行政院國家科學委員會補助專題研究計畫成果報告
※※※※※※※※※※※※※※※※※※※※※※※
※
氧化性低密度脂蛋白誘發動脈硬化粥狀硬化之
※
※
致病機轉氧化性脂蛋白對血管通透性之病生理影響
※
※※※※※※※※※※※※※※※※※※※※※※※
計畫類別:□個別型計畫
■整合型計畫-子計劃
計畫編號:NSC90-2314-B002-284-M52
執行期間:1999 年 08 月 01 日 至 2002 年 07 月 31 日
計畫主持人:吳造中
共同主持人:李源德
執行單位:國立台灣大學醫學院一般醫學科
中
華
民
國
2002
年
10
月
25
日
行政院國家科學委員會專題研究計畫成果報告
計畫編號:NSC90-2314-B002-284-M52 執行期間:1999 年 08 月 01 日 至 2002 年 07 月 31 日 主持人:吳造中 國立台灣大學醫學院一般醫學科 共同主持人:李源德 國立台灣大學醫學院附設醫院內科部 中英文摘要, 為了研究食物中的脂肪與血管內皮細胞通透性之關係,我們以 12 週之高脂飲 食餵食 60 隻紐西蘭白兔後,再以 80 週之標準兔飼料作飲食治療。另外 60 隻兔 子則飼以 92 週之標準兔飼料(對照組)。吾人施以一系列的血清脂質定量,眼前 房螢光光度計測量(血管通透性),及眼睛形態學及顯微組織檢查。研究結果發 現當食物中脂質含量增加時,血中脂質(膽固醇及三酸甘油脂)首先上升,隨著 IL-8 上升,內皮細胞間連結不良,局部血管新生,最後造成血管通透性增加。而 飲食治療可有效地使血管內皮屏障功能回復。此研究闡明了高脂飲食誘發血管屏 障功能失全之病生理機轉。 關鍵字:血管通透性、高血脂症、飲食、血管新生、IL-8。
To study the mechanisms linking dietary lipid to changes in endothelial permeability, adult NZW rabbits (n=120) were fed with either lipid-enriched diet or standard chow for12 weeks, followed by standard chow for 80 weeks. Sequential anterior chamber fluorophotometric examinations along with morphopathological characterization of iris were carried out. With increased lipid intake, elevated serum lipid level and subsequent IL-8 surge precede the disrupted organization of interendothelial junctions, induce angiogenesis, and finally alter endothelial permeability. Diet therapy is effective to restore normal endothelial barrier function. This study proposes a possible mechanism how dietary lipid modulates the microvascular endothelial barrier.
Key words: Vascular permeability, hyperlipidemia, diet, angiogenesis, IL-8
計畫緣由與目的、
The endothelium forms a highly selective permeability barrier between blood and vascular wall.1 Progressive deterioration of endothelial barrier integrity, which leads to low-density lipoprotein (LDL) deposition in the arterial wall, contributes to the development of atherosclerosis.1,2 Although sites of increased LDL transport have been identified in normal arteries,3 insults to endothelium seem to be a prerequisite for LDL to deposit.4,5,6,7 It has been well known that hypercholesterolemia, even in the absence of plaque formation, also results in endothelial dysfunction.8,9 Increased dietary lipid intake has been shown to induce hyperlipidemia and increase the
vascular permeability. 10 , 11 Diet therapy has been recommended as one of the first-step management of hyperlipidemia,12 However, the mechanisms linking dietary lipid to changes in endothelial permeability remain undisclosed.
The purpose of this study was to use a rabbit model to test the hypothesis that during hyperlipidemia, endothelial dysfunction and barrier remodeling lead to increased vascular permeability and this process could be reversed with diet therapy. To elucidate the underlying pathophysiologic mechanisms, serial examinations for the iridic microvascular permeability, serum lipid profile, and microscopic and ultrastructural changes of iridic microvascular endothelium were carried out. The serum level of oxidized phosphatidylcholine (ox-PC), a major fraction of oxidized phospholipid in oxidized LDL, was determined to estimate the oxidative stress of hyperlipidemic animals. The changes of angiogenic cytokines, including interleukin-8 (IL-8), vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), were also investigated in serum from the same group.
