INTRODUCTION
The family Megalopidae has 2 species of tarpon: the Pacific tarpon Megalops cyprinoides (Broussonet 1782) of the Indo-Pacific Ocean and the Atlantic tarpon Megalops atlanticus (Valenciennes 1847) of the At-lantic Ocean. Both species have a leptocephalus stage in their early life, as do other members of the Super-order Elopomorpha (Elopiformes, Albuliformes, An-guilliformes and Saccopharyngiformes) (Hulet & Robin 1989). The tarpon is the most primitive of the Elopomor-pha (Tsukamoto & Okiyama 1997, Wang et al. 2003) and also the most ancestral among teleost fishes, as
evi-denced by its morphological features and results of molecular study (Forey et al. 1996). Tarpon have a mod-ified air bladder that allows them to inhale atmospheric oxygen and tolerate oxygen-poor environments (Wells et al. 2003). This enables them to live in polluted envi-ronments, and makes them useful as an indicator spe-cies for heavy stream pollution (Wang 2002). Although both species of tarpon are marine spawners, they can tolerate a wide range of salinities. After hatching, tar-pon larvae are advected to an inshore nursery area ~25 to 30 d later (Tzeng et al. 1998) and after metamor-phosing, young tarpon are frequently found in rivers, bays, mangrove areas and even in the upper reaches
© Inter-Research 2009 · www.int-res.com *Corresponding authors. Email: changcw@nmmba.gov.tw;
wnt@ntu.edu.tw
Facultative habitat selection in Pacific tarpon
Megalops cyprinoides as revealed by otolith
Sr:Ca ratios
K. N. Shen
2, C. W. Chang
3, 4,*, Y. Iizuka
5, W. N. Tzeng
1, 2,*
1Department of Life Science, and 2Institute of Fisheries Science, College of Life Science, National Taiwan University,
Taipei, Taiwan 106, ROC
3National Museum of Marine Biology and Aquarium, Pingtung, Taiwan 944, ROC
4Institute of Marine Biodiversity and Evolutionary Biology, National Donghwa University, Hualien, Taiwan 974, ROC 5Institute of Earth Sciences, Academia Sinica, Nankang, Taipei, Taiwan 115, ROC
ABSTRACT: The Pacific tarpon Megalops cyprinoides is a euryhaline fish that is widely distributed in offshore waters of the tropical and subtropical Indo-Pacific Ocean. It spawns in offshore waters and the larvae drift with tidal currents before recruiting to estuarine nursery areas at ~25 to 30 d post-hatching. Young Pacific tarpon are found in coastal waters, rivers, estuaries, inner bays, and man-grove areas. However, their age-specific migratory behavior and habitat selection are still unclear. The strontium (Sr) concentration in seawater is ~100× higher than in fresh water, and the Sr:Ca ratios in fish otoliths are positively correlated with ambient salinity and are thus widely used in studying fish migration between seawater and freshwater environments. To understand the migratory envi-ronmental history of the Pacific tarpon, otolith Sr:Ca ratios of leptocephali collected from the estuary and sub-adults collected from both Tadu Creek and offshore waters on the west coast of Taiwan were examined using an electron probe microanalyzer (EPMA). Fish were aged by counting otolith annuli. The Sr:Ca ratios in leptocephalus otoliths were high (7.4 ± 0.03 × 10– 3). However, the patterns of oto-lith Sr:Ca ratios in 1 to 3 yr old sub-adults were diverse and can be divided into 3 types: largely brack-ishwater residents, largely freshwater residents, and vagrants between brackish and fresh waters. Fish older than 4 to 5 yr all return to the marine environment. This study demonstrates that habitat selection in the Pacific tarpon after the leptocephalus stage is facultative and that their migratory environmental history can be reconstructed from otolith Sr:Ca ratios.
KEY WORDS: Megalops cyprinoides · Pacific tarpon · Leptocephalus · Otolith · Sr:Ca ratio Resale or republication not permitted without written consent of the publisher
of rivers (Merrick & Schmida 1984, Tzeng & Yu 1986, Coates 1987).
During spring and until late summer, large numbers of metamorphosing Pacific tarpon leptocephali are found schooling in the estuaries of western Taiwan (Tzeng et al. 2002b). However, little is known about their migratory history and habitat selection (Tsuka-moto & Okiyama 1993, 1997). As such, it is unknown whether Pacific tarpon obligate or facultative migra-tory fishes after the leptocephalus stage.
Fish migratory behavior and habitat selection usu-ally change with life stage (Secor 1992). Such informa-tion can be retrieved using different means that in-clude traditional census of tagged individuals (Zeller & Russ 2000), advanced tagging and marking tech-niques, acoustic, archival and satellite tagging (Sed-berry & Loefer 2001) and use of natural markers such as chemical signatures within calcified structures like fish otoliths (Secor & Rooker 2000, Tsukamoto & Arai 2001, Tzeng et al. 2007).
