparticles measured at room temperature. The as-synthesized magnetites indicate a
Research Express@NCKU - Articles Digest
superparamagnetic behavior, as evidenced by zero coercivity and remanance on the magnetization loop. A saturation magnetization of ~40 emu/g was determined for the fine Fe3O4 particles.
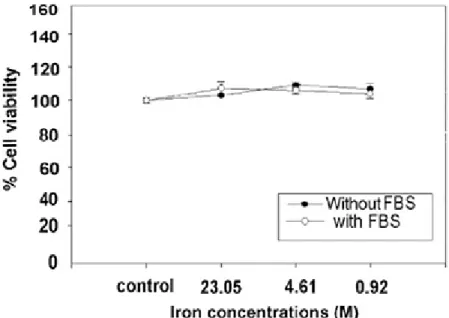
The viability of cells evaluated by MTT assay was apparently unaltered upon exposure to various concentrations of Fe3O4 nanoparticles for 4 h, ranging from 0.92 to 23.05 mM of iron concentrations (Fig. 2). According to cell viability assay, Fe3O4 nanoparticles are generally considered to be biocompatible. The in vitro hemolysis test, by detecting free hemoglobin in the serum after incubation with various concentrations of the nanoparticles, indicated that significant hemolysis (0.5 g/dL) could only be detected in 0.1 M iron concentration of the nanoparticle. Other samples presented undetectable hemoglobin concentration to the instrument’s detection limit. 0.1 M concentration was below the dose required for MR contrast enhancement in whole blood.
Fig 2. Viability of Cos-7 monkey kidney cells exposed to the ferrofluids at various iron concentrations. Cell viability is expressed as the mean ± S.E. of the percentage of absorbance of controls where
100% equals viability of untreatedcontrol cells.
Superparamagnetic nano-ferrofluids have been recognized to hold great potential in clinical diagnostic applications as the magnetic resonance (MR) imaging contrast agents that could exhibit the ability to alter the proton relaxivity of water. Water proton relaxation for the as-synthesized Fe3O4 was performed using standard and solid state NMR spectrometer at 9.4 T. The magnetic nanoparticles strongly reduce both relaxation times (T1 and T2) with T2 significantly lower than T1. At a concentration of 0.86 mM magnetite particles (4.61mM of iron concentration), the longitudinal relaxation time (T1) was reduced from 3000 ms for pure water to 22.88 ms, while the T2
relaxation time was reduced from 212.8 to 0.36 ms. In conclusion, the calculated r1 and r2 relaxivities of the newly synthesizednanoparticles were 9.4 s-1mM-1 and605.5 s-1mM-1, respectively. Both r1 and r2 of the synthesized
nanoparticles were much shorter than Resovist(commercial product). These results present the evidence that the newly prepared Fe3O4 nanoparticles strongly reduce both T1 and T2 relaxation times. Therefore, the magnetite material presented here may have great potential for clinical MR imaging as a contrast agent.
Conclusion
Newly formed Fe3O4 nanoparticles of 9 nm diameter were developed using ferrous and ferric ions with N(CH3)
Research Express@NCKU - Articles Digest
4OH. The resulting superparamagnetic magnetite exhibited a well-dispersed property. From the FT-IR and XPS
analysis, the surface nature of the iron oxides was viewed as the electrostatic interaction between quaternary (CH3)
4N+ cations and the surface hydroxyl groups. The Cos-7 monkey kidney cells were used for estimating the
biological effect of the superparamagnetic fluids on cell viability and proliferation. No apparent cytotoxic effects were observed at various Fe3O4 doses.