結果與討論、
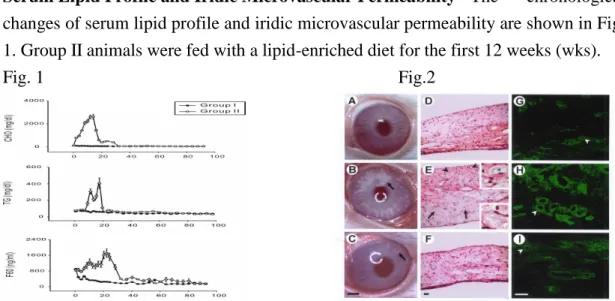
Ser um Lipid Profile and Ir idic Microvascular Per meability The chronological changes of serum lipid profile and iridic microvascular permeability are shown in Fig. 1. Group II animals were fed with a lipid-enriched diet for the first 12 weeks (wks).
Fig. 1 Fig.2
Gross, Microscopic and Ultr astr uctur al Mor phology of Ir is Fig. 2. shows the
external eye morphology (left panel), H&E stain (middle panel) and lectin labeling of the iris (right panel) in an animal treated with a normal diet and sacrificed at the 16th week (upper row), one with cessation of a lipid-enriched diet for 4 weeks (middle row), and one with cessation of a lipid-enriched diet for 36 weeks (lower row). Arrowheads in E indicate microvasculature, a high magnification view of which is in the right upper inset. Arrows in E indicate foam cells, a high magnification view of which is in the right lower inset. Arrowheads in G-I indicate lectin-binding blood vessels. Images in each column are of the same magnification. So as insets in E (Bars, 2 mm in C, 10 ìm in inset of E, 25 ìm in F and I). Fig. 3. shows the
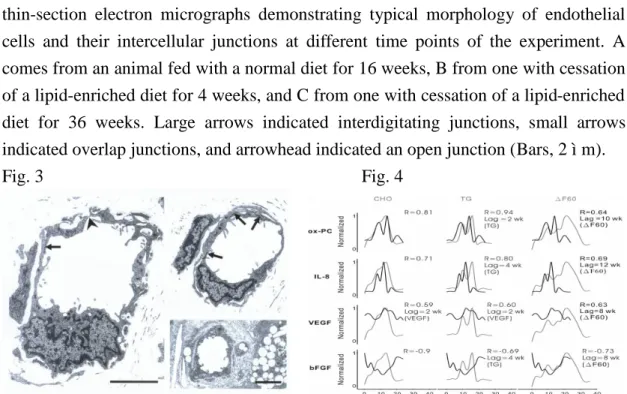
thin-section electron micrographs demonstrating typical morphology of endothelial cells and their intercellular junctions at different time points of the experiment. A comes from an animal fed with a normal diet for 16 weeks, B from one with cessation of a lipid-enriched diet for 4 weeks, and C from one with cessation of a lipid-enriched diet for 36 weeks. Large arrows indicated interdigitating junctions, small arrows indicated overlap junctions, and arrowhead indicated an open junction (Bars, 2 ìm).
Fig. 3 Fig. 4
Ser um Levels of bFGF, VEGF, IL-8 and ox-PC Fig. 4. shows the trends of serum
lipid profile, angiogenic cytokines and iridic microvascular permeability in group II animals. Ox-PC, IL-8, VEGF, and bFGF are depicted in dark lines in corresponding rows, and serum levels of cholesterol (TC), triglyceride (TG) and difference of iridic microvascular permeability between 2 groups (Ä F60) are depicted in gray lines in corresponding columns. The best correlation coefficients and relevant lag correction are shown in each panel. Lag period is expressed in weeks, and is not shown when no lag is needed to achieve the best correlation. Data is normalized to its own maximum value within the 24 weeks.