Fish otoliths are primarily composed of calcium car-bonate with minor organic matrices that are deposited on a daily basis (Pannella 1971, Campana & Neilson 1985, Jones 1986), which permit the determination of daily fish age. The use of calcium (Ca) together with elements deposited in otolith increments enables the reconstruction of the past migratory environmental history of fish (Secor 1992, Bath et al. 2000). At least 31 elements have been found in fish otoliths (Campana 1999) and among these elements, strontium (Sr) and calcium are widely used in determining migratory his-tory. Strontium ions have the same valence and similar ionic radius as calcium ions and are readily incorpo-rated into aragonite otolith by substitution for calcium (Amiel et al. 1973). The Sr concentration in seawater is 100× higher than in fresh water (Campana 1999, Mil-ton & Chenery 2001), permitting the use of otolith Sr:Ca ratios as a natural marker for examining the migratory environmental history, life history strategies and migratory behavior of diadromous fishes (Kalish 1990, Secor et al. 1995, Tzeng et al. 1997, Tsukamoto & Arai 2001). The relationship between the Sr:Ca ratio in tarpon otolith and the ambient salinity after metamor-phosis was validated by Chen et al. (2008) using wild-caught leptocephali reared in different salinities. Therefore, the migratory behavior and habitat selec-tion of the Pacific tarpon can be reconstructed from otolith Sr:Ca profiles.
The aims of the present study were to use otolith microchemistry to clarify the migratory behavior of the Pacific tarpon between freshwater and marine habitats by analyzing temporal changes in Sr:Ca ratio in the otolith using an electron probe microanalyzer (EPMA). Fish age was determined by counting otolith annuli to evaluate whether habitat selection is age-specific.
MATERIALS AND METHODS
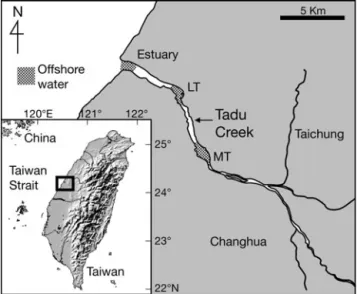
Sampling design. A total of 1106 tarpon leptocephali were collected from the estuary of Tadu Creek in 1998, 62 sub-adults were collected from the freshwater area of the lower (LT, n = 54) and middle (MT, n = 8) reaches of Tadu Creek from 1998 to 1999, and 19 sub-adults were collected in the offshore waters adjacent to Tadu Creek in 2004 to 2005 (Fig. 1). The leptocephali were collected using a net set against the tidal current in the estuary, while the sub-adults from the lower and mid-dle reaches of Tadu Creek were collected using a trammel net (50 m long × 1.5 m wide) with 3 layers of different mesh sizes (15–20–15 cm). Sub-adults were collected during the daytime monthly from March 1998 through March 1999. Two to 4 hauls from the middle and lower reaches of Tadu Creek were made each month. The sub-adults from offshore waters were collected using a gill net.
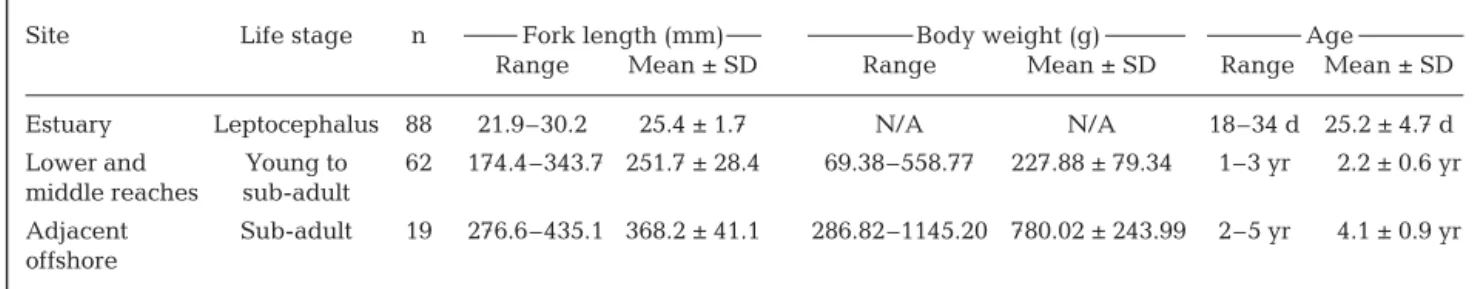
After collection, all specimens were preserved in ice in the field. The fork length of leptocephali and sub-adults and the body weight of sub-adults were measured to the nearest 0.1 mm and 0.01 g. The number of fish collected and biological measurements can be seen in Table 1. The gonads of sub-adult fish were visually examined to determine sex and maturation stage.
Otolith preparation for age determination. Sagittae, the biggest of the 3 pairs of otoliths, were removed from the sacculus of the inner ear. The right sagittal otolith was cleaned with distilled water, air dried, embedded in Epofix resin, ground and then polished along the sagittal plane with 0.05 µm alumina powder until the primordium was exposed. The annuli were
Fig. 1. Sampling locations (dotted areas) for Pacific tarpon Megalops cyprinoides in the estuary, the lower (LT) and mid-dle (MT) reaches, and the offshore waters adjacent to Tadu
examined under a microscope with a black back-ground to determine age. Annual rings, which are dis-cernible as shown in Fig. 2a, were validated by count-ing the number of otolith daily increments between the metamorphosis check and the first annulus during the period from recruitment to the first winter (Fig. 2b). The annulus in each otolith was counted by 2 different readers. Previous studies have shown that the otolith growth increment in Pacific tarpon is deposited daily (Tsukamoto & Okiyama 1993, Chen & Tzeng 2006); thus, the daily growth increments in leptocephali oto-liths were counted to determine the marine larval du-ration before estuarine arrival. In total, random sub-samples of 88 leptocephali and 81 sub-adults collected from fresh water (n = 62) and offshore waters (n = 19) were aged.
Measurement of otolith Sr:Ca ratios. The temporal pattern of Sr:Ca ratios (wt %) along the otolith transect was used to evaluate the environmental history of each Pacific tarpon, and the measurement procedures were similar to those used by Tzeng et al. (2002a, 2003). For comparison, 5 leptocephali were randomly selected for Sr:Ca ratio analysis, while the 81 sub-adults collected from freshwater (n = 62) and offshore (n = 19) habitats were all used in age determination and otolith Sr:Ca
ratio analyses. The otolith Sr:Ca ratios of the sub-adults were measured along the shorter posterior axis of the otolith (Fig. 2b). Otolith Sr and Ca concentrations were measured using an EPMA (JXA-8900R, JEOL) at an interval of 10 µm from the primordium to the otolith edge, with an electron beam size of 5 × 4 µm2 rectan-gle, and accelerating voltage and probe current set at 15 kV and 5 nA, respectively. Strontianite ([Sr0.95Ca0.05] CO3) and aragonite (CaCO3) were used as standards for the calibration of Sr and Ca concentration in the otolith.
Life history determination and data analysis. An aver-age Sr:Ca ratio of ≤4 × 10– 3was considered as the crite-rion to discriminate freshwater from brackishwater envi-ronments of the tarpon, as validated by a salinity control experiment with tarpon reared at a salinity of 0 by Chen et al. (2008). This criterion was also supported by the av-erage Sr:Ca ratio of 3.8 ± 0.54× 10– 3calculated from the Sr:Ca ratios in the otolith edge of 62 wild sub-adult Pa-cific tarpon caught in the freshwater reaches of Tadu Creek. The average Sr:Ca ratio range of tarpon reared in salinities of 10 and 35 were 6× 10– 3to 8× 10– 3 and 7× 10– 3to 11× 10– 3, respectively (Chen et al. 2008). Average Sr:Ca ratios > 8× 10– 3were considered as the criterion separating exclusively marine and brackishwater envi-ronments. The average Sr:Ca ratio (8.6 ± 0.98 × 10– 3) in the otolith edge of 19 off-shore sub-adults supports this criterion. Thus, tarpon with otolith Sr:Ca ratios ≤4 × 10– 3were considered as freshwater resi-dents, those with Sr:Ca ratios > 4× 10– 3 and ≤8 × 10– 3were regarded as brackish-water residents, and those with Sr:Ca ratios > 8 × 10– 3were considered as sea-water residents. Differences in age distri-bution between habitats were tested for significance using the Kolmogorov-Smirnov 2-sample test.
RESULTS
Length, weight and age of tarpon in different habitats
The fork lengths of 88 randomly se-lected Pacific tarpon leptocephali from the Tadu Creek estuary ranged from 21.9 to 30.2 mm. The fork length and body weight of 62 sub-adult tarpon col-lected in both the lower (LT) and middle (MT) reaches of Tadu Creek ranged from 174.4 to 343.7 mm and 69.38 to 558.77 g, while those for the 19 tarpon collected in waters offshore of Tadu Creek ranged Fig. 2. Megalops cyprinoides. External features of the otolith and (a) annual rings
under reflected light, and (b) daily growth increments and annual rings under transmitted light after polishing of a sub-adult (390 mm fork length). (
n
) Otolith primordium, MC: metamorphosis check. The area within the dashed lines indi-cates the location of Sr:Ca ratio analysis. A: anterior, P: posterior, D: dorsal,from 276.6 to 435.1 mm and 286.82 to 1145.20 g, respec-tively. The newly recruited leptocephali (n = 88) ranged from 18 to 34 d old, while sub-adults collected from Tadu Creek and from offshore waters were estimated to be 1 to 3 and 2 to 5 yr old, respectively (Table 1).
Temporal changes in otolith Sr:Ca ratios of leptocephali and sub-adults (ages 1 to 3) in fresh water
Sr:Ca ratios from the primordium to the otolith edge of 5 newly recruited leptocephali ranged from 5.1 × 10– 3 to 10.9 × 10– 3, with a mean of 7.4 ± 0.03 × 10– 3 (Fig. 3), indicating that they lived in a marine and brackishwater environment before estuarine arrival. Sr:Ca ratios from the primordium to the otolith edge of 5 sub-adults collected from the freshwater lower reach of Tadu Creek (LT) also ranged from 5 × 10– 3to 11 × 10– 3in the leptocephalus stage (Fig. 4). After metamor-phosis, however, the patterns of Sr:Ca ratios varied greatly and can be classified into 3 types of migration behavior: Type I: largely brackishwater residents with Sr:Ca ratios that fluctuated mostly between 4 × 10– 3 and 8 × 10– 3, indicating stay in a brackishwater
envi-ronment after metamorphosis but with possible occa-sional invasion of marine or fresh waters (exhibited by 12 out of 54 ind.; Fig. 4a,b); Type II: largely freshwater residents, with Sr:Ca ratios mostly < 4 × 10– 3, indicating stay in fresh water after metamorphosis but with possi-ble occasional invasion of brackish waters (exhibited by 34 out of 54 ind.; Fig. 4c,d); and Type III: vagrants that migrated between fresh and brackish waters for longer periods after metamorphosis (exhibited by 8 out of 54 ind.; Fig. 4e). Tarpon collected in the middle reach of Tadu Creek (MT), however, did not have Type I residents. Out of 8 ind., 6 were Type II (e.g. Fig. 5a,b) and 2 were Type III vagrants (Fig. 5c,d).
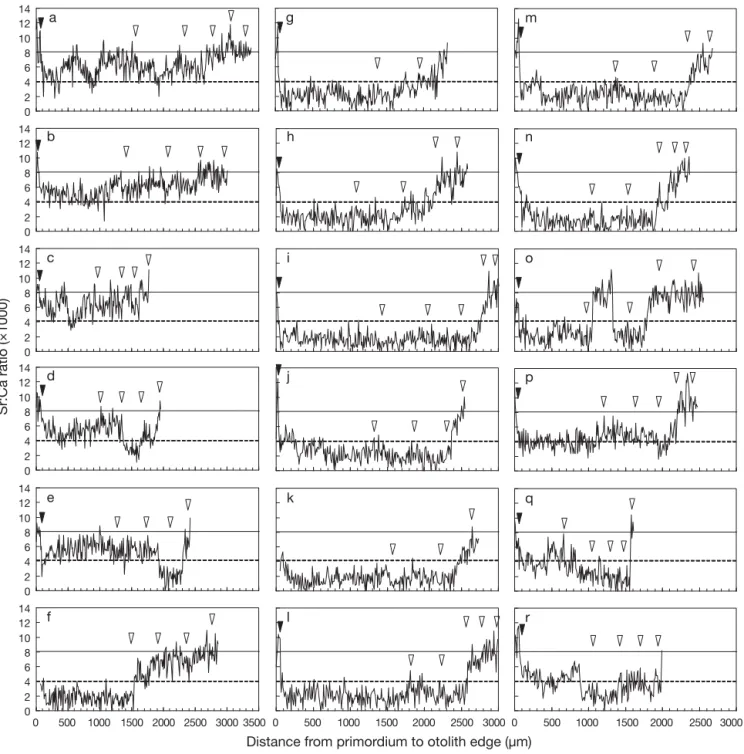
Temporal changes in otolith Sr:Ca ratios of sub-adults (ages 2 to 5) in offshore waters Otolith Sr:Ca ratios from the primordium to the edge for 18 of the 19 tarpon collected in waters offshore of Tadu Creek ranged from 4.8 × 10– 3to 12.8 × 10– 3in the leptocephalus stage, except for some individuals where the marine larval stage was not measured. The tarpon caught in offshore waters were all Type III because after 2 to 3 yr, most fish gradually moved to the marine environment regardless of their earlier migratory type. For example, tarpon of Type I for the first 2 yr might gradually go back to the sea (Fig. 6a–c) or move to fresh water for a period before finally going back to the sea (Fig. 6d,e). Fig. 6f –o are examples of Type II tarpon for the first 1 to 3 yr that stayed in brack-ish water for another 1 to 2 yr and then went back to seawater (Fig. 6f –n), except for 1 ind. that went back to the sea for a period and then back to fresh water before finally going to brackish and seawater (Fig. 6o). Fig. 6p–r are examples of Type III tarpon for the first year. One individual frequently migrated between fresh and brackish water during its first year, and did not move to seawater until its 4th year (Fig. 6p). Another 2 ind. were also vagrants between fresh and brackish waters during their first year that stayed in the freshwater environment for another 1 to 3 yr before finally moving back to seawater quickly (Fig. 6q,r). 0 2 4 6 8 10 12 14 0 10 20 30 40 50 60 70 a b c d e Sr:Ca ratio ( × 100)
Distance from primordium to otolith edge (µm)
Fig. 3. Megalops cyprinoides. Temporal changes in otolith Sr:Ca ratios of 5 newly recruited leptocephali collected from Tadu Creek estuary. a–e: sizes in mm fork length (a: 25.8,
b: 26.5, c: 25.2, d: 26.0, and e: 24.5)
Site Life stage n Fork length (mm) Body weight (g) Age
Range Mean ± SD Range Mean ± SD Range Mean ± SD
Estuary Leptocephalus 88 21.9–30.2 25.4 ± 1.7 N/A N/A 18–34 d 25.2 ± 4.7 d Lower and Young to 62 174.4–343.7 251.7 ± 28.4 69.38–558.77 227.88 ± 79.34 1–3 yr 2.2 ± 0.6 yr middle reaches sub-adult
Adjacent Sub-adult 19 276.6–435.1 368.2 ± 41.1 286.82–1145.20 780.02 ± 243.99 2–5 yr 4.1 ± 0.9 yr offshore
Table 1. Megalops cyprinoides. Range and mean (± SD) fork length, body weight and age by sampling site and life stage of Pacific tarpon from the Tadu Creek system and adjacent offshore waters, Taiwan. n: sample size; N/A: not available
Age-specific habitat selection and migratory behavior
Age 1+ tarpon constituted 9.7% of the total speci-mens collected in the freshwater environment of Tadu Creek, but no age 1+ tarpon were found offshore (Fig. 7a). One age 2+ tarpon was found offshore (5.3% of the total catch for offshore sampling) but most fishes 0 2 4 6 8 10 12 14
a
Type I
0 2 4 6 8 10 12 14b
Type I
0 2 4 6 8 10 12 14c
Type II
0 2 4 6 8 10 12 14d
Type II
0 2 4 6 8 10 12 14e
Type III
0 500 1000 1500 2000 Sr:Ca ratio (×1000)Distance from primordium to otolith edge (µm)
Fig. 4. Megalops cyprinoides. Temporal changes in otolith Sr:Ca ratios of young Pacific tarpon collected in the freshwater lower reach of Tadu Creek (LT in Fig. 1). (a) 208.3, (b) 190, (c) 225.5, (d) 287.9, and (e) 174.9 mm fork length. (
z
) Metamorpho-sis check; (y
) annual rings. The freshwater–brackishwater boundary (dashed lines) was set at 4 × 10– 3, while the brackish– seawater boundary (solid lines) was 8 × 10– 3. Type I: brackish-water residents; Type II: freshbrackish-water residents; Type III:vagrants between brackish and fresh waters
0 2 4 6 8 10 12 14
a
Type II
0 2 4 6 8 10 12 14b
Type II
0 2 4 6 8 10 12 14c
Type III
0 2 4 6 8 10 12 14d
Type III
0 500 1000 1500 Sr:Ca ratio (×1000)Distance from primordium to otolith edge (µm)
Fig. 5. Megalops cyprinoides. Temporal changes in otolith Sr:Ca ratios of young Pacific tarpon collected in the middle reach of Tadu Creek (MT in Fig. 1). (a) 264.1, (b) 252.9, (c) 247.1, and (d) 226.3 mm fork length. (
z
) Metamorphosis check; (y
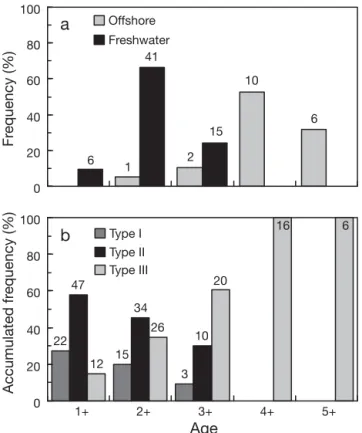
) annual rings. The freshwater–brackishwater boundary (dashed lines) was set at 4 × 10– 3, while the brackishwater– seawater boundary (solid lines) was 8 × 10– 3. Type I: brackish-water residents; Type II: freshbrackish-water residents; Type III:stayed in the fresh water (66.1% of specimens collected in fresh water) of Tadu Creek. At age 3+, 24.2% of tar-pon remained in fresh water while 10.5% moved off-shore. All tarpon moved offshore at ages 4+ and 5+. The frequency distributions of age-specific habitat selection differed significantly between fresh and offshore waters (Kolmogorov-Smirnov D = 0.8421, p < 0.001).
The accumulated frequency of freshwater residents (Type II) was higher (58%) than for brackishwater resi-dents (Type I) (27.2%) and for vagrants (Type III) (14.8%) at age 1+. The accumulated frequencies of Type II and Type I gradually decreased with age until age 3+ but gradually increased with age for Type III. At age 4+ and 5+, all tarpon were Type III (Fig. 7b). g h i j k l 0 500 1000 1500 2000 2500 3000 0 2 4 6 8 10 12 14 a 0 2 4 6 8 10 12 14 b 0 2 4 6 8 10 12 14 c 0 2 4 6 8 10 12 14 d 0 2 4 6 8 10 12 14 e 0 2 4 6 8 10 12 14 f 0 500 1000 1500 2000 2500 3000 3500 m n o p q r 0 500 1000 1500 2000 2500 3000 Sr:Ca ratio (×1000)
Distance from primordium to otolith edge (µm)
Fig. 6. Megalops cyprinoides. Temporal changes in otolith Sr:Ca ratios of sub-adult Pacific tarpon collected in the waters offshore of Tadu Creek. (a) 377.3, (b) 407.2, (c) 364.5, (d) 347.2, (e) 391.9, (f) 419.3, (g) 300.2, (h) 391.2, (i) 378.3, (j) 345.7, (k) 276.1, (l) 435.1, (m) 365.3, (n) 401.8, (o) 349.5, (p) 384.3, (q) 405.1, and (r) 346.7 mm fork length. (
z
) Metamorphosis check; (y
) annual rings. The freshwater–brackishwater boundary (dashed lines) was set at 4 × 10– 3, while the brackishwater–seawater boundary (solid lines)DISCUSSION
Migratory history reconstruction from otolith Sr:Ca ratios
Fish movements between marine, estuarine, and freshwater habitats have been reconstructed from tem-poral changes in otolith Sr:Ca ratios for anguillid eels (Japanese eel Anguilla japonica: Tzeng & Tsai 1994, Tzeng et al. 2002a, 2003; European eel Anguilla an-guilla: Tzeng et al. 1997; American eel Anguilla rostra-ta (Jessop et al. 2002, 2004) striped bass Morone saxi-tilis (Secor & Piccoli 1996) and amphidromous goby Sicyopterus japonicus (Shen & Tzeng 2002). The incor-poration of Sr into the otolith is a complex biogeochem-ical process which is influenced by both intrinsic and exogenous factors (Fowler et al. 1995) as well as the crystalline structure of the otolith (Tzeng et al. 2007). Although the ambient temperature may affect the Sr:Ca ratio of the otolith (Townsend et al. 1992, Elsdon & Gillanders 2004), the effect of temperature on otolith Sr:Ca ratios in Pacific tarpon was found to be negligi-ble (Tzeng 1996, Chen et al. 2008). Ambient salinity is the main factor affecting otolith Sr:Ca ratios in reared leptocephali (Chen & Tzeng 2006, Chen et al. 2008). The Sr:Ca ratios used to judge Pacific tarpon
migra-tions between freshwater, brackishwater and offshore environments were similar to those used for other fish species. For example, Jessop et al. (2002, 2004) divided the American eel into freshwater and estuarine resi-dents when otolith Sr:Ca ratios were < 4 × 10– 3 and > 5× 10– 3, with the intermediate values representing a change in habitat. Tzeng et al. (2002a, 2003) used a Sr:Ca ratio of 4 × 10– 3in the otoliths of the Japanese eel as a criterion to distinguish freshwater and seawater contingents. Shiao et al. (2003) also used the upper limit of 4 × 10– 3as a criterion to separate the Japanese eel and the giant mottled eel Anguilla marmorata into freshwater, estuarine or marine residents. Kawakami et al. (1998) reported Sr:Ca ratios in Japanese eel elvers that averaged ~4.5 × 10– 3 in fresh water and 8.3× 10– 3in seawater. Chang et al. (2004), however, used the 95% CI of the mean Sr:Ca ratio at the estuar-ine check of the adult mullet otolith (3 × 10– 3 and 7 × 10– 3) as the criterion to distinguish habitat change between fresh water, brackish water and offshore high-salinity water for the grey mullet Mugil cephalus. The similarities in criteria used for different fishes liv-ing in the same environment suggest that plasma ionic homeostasis may occur for different fishes in certain environments. The minor differences observed among the studies may result from differences in analytical methods, sampling sites and species (Jessop et al. 2002).
Facultative migratory behavior and euryhalinity in sub-adult Pacific tarpon The tarpon, like many other estuarine-dependent marine fishes, recruit to an estuarine nursery habitat during the age 0+ period of their life history, but the mi-gratory behavior of young and sub-adult tarpon is not yet fully understood. After the reconstruction of the mi-gratory environmental history of the Pacific tarpon us-ing Sr:Ca ratios, all 3 migratory types were found in the lower reach of the freshwater stream, but Type I was not found in the middle reach of Tadu Creek. This means that Type I individuals preferred to stay in brackish water, might move to the lower reaches of the freshwater stream, but would not get into the middle reach. In contrast, Type II individuals preferred the freshwater environment, and might stay in either low or middle reaches of the Creek, suggesting higher adapt-ability to the freshwater environment. Type III individu-als had the ability to migrate between brackish and freshwater environments within age 0+, enabling them to select a wider range of habitats. The sub-adult tarpon caught in offshore waters all had higher Sr:Ca ratios at the otolith edge, but their past migratory history shows that they fit the 3 migratory behaviors of the tarpon in the creek. Most tarpon (53%) stayed in a freshwater en-0 20 40 60 80 100 Offshore Freshwater 0 20 40 60 80 100 1+ 2+ 3+ 4+ 5+ Type I Type II Type III
a
b
Age
Accumulated fr equency (%) Fr equency (%) 15 22 47 10 6 2 10 1 6 41 12 15 34 3 26 20 16 6Fig. 7. Megalops cyprinoides. (a) Frequency distributions of age-specific habitat use (b) and the accumulated frequencies of age-specific migratory patterns (Types I, II and III refer to
vironment for 1 to 3 yr, but some tarpon still stayed in a brackishwater environment for 2 to 3 yr before moving offshore or to a freshwater environment, or migrated between freshwater and brackishwater environments. Therefore, the migratory behavior of tarpon is highly variable and facultative. Tarpon tended to stay in fresh water at ages 0+ to 1+ but as age increased, the propor-tion of fish inhabiting fresh water decreased and no fish at age 4+ (n = 16) or older (n = 6) inhabited fresh water. Tarpon occupy a high trophic level and are predom-inately piscivorous. Atlantic tarpon, for example, prey on mullet juveniles during their migrations along the Caribbean coast from March to July (Catano & Garzon-Ferreira 1994). Pacific tarpon also prey on mullet in both brackish and fresh waters and on milk-fish in culture ponds of Taiwan (Tzeng & Yu 1986), suggesting a euryhaline habit.
Where do the mature Pacific tarpon go? Mature Atlantic tarpon are usually >1000 mm in stan-dard length and around 10 yr old (Crabtree et al. 1997), with 1 female Atlantic tarpon being reported to be 2032 mm in standard length (Wade 1962). Few Pacific tarpon in Taiwan are > 600 mm in total length (TL), but a Pa-cific tarpon of 1500 mm TL was recorded in Bangladesh (Rahman 1989). All tarpon collected in the present study were immature because no obvious gonads were found. The newly recruited leptocephalus had daily ages ranging from 18 to 34 d, suggesting that the spawning ground was nearby. In addition, adult Pacific tarpon may go deeper for spawning just like the At-lantic tarpon (90 to 1400 m) (Wade 1962, Crabtree et al. 1992) and other members of the Superorder Elopomor-pha (Tsukamoto et al. 2002). The 19 sub-adults col-lected offshore from Tadu Creek were estimated to be 2 to 5 yr old based on the examination of annual rings in their otoliths. While our study does not directly an-swer the question of where mature Pacific tarpon live, the high Sr:Ca ratios in the leptocephalus stage and the spawning behavior of its congeners support the hypo-thesis of deep-sea spawning in offshore waters.
In conclusion, we found that (1) after metamorphos-ing from the leptocephalus to the juvenile stage, Pacific tarpon found in Tadu Creek could inhabit fresh and brackish waters or migrate facultatively between these habitats, (2) at age 0+, tarpon preferentially oc-cupy fresh water, (3) tarpon gradually move back to marine waters after 4 yr of age, indicating euryhaline behavior and facultative habitat selection by sub-adults, and (4) the spawning migration of the tarpon is still unknown but the high Sr:Ca ratio during the lepto-cephalus stage supports the hypothesis of offshore spawning by Pacific tarpon.
