Protective effect of ascorbic acid and glutathione
on AlCl
3-inhibited growth of rice roots
J.-W. WANG and C.H. KAO*
Department of Agronomy, National Taiwan University, Taipei, Taiwan 106, Republic of China
Abstract
The effect of AlCl3 on the antioxidant system of rice roots and the role of applied antioxidants ascorbic acid (AsA) and
glutathione (GSH) in AlCl3-inhibited growth of rice roots were investigated. AlCl3 treatment resulted in a rapid
inhibition of root growth but had no effect on lipid peroxidation and antioxidative enzyme activities in rice roots. AlCl3
treatment resulted in lower content of H2O2, AsA, and GSH than in controls. Exogenous AsA or GSH counteracted
growth inhibition of rice roots induced by AlCl3. AlCl3 treatment increased syringaldazine peroxidase (SPOX) activities
and lignin content in rice roots. Exogenous AsA or GSH prevented the decrease in H2O2 content and the increase in
SPOX activities and lignin content in rice roots caused by AlCl3. Results suggest that lignification induced by low AsA
or GSH content may explain the mechanism of Al-inhibited growth of rice roots.
Additional key words: hydrogen peroxide, lignin, Oryza sativa, reactive oxygen species.
Introduction
The primary effect of aluminum (Al) toxicity is the inhibition of root growth (Tamás et al. 2006). Several mechanisms of Al toxicity have been proposed (Zheng and Yang 2005). However, the precise physiological and molecular bases are not completely understood (Matsumoto 2000, Kochian et al. 2005, Zheng and Yang 2005).
It has been shown that Al induces reactive oxygen species (ROS) and enhances lipid peroxidation in
Hordeum vulgare (Sakihama and Yamasaki 2002,
Šimonovicová et al. 2004a,b), Oryza sativa (Kuo and Kao 2003, Meriga et al. 2004), Pisum sativum (Yamamoto et al. 2001, 2002), Triticum aestivum (Darkó
et al. 2004), and Zea mays (Boscolo et al. 2003). In Arabidopsis and cultured tobacco cells, Al induced the
expression of several genes (e.g. for peroxidase and SOD) that are induced by oxidative stress (Ezaki et al. 1995, 1996, Richards et al. 1998). Thus a possible induction of oxidative stress by Al was suggested.
ROS can damage essential membrane lipids as well as proteins and nucleic acids (Inzé and Van Montagu 1995, Noctor and Foyer 1998). Levels of ROS in plant cells are normally controlled by protective antioxidant system. Various associations between Al and endogenous levels of antioxidant enzymes have been reported (Kuo and Kao
2003, Darkó et al. 2004, Meriga et al. 2004, Šimonovicová et al. 2004a). Darkó et al. (2004) demon-strated that the roots of Al-tolerant wheat exhibited more intensive growth, while accumulating less Al and ROS than Al-sensitive wheat under Al stress condition. They also found that among the antioxidant enzymes induced by Al stress, CAT and glutathione-S-transferase may play an important role in the detoxification of ROS in Al-tolerant wheat.
Ascorbic acid (AsA) and glutathione (GSH) have been implicated in the regulation of plant cell growth and division (e.g. Conklin et al. 1996, Córdoba-Pedregosa
et al. 1996, 2005, May et al. 1998, Potters et al. 2000,
2002, Vernoux et al. 2000). Lukaszewski and Blevins (1996) demonstrated that increasing concentration of aluminum caused progressive inhibition of root growth and a parallel reduction in AsA concentration of
Cucurbita pepo. Recently, Devi et al. (2003) reported that
the higher content of AsA in Al tolerant cell line of tobacco than in Al sensitive cell line are responsible for its higher tolerance to Al. Yamaguchi et al. (1999) observed that total GSH concentration in tobacco cell suspension treated with a combination of Al and iron was lower than in the control cells. High content of GSH has been shown to be responsible for the tolerance
⎯⎯⎯⎯
Received 19 July 2005, accepted 10 May 2006.
Abbreviations: APX - ascorbate peroxidase; AsA - ascorbic acid; CAT - catalase; DHA - dehydroascorbate; d.m. - dry mass;
GR - glutathione reductase; GSH - reduced glutathione; GSSG - glutathione disulfide; MDA - malondialdehyde; ROS - reactive oxygen species; SOD - superoxide dismutase; SPOX - syrinaldazine peroxidase.
Acknowledgements: This work was supported by the National Science Council of the Republic of China.
mechanism of tobacco cells (Devi et al. 2003) and wheat plant (Dong et al. 2002) to Al. All these results suggest that AsA and GSH play an important role in Al-inhibited growth.
In the present paper, we have studied the effect of AlCl3 on the antioxidant system of rice roots and the role
of antioxidant (AsA and GSH) in Al Cl3-inhibited root
growth of rice.
Materials and methods
Uniformly germinated rice (Oryza sativa L., cv. Taichung Native 1) caryopses were grown in a Petri dish (9 cm) containing filter paper moistened with 10 cm3 of distilled water for 2-d at 27 °C in darkness. Then, 2-d-old seedlings were treated with distilled water or AlCl3
solution. In previous work, we observed that increasing concentration of AlCl3 from 0.25 to 0.5 mM at pH 4.0
progressively decreased root growth of rice seedlings and no further decrease was observed at 0.75 and 1 mM AlCl3
(Wang and Kao 2004). Thus, 0.5 mM AlCl3 was used in
the present investigation. Root growth of rice seedlings grown in distilled water is similar to that grown in medium containing inorganic salts and so seedlings grown in distilled water were used as the controls. Each Petri dish contained 10 seedlings and each treatment was replicated four times.
For the determination of Al, roots were dried at 65 °C for 48 h. Dried material was ashed at 550 °C for 20 h. Ash residue was incubated with 31 % HNO3 and 17.5 %
H2O2 at 70 °C for 12 h, and dissolved in 0.1 M HCl. Al
was then quantified using an atomic absorption spectrophotometer (Model AA-680, Shimadzu, Kyoto, Japan).
The H2O2 content was measured colorimetrically as
described by Jana and Choudhuri (1981). H2O2 was
extracted by homogenizing 50 mg root tissue with 3 cm3 of phosphate buffer (50 mM, pH 6.5) containing 1 mM hydroxylamine. The homogenate was centrifuged at 6 000 g for 25 min. To determine H2O2 content, 3 cm3 of
extracted solution was mixed with 1 cm3 of 0.1 % titanium sulphate in 20 % (v/v) H2SO4. The mixture was
then centrifuged at 6 000 g for 15 min. The absorbance was measured at 410 nm. Malondialdehyde (MDA) was extracted with 5 % (m/v) trichloroacetic acid and determined according to Heath and Packer (1968).
For extraction of enzymes, roots were homogenized with 0.1 M phosphate buffer (pH 6.8) in a chilled pestle and mortar. The homogenate was centrifuged at 12 000 g for 20 min and the resulting supernatant was used for the determination of enzyme activity. The whole extraction procedure was carried out at 4 °C. CAT activity was assayed by measuring the initial rate of disappearance of H2O2 (Kato and Shimizu 1987). The decrease in H2O2
was followed as the decline in absorbance at 240 nm. One unit (U) of CAT was defined as the amount of enzyme which breaks down 1 nmol H2O2 per min. SOD was
determined to Paoletti et al. (1986). One U of SOD was defined as the amount of enzyme which inhibits by 50 % the rate of NADH oxidation observed in blank. APX was determined according to Nakano and Asada (1981). The decrease in AsA concentration was followed at 290 nm. One U of APX was defined as the amount of enzyme which breaks down 1 μmol of AsA per min. GR was determined by the method of Foster and Hess (1980). One U of GR was defined as the amount of enzyme which decreases A340 (1 unit per min). Syringaldazine
peroxidase (SPOX) was assayed according to Grison and Pilet (1985). The oxidation of syringaldazine was measured followed the absorbance decrease at 530 nm. One U of SPOX was defined as the amount of enzyme which decreases A530 (1 unit per min).
Contents of ascorbate (AsA) and dehydroascorbate (DHA) in 5 % (m/v) trichloroacetic acid extract and GSH and glutathione disulfide (GSSG) in 3 % sulfosalicylic acid extract were determined as described by Laws et al. (1983) and Smith (1985), respectively. The lignin content in roots was measured by the Sasaki et al. (1996) method, a method originally described by Morrison (1972). Roots were homogenized with a pestle and mortar in 95 % ethanol. The homogenate was centrifuged at 1 000 g for 5 min. The pellet was washed three times with 95 % ethanol and twice with a mixture of ethanol and hexane (1:2, v/v). The material was allowed to air dry and its lignin content measured. The dried sample was washed one time with 2 cm3 acetyl bromide in acetic acid (1:3, v/v). Then 1 cm3 acetyl bromide in acetic acid (1:3, v/v) was added to the pellet and incubated at 70 °C for 30 min. After cooling of the mixture to room temperature, 0.9 cm3 of 2 M NaOH and 0.1 cm3 7.5 M hydroxylamine hydrochloride were added, and the volume was made up to 10 cm3 with acetic acid. After centrifugation at 1 000 g for 5 min, the absorbance of the supernatant was measured at 280 nm (A280).
Statistical differences between measurements (n = 4) on different treatment or on different times were analyzed by Duncan’s multiple range test or Student’s t-test.
Results
The reduction of root growth by AlCl3 was evident 8 h
after treatment (Fig. 1A). Al concentration in control roots remained unchanged during 12 h of incubation.
However, Al concentration in AlCl3-treated roots
increased with increasing duration of incubation (Fig. 1B). The increase in Al concentration in AlCl3-
Fig. 1. Changes in root length (A) and Al concentration (B) in rice roots treated with AlCl3 (0.5 mM, pH 4.0) or H2O (pH 4.0) for 4, 8 and 12 h. Means ± SD (n = 4). Asterisks indicate values that are significantly different between H2O and AlCl3 treatments at P < 0.05 according to Student’s t-test.
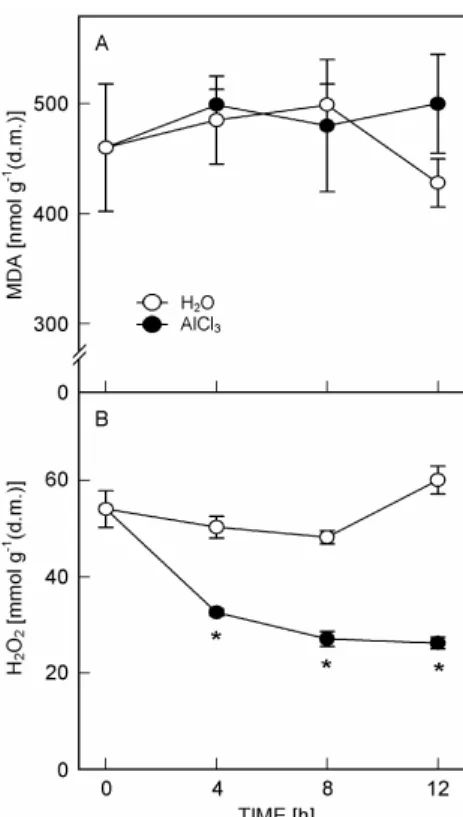
Fig. 2. Changes in MDA (A) and H2O2 (B) contents in rice roots treated with AlCl3 (0.5 mM, pH 4.0) or H2O (pH 4.0) for 4, 8 and 12 h. Means ± SD (n = 4). Asterisks indicate values that are significantly different between H2O and AlCl3 treatments at
P < 0.05 according to Student’s t-test.
Fig. 3. Changes in antioxidative enzyme activities in rice roots treated with AlCl3 (0.5 mM, pH 4.0) or H2O (pH 4.0) for 4, 8 and 12 h. Means ± SD (n = 4). Asterisks indicate values that are significantly different between H2O and AlCl3 treatments at P < 0.05 according to Student’s t-test.
Fig. 4. Changes in antioxidants in rice roots treated with AlCl3 (0.5 mM, pH 4.0) or H2O (pH 4.0) for 4, 8, and 12 h. Means ± SD (n = 4). Asterisks indicate values that are significantly different between H2O and AlCl3 treatments at P < 0.05 according to Student’s
t-test.
treated roots was evident at 4 h after treatment (Fig. 1B). MDA is routinely used as an indicator of lipid peroxidation. No difference in MDA content was observed between H2O- and AlCl3-treated roots (Fig. 2A).
However, H2O2 content in AlCl3-treated roots was lower
than that in control roots (Fig. 2B). For the activities of SOD, APX, and GR, no difference was observed between AlCl3- and H2O-treated roots (Fig. 3A,B,C). The increase
in CAT activity in AlCl3-treated roots was only observed
12 h after treatment (Fig. 3D).
When 2-d-old rice seedling roots were treated with 0.5 mM AlCl3, AsA content was significantly lower than
in control roots (Fig. 4B). However, AlCl3 had no effect
on AsA + DHA and DHA contents in roots (Fig. 4A,C). It was also observed that GSH, GSSG, and GSH + GSSG
contents in AlCl3-treated roots were lower than those of
control roots (Fig. 4D,E,F). If AsA or GSH plays an important role in regulating AlCl3-induced growth
inhibition of rice roots, then growth of roots in AlCl3 is
expected to be enhanced by adding AsA or GSH. Adding AsA, which increased AsA but not GSH content (Fig. 5A,B), or GSH, which increased GSH but not AsA content (Fig. 5A,B), significantly enhanced growth of roots treated with AlCl3 for 12 h (Fig. 5C). This
protective effect on AlCl3-inhibited root growth was also
observed in a long (48 h) AsA or GSH treatment (Fig. 6A).
Since AsA or GSH was added simultaneously with AlCl3, thus AsA- or GSH-reduced growth inhibition of
Fig. 5. Effect of AsA (0.5 mM, pH 4.0) and GSH (0.5 mM, pH 4.0) on the contents of AsA (A) and GSH (B) in roots and root growth (C) of rice seedlings in the presence of AlCl3 (0.5 mM, pH 4.0). All measurements were made 12 h after treatment. Values with the same letter are not significantly different at P < 0.05 according to Duncan’s multiple range test.
blockage of Al uptake. Al concentration in roots treated with AlCl3 was similar to that treated with AlCl3 + AsA
(Fig. 6B). Although Al concentration in roots treated with AlCl3 + GSH was lower than that treated with AlCl3
alone (Fig. 6B), the amount of Al [about 550 μg g-1(d.m.)] found in roots treated with AlCl3 + GSH is still high
enough to inhibit growth of rice roots (Fig. 1B). Thus, the protective role in counteracting AlCl3-inhibited growth of
roots is unlikely caused by blockage of Al uptake. This conclusion is supported further by the observations that the protective effect of AsA or GSH was also observed when rice roots were exposed to AsA or GSH and AlCl3
separately (Fig. 7).
It was observed that both lignin content and
Fig. 6. Effect of AsA and GSH on root growth (A) and Al concentration (B) in roots of rice seedlings treated with AlCl3. Two-d-old rice seedlings were treated with distilled H2O (pH 4.0) and 0.5 mM AlCl3 ( pH 4.0), 0.5 mM AlCl3+ 0.5 mM AsA (pH 4.0) and 0.5 mM AlCl3 + 0.5 mM GSH (pH 4.0) for 48 h. Values with the same letter are not significantly different at P < 0.05 according to Duncan’s multiple range test.
Fig. 7. Effect of pre-treatments of AsA and GSH on root growth of rice seedlings exposed to AlCl3. Two-d-old rice seedlings were pre-treated with distilled water (pH 4.0), 0.5 mM AsA (pH 4.0) and 0.5 mM GSH (pH 4.0), respectively, for 12 h and then treated with distilled water or 0.5 mM AlCl3 for 12 h. Values with the same letter are not significantly different at
syringaldazine peroxidase (SPOX) activity in rice roots increased during AlCl3 treatment (Fig. 8A,B). The
decrease in H2O2 content, the increase in lignin content,
and the increase in SPOX activity caused by AlCl3 were
significantly prevented by adding AsA or GSH (Fig. 9).
Discussion
Several of our observations suggest that Al treatment does not lead to oxidative stress in rice roots: 1) no accumulation of H2O2 was observed in AlCl3-treated rice
roots (Fig. 2B); 2) AlCl3 treatment had no effect on lipid
peroxidation in rice roots (Fig. 2A); 3) no general up-regulation of antioxidative enzymes by AlCl3 was
observed (Fig. 3). It is clear that our results are in contrast with those researchers, who demonstrated that Al induced oxidative stress in plants (Yamamoto et al. 2001, 2002, Sakihama and Yamasaki 2002, Boscolo et al. 2003, Kuo and Kao 2003, Darkó et al. 2004, Meriga et al. 2004, Šimonovicová et al. 2004a,b). Using leaves of the same rice cultivar as used in the present study (Kuo and Kao 2003), we observed that AlCl3 was able to induce
oxidative stress. It appears that Al-induced oxidative stress in rice plants is organ-specific.
Fig. 8. Changes in SPOX activity (A) and lignin content (B) in rice roots treated with AlCl3 (0.5 mM, pH 4.0) or H2O (pH 4.0) for 4, 8 and 12 h. Means ± SD (n = 4). Asterisks indicate values that are significantly different between H2O and AlCl3 treatments at P < 0.05 according to Student’s t-test.
It has been documented that AsA or GSH plays a crucial role in plant growth (e.g. Conklin et al. 1996, Córdoba-Pedregosa et al. 1996, 2005, Sánchez-Fernández
et al. 1997, May et al. 1998, Potters et al. 2000, 2002,
Vernoux et al. 2000). In the present study, two lines of evidence indicated that AsA or GSH seems to be involved in root growth inhibition of rice seedlings caused by AlCl3. Firstly, treatment of AlCl3 decreased
AsA or GSH content in rice roots (Fig. 4B,E). Secondly, the growth of roots in AlCl3 can be enhanced by adding
AsA or GSH (Fig. 5C, 6A). Low content of AsA has
Fig. 9. Effect of AsA (0.5 mM, pH 4.0) and GSH (0.5 mM, pH 4.0) on the contents of H2O2 (A), the activity of SPOX (B), and the content of lignin (C) in rice roots treated with AlCl3 (0.5 mM, pH 4.0). All measurements were made 12 h after treatment. Values with the same letter are not significantly different at P < 0.05 according to Duncan’s multiple range test.
also been described to be responsible for Al-inhibited growth of Cucurbita pepo (Lukaszewski and Belvins 1996) and Nicotana tabacum (Devi et al. 2003). The tolerance mechanism to Al in tobacco cells (Devi et al. 2003) and wheat plant (Dong et al. 2003) has been shown to be due to high GSH content. It appears that low AsA or GSH content is responsible for AlCl3-inhibited root
growth of rice seedlings.
The lower content of AsA or GSH in AlCl3-treated
rice roots is possible due to the reduction of the rate of AsA or GSH synthesis. However, the possibility that utilization, regeneration, catabolism and transport of AsA or GSH are altered by AlCl3 in rice roots cannot be
excluded.
Both AsA and GSH can function as antioxidants in plant cells. It has been shown that high content of AsA is responsible for tolerance mechanism of tobacco cells to Al by protecting cells from lipid peroxidation (Devi et al. 2003) and GSH is able to protect cells from either peroxidation or H2O2 commonly enhanced by Al (Devi
et al. 2003, Dong et al. 2002, Yamaguchi et al. 1999).
However, AlCl3 treatment did not increase lipid
peroxidation and decreased H2O2 content in rice roots
(Fig. 2). Thus, AlCl3-inhibited growth of rice roots is
unlikely mediated through decreased antioxidant capacity of AsA or GSH.
In the present study we observed that AlCl3 treatment
resulted in a lower content of H2O2 in rice roots than
controls (Fig. 2B). No accumulation of H2O2 was also
observed in drought, excess Fe- and NaCl-treated leaves (Moran et al. 1994, Lin and Kao 2000, Fang et al. 2001). Lignification is part of cell differentiation and irreversibly
inhibits cell elongation. It has been shown that lignification in the elongation region coincided with the extent of inhibition of root growth by Al in two wheat cultivars that differed in their sensitivity to Al (Sasaki
et al. 1996). Here, we show that AlCl3 treatment resulted
in an increase in lignin content (Fig. 8B). H2O2 is
required for lignin synthesis. It is possible that H2O2 is
being utilized in the formation of lignin in AlCl3-treated
rice roots. This would explain why H2O2 content
decreased in rice roots exposed to AlCl3. It has been
shown that syringaldazine, a hydrogen donor, has a particularly high affinity for peroxidation associated with lignification (Goldberg et al. 1983). In the present study, SPOX activity in AlCl3-treated root was also observed to
be higher than that in control roots (Fig. 8A). The increase in SPOX activity caused by AlCl3 was also
observed to be prior to that in lignin content (Fig. 8). All these results strongly suggest that lignification is responsible for Al-inhibited growth of rice roots.
Our data indicated that AsA and GSH prevented the decrease in H2O2 content, the increase in lignin content,
and the increase in SPOX activity in AlCl3-treated rice
roots (Fig. 9). It appears that lignification induced by low AsA or GSH content may explain the mechanism of Al-inhibited growth of rice roots. Veljovic-Jovanovic et al. (2001) suggested that low AsA in the vtc-1 mutant of
Arabidopsis, which is deficient in AsA biosynthesis, will
create an environment that markedly favors cell wall cross linking. Thus, the possibility that Al-inhibited growth of rice roots mediated through cell wall cross linking cannot be excluded.
References
Boscolo, P.R.S., Menossi, M., Jorge, R.A.: Aluminum-induced oxidative stress in maize. - Phytochemistry 62: 181-189, 2003.
Conklin, R.L., Williams, E.H., Last, R.L.: Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. - Proc. nat. Acad. Sci. USA 93: 9774-9974, 1996.
Córdoba-Pedregosa, M. del C., González-Reyes, J.A., Cañadillas, S., Navas, P., Córdoba, F.: Role of apoplastic and cell-wall peroxidase on the stimulation of root elongation by ascorbate. - Plant Physiol. 112: 1119-1125, 1996.
Córdoba-Pedregosa, M. del C., Villalba, J.M., Córdoba, F., González-Reyes, J.A.: Changes in intracellular and apoplastic peroxidase activity, ascorbate redox status, and root elongation induced by enhanced ascorbate content in
Allium cepa L. - J. exp. Bot. 56: 685-694, 2005.
Darkó, É., Ambrus, H., Stefanovits-Bányai, É., Fodor, J., Bakos, F., Barnabás, B.: Aluminum toxicity, Al tolerance and oxidative stress in an Al-sensitive wheat genotype and in Al-tolerance lines developed by in vitro microspore selection. - Plant Sci. 166: 583-591, 2004.
Devi, S.R., Yamamoto, Y., Matsumoto, H.: An intracellular mechanism of aluminum tolerance associated with high antioxidant status in cultured tobacco cells. - J. inorg. Chem. 97: 59-68, 2003.
Dong, B., Sang, W.L., Jiang, X., Zhou, J.M., Kong, F.X., Hu, W., Wang, L.S.: Effect of aluminum on physiological metabolism and antioxidant system of wheat (Triticum
aestivum L.). - Chemosphere 47: 87-92, 2002.
Ezaki, B., Yamamoto, Y., Matsumoto, H: Cloning and sequencing of the cDNAs induced by aluminum treatment and Pi starvation in cultured tobacco cells. - Physiol. Plant. 93: 11-18, 1995.
Ezaki, B., Tugita, S., Matsumoto, H.: Expression of a moderately anionic peroxidase is induced by aluminum treatment in tobacco cells: possible involvement of peroxidase isozymes in aluminum ion stress. - Physiol. Plant. 96: 21-28, 1996.
Fang, W.C., Wang, J.-W., Lin, C.C., Kao, C.H.: Iron induction of lipid peroxidation and effects on antioxidative enzyme activities in rice leaves. - Plant Growth Regul. 35: 75-80, 2001.
Foster, J.G., Hess, J.L.: Responses of superoxide dismutase and glutathione reductase activities in cotton leaf exposed to atmosphere enriched in oxygen. - Plant Physiol. 66: 482-487, 1980.
Goldberg, R., Catesson, A.M., Czaninski, Y.: Some properties of syringaldazine oxidase, a peroxidase specifically involved in lignification processes. - Z. Pflanzenphysiol. 110: 267-279, 1983.
Grison, R., Pilet, P.M.: Properties of syringaldazine oxidase/peroxidase in maize roots. - J. Plant Physiol. 118: 201-208, 1985.
Heath, R.L., Packer, L.: Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. - Arch. Biochem. Biophys. 125: 189-198, 1968.
Inzé, D., Van Montagu, M.: Oxidative stress in plants. - Curr. Opin. Biotechnol. 6: 153-158, 1995.
Jana, S., Choudhuri, M.A.: Glycolate metabolism of three submerged aquatic angiosperm during aging. - Aquat. Bot. 12: 345-354, 1981.
Kato, M., Shimizu, S.: Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. - Can. J. Bot. 65: 729-735, 1987.
Kochian, L.V., Piñeros, M.A., Hoekenga, O.A.: The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. - Plant Soil 274: 175-195, 2005.
Kuo, M.C., Kao, C.H.: Aluminum effects on lipid peroxidation and antioxidative enzyme activities in rice leaves. - Biol. Plant. 46: 149-152, 2003.
Laws, M.Y., Stephen, Y., Charles, A., Halliwell, B.: Glutathione and ascorbic acid in spinach (Spinacia
oleracea) chloroplasts. The effect of hydrogen peroxide and
paraquat. - Biochem. J. 210: 899-903, 1983.
Lin, C.C., Kao, C.H.: Effect of NaCl stress on H2O2 metabolism in rice leaves. - Plant Growth Regul. 30: 151-155, 2000. Lukaszewski, K., Blevins, D.G.: Root growth inhibition in
boron-deficient or aluminum-stressed squash may be a result of impaired of ascorbate metabolism. - Plant Physiol. 41: 1135-1140, 1996.
Matsumoto, H.: Cell biology of aluminum toxicity and tolerance in higher plants. - Int. Rev. Cytol. 200: 1-46, 2000.
May, M.J., Vernoux, T., Leaver, C., Van Montagu, M., Inzé, D.: Glutathione homeostasis in plants: implications for environmental sensing and plant development. - J. exp. Bot. 49: 649-667, 1998.
Meriga, B., Reddy, B.K., Rao, K.R., Reddy, A., Kavi Kishor, P.B.: Aluminum-induced production of oxygen radicals, lipid peroxidation and DNA damage in seedlings of rice (Oryza sativa). - J. Plant Physiol. 161: 63-68, 2004.
Moran, J.F., Becana, M., Iturbe-Ormaetxe, I., Frechilla, S., Klucas, R.V., Aspariciv-Tejo, P.: Drought induces oxidative stress in pea plants. - Planta 194: 346-352, 1994.
Morrison, I.M.: A semi-micro method for the determination of lignin and its use in predicting the digestibility of forage crops. - J. Sci. Food Agr. 23: 455-463, 1972.
Nakano, Y., Asada, K.: Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. - Plant Cell Physiol. 22: 867-880, 1981.
Noctor, G., Foyer, C.H.: Ascorbate and glutathione: keeping active oxygen under control. - Annu. Rev. Plant Physiol. Plant mol. Biol. 49: 249-279, 1998.
Paoletti, F., Aldinucci, D., Mocali, A., Capparini, A.: A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. - Anal. Biochem. 154: 536-541, 1986.
Potters, G., Horemans, N., Caubergs, R.J., Assad, H.: Ascorbate and dehydroascorbate influence cell cycle progression in a tobacco cell suspension. - Plant Physiol. 124: 17-20, 2000.
Potters, G., De Gara, L., Assad, H., Horemans, N.: Ascorbate and glutathione: guardians of the cell cycle, partners in crime? - Plant Physiol. Biochem. 40: 537-548, 2002. Richards, K.D., Schott, E.J., Sharma, Y.K., Davis, K.R.,
Gardner, R.C.: Aluminum induces oxidative stress genes in
Arabidopsis thaliana. - Plant Physiol. 116: 409-418, 1998.
Sakihama, Y., Yamasaki, H.: Lipid peroxidation induced by phenolics in conjunction with aluminum ions. - Biol. Plant. 45: 249-254, 2002.
Sánchez-Fernández, R., Fricker, M., Corben, L.B., White, N.S., Sheard, N., Leaver, C.J., Van Montagu, M., Inzé, D., May, M.J.: Cell proliferation and hair tip growth in the
Arabidopsis root are under mechanistically different forms
of redox control. - Proc. nat. Acad. Sci. USA 94: 2745-2750, 1997.
Sasaki, M., Yamamoto, Y., Matsumoto, H.: Lignin deposition induced by aluminum in wheat (Triticum aestivum) roots. - Physiol. Plant. 96: 193-198, 1996.
Šimonovicová, M., Huttová, J., Mistrík, I., Široká, B., Tamás, L.: Root growth inhibition by aluminum is probably caused by cell death due to peroxidase-mediated hydrogen peroxide production. - Protoplasma 224: 91-98, 2004b.
Šimonovicová, M., Tamás, L., Huttová, J., Mistrík, I.: Effect of aluminum on oxidative stress related enzymes activities in barley roots. - Biol. Plant. 48: 261-266, 2004a.
Smith, I.K.: Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. - Plant Physiol. 79: 1044-1047, 1985.
Tamás, L., Budíková, S., Šimonovičová, M., Huttová, J., Široká, B., Mistrík, I.: Rapid and simple method for Al-toxicity analysis in emerging barley roots during germination. - Biol. Plant. 50: 87-93, 2006.
Veljovic-Jovanovic, S.D., Pignocchi, C., Noctor, G., Foyer, C.H.: Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intercellular redistribution of the antioxidant system. - Plant Physiol. 127: 426-435, 2001.
Vernoux, T., Wilson, R.C., Seeley, K.A., Reichheld, J.-P., Muroy, S., Brown, S., Maughan, S.C., Cobbett, C.S., Van Montagu, M., Inzé, D., May, M.J., Sung, Z.R.: The ROOT
MERISTEMLESS/CADMIUM SENSITIVE 2 gene defines a
glutathione-dependant pathway involved in inhibition and maintenance of cell division during postembryonic root development. - Plant Cell 12: 97-109, 2000.
Wang, J.-W., Kao, C.H.: Reduction of aluminum-inhibited root growth of rice seedlings with supplemental calcium, magnesium and organic acids. - Crop Environ. Bioinfo. 1: 191-198, 2004.
Yamaguchi, Y., Yamamoto, Y., Ikagawa, H., Matsumoto, H.: Protective effect of glutathione on the cytotoxicity caused by a combination of aluminum and iron in suspension-cultured tobacco cells. - Physiol. Plant. 105: 417-422, 1999. Yamamoto, Y., Kobayashi, Y., Devi, S.R., Rikishi, S.,
Matsumoto, H.: Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. - Plant Physiol. 128: 63-72, 2002.
Yamamoto, Y., Kobayashi, Y., Matsumoto, H.: Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. - Plant Physiol. 125: 199-208, 2001.
Zheng, S.J., Yang, J.L.: Target sites of aluminum phytoxicity. - Biol. Plant. 49: 321-331, 2005.