Discussion
In this study, we modulated dietary lipid content to obtain serial observations of the rabbit iridic endothelial barrier integrity. Our principal findings include: (1) feeding with lipid-enriched diet leads to increased fluorescein sodium leakage through the iridic blood-aqueous barrier; (2) functional changes responded to dietary lipid content prior to structural alteration, such as foam cells infiltration, increased vessel density, loosening of interendothelial junctions, and atheroma formation; (3) increased dietary lipid content led to hyperlipidemia, increased serum levels of ox-PC, IL-8, and VEGF, and decreased serum level of bFGF. Trend of fluorescein leakage throughout the experiment resembled trends of serum lipid profile and angiogenic cytokines with 8-12 weeks’ lag; (4) diet therapy restored serological, functional and structural changes.
Previously mentioned explanation for altered endothelial permeability during hyperlipidemia includes increased pinocytosis in the presence of oxidized LDL,13
accelerated endothelial cell turnover and associated transient open junctions,14 increased endothelial contraction due to RhoA mediated F-actin polymerization,15 and enhanced leukocytes-endothelial cells interaction.16 In addition to the theories mentioned above, we observed increased small vessel density in our model. As our results point out, ultrastructural and microscopic changes lagged behind functional changes. It implies that anatomical as well as physiological factors contribute to the iridic endothelial barrier disruption during diet-induced hyperlipidemia.
According to our correlation analysis, TC level is correlated with serum levels of ox-PC, IL-8, VEGF and bFGF. The IL-8 and bFGF are also highly correlated with the lagged Ä F60. Best predictor of Ä F60 comes from a weighted combination of TC level 10 weeks earlier and TG level. Our interpretation of such results is as follows: increased serum level of cholesterol triggers inflammation response and IL-8 is secreted as a result. Increased IL-8 may recruit leukocytes, augmenting lipid peroxidation as evidenced by subsequently elevated serum level of ox-PC. IL-8 is also capable of inducing angiogenesis,17 and therefore contributes to increased microvascular permeability. At the mean while, elevated serum level of TG may immediately lead to increased release of non-esterified fatty acid.18 Endothelial cells are stressed, and cytoplasmic vacuolation develops. Therefore, interendothelial junction loosening ensues. After dietary source of excess lipid is removed, inflammation gradually fades out, and oxidative stress diminishes. As a result, changes in angiogenic cytokines exponentially decay, endothelial barrier is restored, and microvascular permeability returns to normal status. The increased VEGF and decreased bFGF in serum may be due to ox-LDL related macrophage activation and endothelial damages.19 , 20 Although decreased bFGF can explain the impaired angiogenic capability upon vasoocclusive challenges as most in vivo works described,
the elevated IL-8 and VEGF in our model might induce endothelial proliferation and increase vascular permeability.21
計畫成果自評
Most studies regarding the impact of hyperlipidemia on endothelial permeability were performed in an invasive manner, which limited the clinical application of microvascular permeability to assess the status of endothelium. Our results integrate and reconfirm the functional and structural changes previously described. We further propose a new mechanism on how dietary lipid content modulates endothelial permeability, and add an evidence of beneficial effects of diet therapy. Increased endothelial permeability is an early sign of atherosclerosis. Therefore this non-invasive monitoring modality warrants further investigation in order to provide clinical information on vascular diseases.
1. Heinecke JW. Mechanisms of oxidative damage of low-density lipoprotein in
human atherosclerosis. Curr Opin Lipidol. 1997;8:268-274.
2. Huttner I, Boutet M, More RH. Studies on protein passage through arterial endothelium, I: structural correlated of permeability in rat arterial endothelium.
Lab Invest. 1973;28:672-677.
3. Herrmann RA, Malinauskas RA, Truskey GA. Characterization of sites with elevated LDL permeability at intercostal, celiac, and iliac branches of the normal rabbit aorta. Arterioscler Thromb. 1994;14:313-323.