Acknowledgement. We thank H. Y. Teng for specimen sorting and aging, and N. J. Leander, B. M. Jessop and several anonymous reviewers for comments on the early draft of the manuscript. This study was conducted with the financial sup-port of the National Science Council, Republic of China (Pro-ject No. NSC 95-2313-B002-057).
LITERATURE CITED
Amiel AJ, Friedman GM, Miller DS (1973) Distribution and nature of incorporation of trace elements in modern arag-onite corals. Sedimentology 20:47–64
Bath GE, Thorrold SR, Jones CM, Campana SE, McLaren JW, Lam JWH (2000) Strontium and barium uptake in arago-nitic otoliths of marine fish. Geochim Cosmochim Acta 64: 1705–1714
Campana SE (1999) Chemistry and composition of fish oto-liths: pathways, mechanisms, and applications. Mar Ecol Prog Ser 188:263–297
Campana SE, Neilson JD (1985) Microstructure of fish oto-liths. Can J Fish Aquat Sci 42:1014–1032
Catano S, Garzon-Ferreira J (1994) Tropic ecology of the tarpon Megalops atlanticus (Pisces: Megalopidae) in the area of Cienaga Grande de Santa Marta, Colombian Carib-bean. Rev Biol Trop 42:673–684
Chang CW, Iizuka Y, Tzeng WN (2004) Migratory environ-mental history of the grey mullet Mugil cephalus as revealed by otolith Sr:Ca ratios. Mar Ecol Prog Ser 269: 277–288
Chen HL, Tzeng WN (2006) Daily growth increment forma-tion in otoliths of Pacific tarpon Megalops cyprinoides during metamorphosis. Mar Ecol Prog Ser 312:255–263 Chen HL, Shen KN, Chang CW, Iizuka Y, Tzeng WN (2008)
Effects of water temperature, salinity and feeding regimes on metamorphosis, growth and otolith Sr:Ca ratios of Megalops cyprinoides leptocephali. Aquat Biol 3:41–50 Coates D (1987) Observations on the biology of tarpon,
Mega-lops cyprinoides (Broussonet) (Pisces: Megalopidae), in the Sepik River, northern Papua New Guinea. Aust J Mar Freshwater Res 38:529–535
Crabtree RE, Cyr EC, Bishop RE, Falkenstein LM, Dean JM (1992) Age and growth of tarpon, Megalops atlanticus, lar-vae in the eastern Gulf of Mexico with notes on relative abundance and probable spawning areas. Environ Biol Fishes 35:361–370
Crabtree RE, Cyr EC, Chaverri DC, McLarney WO, Dean JM (1997) Reproduction of tarpon, Megalops atlanticus, from Florida and Costa Rican waters and notes on their age and growth. Bull Mar Sci 61:271–285
Elsdon TS, Gillanders BM (2004) Fish otolith chemistry influ-enced by exposure to multiple environmental variables. J Exp Mar Biol Ecol 313:269–284
Forey PL, Littlewood DTJ, Ritchie P, Meyer A (1996) Interre-lationships of elopomorph fishes. In: Stiassny MLJ, Parenti LR, Johnson GD (eds) Interrelationships of fishes. Acade-mic Press, San Diego, CA, p 175–191
Fowler AJ, Campana SE, Jones CM, Thorrold SR (1995) Experimental assessment of the effect of temperature and salinity on elemental composition of otoliths using laser ablation ICPMS. Can J Fish Aquat Sci 52:1431–1441 Hulet WH, Robin CR (1989) The evolutionary significance of
the leptocephalus larva. In: Bohlke, EB (ed) Fishes of the western North Atlantic. Sears Foundation for Marine Research (Memoir No. I, Part 9, Vol 2), New Haven, CT, p 669–677
Jessop BM, Shiao JC, Iizuki Y, Tzeng WN (2002) Migratory
➤
➤
➤
➤➤
➤
➤
➤
➤
➤
➤
➤
behaviour and habitat use by American eels Anguilla ros-trata as revealed by otolith microchemistry. Mar Ecol Prog Ser 233:217–229
Jessop BM, Shiao JC, Iizuki Y, Tzeng WN (2004) Variation in the annual growth, by sex and migration history, of silver American eels Anguilla rostrata. Mar Ecol Prog Ser 272: 231–244
Jones CM (1986) Determining age of larval fish with the otolith increment technique. Fish Bull US 84:91–103 Kalish JM (1990) Use of otolith microchemistry to distinguish
progeny of sympatric anadromous and non-anadromous salmonids. Fish Bull US 88:657–666
Kawakami Y, Mochioka N, Morishita K, Tajima T, Nakagawa H, Toh H, Nakazono A (1998) Factors influencing oto-lith strontium/calcium ratios in Anguilla japonica elvers. Environ Biol Fishes 52:299–303
Merrick JR, Schmida GE (1984) Australian freshwater fishes — biology and management. Griffin Press, Netley, SA Milton DA, Chenery SR (2001) Sources and uptake of trace
metals in otoliths of juvenile barramundi (Lates calcarifer). J Exp Mar Biol Ecol 264:47–65
Pannella G (1971) Fish otoliths: daily growth layers and peri-odical patterns. Science 173:1124–1127
Rahman AKA (1989) Freshwater fishes of Bangladesh. Zoo-logical Society of Bangladesh. Department of Zoology, University of Dhaka, p 364
Secor DH (1992) Application of otolith microchemistry analy-sis to investigate anadromy in Chesapeake Bay striped bass Morone saxatilis. Fish Bull (Wash DC) 90:798–806 Secor DH, Piccoli PM (1996) Age- and sex-dependent migra-tions of striped bass in the Hudson River as determined by chemical microanalysis of otoliths. Estuaries 19:778–793 Secor DH, Rooker JR (2000) Is otolith strontium a useful scalar
of life cycles in estuarine fishes? Fish Res 46:359–371 Secor DH, Henderson-Arzapalo A, Piccoli PM (1995) Can
oto-lith microchemistry chart patterns of migration and habi-tat utilization in anadromous fishes? J Exp Mar Biol Ecol 192:15–33
Sedberry GR, Loefer JK (2001) Satellite telemetry tracking of swordfish, Xiphias gladius, off the eastern United States. Mar Biol 139:355–360
Shen KN, Tzeng WN (2002) Formation of a metamorphosis check in otoliths of the amphidromous goby Sicyopterus japonicus. Mar Ecol Prog Ser 228:205–211
Shiao JC, Iizuka Y, Chang CW, Tzeng WN (2003) Disparities in habitat use and migratory behavior between tropical eel Anguilla marmorata and temperate eel A. japonica in four Taiwanese rivers. Mar Ecol Prog Ser 261:233–242 Townsend DW, Radtke RL, Corwin S, Libby DA (1992)
Stron-tium:calcium ratios in juvenile Atlantic herring Clupea harengus L. otoliths as a function of water temperature. J Exp Mar Biol Ecol 160:131–140
Tsukamoto K, Arai T (2001) Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habi-tats. Mar Ecol Prog Ser 220:265–276
Tsukamoto Y, Okiyama M (1993) Growth during the early life history of the Pacific tarpon, Megalops cyprinoides. Jpn J Ichthyol 39:379–386
Tsukamoto Y, Okiyama M (1997) Metamorphosis of the
Pacific tarpon, Megalops cyprinoides (Elopiformes, Mega-lopidae) with remarks on development patterns in the Elopomorpha. Bull Mar Sci 60:23–36
Tsukamoto K, Aoyama J, Miller MJ (2002) Migration, specia-tion and the evoluspecia-tion of diadromy in anguillid eels. Can J Fish Aquat Sci 59:1989–1998
Tzeng WN (1996) Effects of salinity and ontogenetic move-ments on strontium: calcium ratios in the otoliths of the Japanese eel, Anguilla japonica Temminck and Schlegel. J Exp Mar Biol Ecol 199:111–122
Tzeng WN, Tsai YC (1994) Changes in otolith microchemistry of the Japanese eel, Anguilla japonica, during its migra-tion from the ocean to the rivers of Taiwan. J Fish Biol 45: 671–683
Tzeng WN, Yu SY (1986) Occurrence of the leptocephalus lar-vae of Elops hawaiensis and Megalops cyprinoides in the Gong-shy-tyan River estuary of north Taiwan with refer-ence to some ecological and taxonomic aspects. Proc Symp Mar Biol Sci, Biology Research Center, Nat Sci Counc Monogr Ser 14: 165–176
Tzeng WN, Severin KP, Wickström H (1997) Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla. Mar Ecol Prog Ser 149: 73–81
Tzeng WN, Wu CE, Wang YT (1998) Age of pacific tarpon, Megalops cyprinoides, at estuarine arrival and growth during metamorphosis. Zool Stud 37:177–183
Tzeng WN, Shiao JC, Iizuka Y (2002a) Use of otolith Sr:Ca ratios to study the riverine migratory behaviours of Japan-ese eel Anguilla japonica. Mar Ecol Prog Ser 245:213–221 Tzeng WN, Wang YT, Chang CW (2002b) Spatial and
tempo-ral variations of the estuarine larval fish community on the west coast of Taiwan. Mar Freshw Res 53:419–430 Tzeng WN, Iizuka Y, Shiao JC, Yamada Y, Oka HP (2003)
Identification and growth rates comparison of divergent migratory contingents of Japanese eel (Anguilla japonica). Aquaculture 216:77–86
Tzeng WN, Chang CW, Wang CH, Shiao JC and others (2007) Misidentification of the migratory history of anguillid eels by Sr/Ca ratios of vaterite otoliths. Mar Ecol Prog Ser 348: 285–295
Wade RA (1962) The biology of the tarpon, Megalops atlanti-cus, and the oxeye, Megalops cyprinoides, with emphasis on larval development. Bull Mar Sci Gulf Caribb 12: 545–622
Wang HC (2002) Indicator fish species for water quality of freshwater streams in Taiwan. Annu Rep NIEA Taiwan ROC 9:207–236 (in Chinese)
Wang CH, Kuo CH, Mok HK, Lee SC (2003) Molecular phylo-geny of elopomorph fishes inferred from mitochondrial 12S ribosomal RNA sequences. Zool Scr 32:231–241 Wells RMG, Baldwin J, Seymour RS, Baudinette RV, Christian
K, Bennett MB (2003) Oxygen transport capacity in the air-breathing fish, Megalops cyprinoides: compensations for strenuous exercise. Comp Biochem Physiol A 134:45–53 Zeller DC, Russ GR (2000) Population estimates and size
structure of Plectropomus leopardus (Pisces: Serranidae) in relation to no-fishing zones: mark-release-resighting and underwater visual census. Mar Freshw Res 51:221–228 Editorial responsibility: Matthias Seaman,
Oldendorf/Luhe, Germany
Submitted: July 20, 2007; Accepted: May 22, 2009 Proofs received from author(s): July 20, 2009