4. Rangaswamy S, Penn MS, Saidel GM, Chisolm GM. Exogenous oxidized low-density lipoprotein injures and alters the barrier function of endothelium in rats in vivo. Circ Res. 1997;80:37-44.
5. Rutledge JC, Woo MM, Rezai AA, Curtis LK, Goldberg IJ. Lipoprotein lipase increases lipoprotein binding to the artery wall and increases endothelial layer permeability by formation of lipolysis products. Circ Res. 1997;80:819-828.
6. Nielsen LB, Nordestgaard BG, Stender S, Kjeldsen K. Aortic permeability to LDL as a predictor of aortic cholesterol accumulation in cholesterol-fed rabbits.
Arteriosclerosis. 1992;12:1402-1409.
7. Rutledge JC, Curry FE, Blanche P, Krauss RM. Solvent drag of LDL across mammalian endothelial barriers with increased permeability. Am J Physiol.
1995;268:H1982-H1991.
8. Osborne JA, Siegman MJ, Sedar AW, Mooers SU, Lefer AM. Lack of endothelium-dependent relaxation in coronary resistance arteries of cholesterol-fed rabbits. Am J Physiol. 1989;256:C591-C597.
9. Verbeuren TJ, Jordaens FH, Zoonekeyn LL, Van Hove CE, Coene MC, Herman AG. Effect of hypercholesterolemia on vascular reactivity in the rabbit, I: endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986;58:552-564.
10. Wu CC, Chang SW, Chen MS, Lee YT. Early change of vascular permeability in hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol. 1995;15:529-533.
11. Menzoian JO, Haudenschild CC, Shipman JL, Nickerson CJ, Fuller RM, Chobanian AV. Correction of enhanced endothelial permeability by cessation of cholesterol feeding. J Vasc Surg. 1987;5:336-41.
12. Kris-Etherton P, Eckel RH, Howard BV, St Jeor S, Bazzarre TL; Nutrition Committee Population Science Committee and Clinical Science Committee of the American Heart Association. AHA Science Advisory. Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Cholesterol Education
Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease.Circulation. 2001;103:1823-5.
13. Chow SE, Lee RS, Shih SH, Chen JK. Oxidized LDL promotes vascular endothelial cell pinocytosis via a prooxidation mechanism. FASEB J.
1998;12:823-30.
14. Lin SJ, Ding YZ. Effects of hyperlipidemia on aortic endothelial cell turnover and transendothelial macromolecular transport in cholesterol-fed rats. Zhonghua Yi Xue Za Zhi (Taipei). 1996;58:235-40.
15. Colangelo S, Langille BL, Steiner G, Gotlieb AI. Alterations in endothelial F-actin microfilaments in rabbit aorta in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1998;18:52-6.
16. Munro JM, Cotran RS. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988;58:249-61.
17. Belperio JA, Keane MP, Arenberg DA, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1-8.
18. Chung BH, Hennig B, Cho BH, et al. Effect of the fat composition of a single meal on the composition and cytotoxic potencies of lipolytically-releasable free fatty acids in postprandial plasma.Atherosclerosis. 1998; 141: 321-32.
19. Inoue M, Itoh H, Tanaka T, Chun TH, Doi K, Fukunaga Y, Sawada N, Yamshita J, Masatsugu K, Saito T, Sakaguchi S, Sone M, Yamahara Ki, Yurugi T, Nakao K. Oxidized LDL regulates vascular endothelial growth factor expression in human macrophages and endothelial cells through activation of peroxisome proliferator-activated receptor-gamma. Arterioscler Thromb Vasc Biol.
2001;21:560-6.
20. Chang PY, Luo S, Jiang T, et al. Oxidized low-density lipoprotein downregulates endothelial basic fibroblast growth factor through a pertussis toxin-sensitive G-protein pathway: mediator role of platelet-activating factor-like phospholipids.
Circulation. 2001; 104: 588-93.
21. Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev.