Effect of adding Ar on the thermal stability of chemical vapor deposited fluorinated
silicon oxide using an indirect fluorinating precursor
Kow Ming Chang, Shih Wei Wang, Chii Horng Li, Ta Hsun Yeh, and Ji Yi Yang
Citation: Applied Physics Letters 70, 2556 (1997); doi: 10.1063/1.118939 View online: http://dx.doi.org/10.1063/1.118939
View Table of Contents: http://scitation.aip.org/content/aip/journal/apl/70/19?ver=pdfcov
Published by the AIP Publishing
Articles you may be interested in
Electrical and reliability performances of nitrogen-incorporated silicon carbide dielectric by chemical vapor deposition
J. Vac. Sci. Technol. B 28, 573 (2010); 10.1116/1.3425633
Thermal stability of low dielectric constant porous silica films
Appl. Phys. Lett. 87, 262909 (2005); 10.1063/1.2159093
Plasma-enhanced chemical vapor deposition of low-k dielectric films using methylsilane, dimethylsilane, and trimethylsilane precursors
J. Vac. Sci. Technol. A 21, 388 (2003); 10.1116/1.1539086
Rapid thermal chemical vapor deposition of zirconium oxide for metal-oxide-semiconductor field effect transistor application
J. Vac. Sci. Technol. B 19, 1782 (2001); 10.1116/1.1396639
Thermal stability enhancement of Cu/WN/SiOF/Si multilayers by post-plasma treatment of fluorine-doped silicon dioxide
J. Appl. Phys. 85, 473 (1999); 10.1063/1.369410
This article is copyrighted as indicated in the article. Reuse of AIP content is subject to the terms at: http://scitation.aip.org/termsconditions. Downloaded to IP: 140.113.38.11 On: Fri, 03 Oct 2014 05:55:25
Effect of adding Ar on the thermal stability of chemical vapor deposited
fluorinated silicon oxide using an indirect fluorinating precursor
Kow Ming Chang, Shih Wei Wang, Chii Horng Li, Ta Hsun Yeh, and Ji Yi Yang Department of Electronic Engineering and Institute of Electronics, National Chiao Tung University, National Nano Device Laboratory, Hsinchu, Taiwan
~Received 4 November 1996; accepted for publication 10 March 1997!
For a low dielectric constant intermetal dielectric application, fluorinated silicon oxide (FxSiOy) films were deposited in an electron cyclotron resonance chemical vapor deposition system, with SiH4, O2, and CF4 as the reaction gases. Since the CF4 is an indirect fluorinating precursor, the
fluorinating mechanism resembles that of the oxide etching by a fluorocarbon plasma. Thermal stability of the incorporated fluorine ~and hence, the dielectric constant! relies heavily on the deposition parameters and technologies. According to experimental results, adding Ar gas during deposition can improve the thermal stability of incorporated fluorine. Such an improvement is due to the fact that Ar sputtering enhances the removal of weakly bonded silicon fluoride on the as-deposited film surface, thereby elevating the mean bonding strength of fluoride remaining in the oxide. © 1997 American Institute of Physics.@S0003-6951~97!00219-2#
Chemical vapor deposition of inorganic fluorinated sili-con oxide (FxSiOy), having various inexpensive precursors and easily integrated properties, has received extensive use in low dielectric constant ~k! intermetal dielectric ~IMD! applications.1Research has shown that a higher fluorine con-tent in the oxide implies a lower k value.1,2However, incor-porating too much fluorine leads to an unstable film, particu-larly when using indirect-fluorinating precursors such as CF4and C2F6. When using these etching gases as
fluorinat-ing sources, the incorporated fluorine originates from the nonvolatile silicon fluoride, which has a formation mecha-nism similar to etching of the oxide. Our previous reports have demonstrated that films deposited at a low temperature have a higher F concentration ~lower k! but lower thermal stability than those deposited at a high temperature.3,4Such a discrepancy is apparently due to the residual nature of weakly bound or physically adsorbed –F bonds buried in the film where the bonds break and diffuse out upon heating. The outgassing of fluorine not only causes the k value to increase, but reliability problems ~e.g., the leaching of –H contained oxide and adhesion degradation! occur as well.5In this letter, Ar gas was added during deposition of FxSiOy film, with SiH4, O2, and CF4 as the reaction gases in an
electron cyclotron resonance chemical vapor deposition
~ECR-CVD! system. Experimental results indicate that
en-hancing the depletion of weakly bound fluorine improves the thermal stability of the FxSiOyfilm by the simultaneous sput-tering effect of the Ar plasma, with a similar effect seen when increasing the deposition temperature.
Electron cyclotron resonance chemical vapor deposited FxSiOy films
3,4 ~SiH
4/O2/CF458/85/10 sccm,
Ar50–16 sccm, MW 300 W, 3 mTorr! were deposited on
n-type Si~100! 4 in. wafers to a thickness around 240 nm, at
temperatures of 25, 100, 200, and 300 °C. After annealing in a nitrogen ambient atmosphere at various temperatures~400, 500, 600, 700, and 800 °C! for half an hour, the films’ ther-mal stability was analyzed from the variation of the Si–F peak (;930 cm21) in the Fourier transform infrared spec-troscopy~FTIR! spectrum. The dielectric constant ~k! of the deposited film was measured from C – V characteristics,
us-ing the metal–insulator–semiconductor ~MIS! structure at 1 MHz.
Figure 1~a! compares the thermal stabilities of FxSiOy films with and without adding Ar gas~12 sccm!. Notably, for the films deposited at 25 °C and without adding Ar, the Si–F (;930 cm21) peak intensity start decaying at 500 °C
~;88% remaining! after half an hour of annealing.3,4
On the other hand, when adding Ar, the FxSiOy film can withstand temperatures above 500 °C without degrading the Si–F peak’s intensity. Figure 1~b! depicts the thermal stability of the Si–F bond in FxSiOyfilms under different Ar flow rates. FxSiOy films can withstand temperature at around 600 °C with only a slight outgassing of fluorine when the Ar exceeds 12 sccm. These results suggest that adding Ar raises the ther-mal stability.
Previous investigations3,4 have indicated that two reac-tions are primarily responsible for Si–F bonds forming in FxSiOyfilm while adding CF4:~1! the homogeneous reaction in the plasma; the active F and O atoms react with SiH4,
thereby causing the formation of oxyfluoride species and deposition, and ~2! the heterogeneous reaction on the film’s surface; the active F absorbs~physically and chemically! on the deposited film and, consequently, the nonvolatile fluoride is buried in the film during the subsequent deposition. On the other hand, the volatile fluoride results in simultaneous etch-ing. The fluorine’s bonding strength for these nonvolatile species buried in the film determines the stability during sub-sequent thermal cycles. Most of the volatile fluoride becomes depleted at a high surface temperature during deposition, leading to a low but stable fluorine concentration remaining in the film. According to Figs. 1~a! and 1~b!, adding Ar yields an effect similar to that observed by increasing the deposition temperature. As is well known, adding Ar en-hances the etching reaction of oxides in a fluorocarbon plasma, due to the depletion of fluoride species on the oxide surface by Ar ion sputtering. Consequently, adding Ar dur-ing the ECR-CVD of FxSiOy film depletes the weakly bonded fluoride and increases the thermal stability of the remaining F bonds, as increasing deposition temperature does as well.
2556 Appl. Phys. Lett. 70 (19), 12 May 1997 0003-6951/97/70(19)/2556/3/$10.00 © 1997 American Institute of Physics This article is copyrighted as indicated in the article. Reuse of AIP content is subject to the terms at: http://scitation.aip.org/termsconditions. Downloaded to IP:
Adding Ar also influences the deposition rate of FxSiOy films. Figure 2 depicts the variation of the FxSiOy film’s deposition rate with CF4 flow rate, at different depo-sition temperatures and Ar flow rates. The depodepo-sition rates generally decrease with an increasing CF4 flow rate and
deposition temperature. Such an event results from the high surface migration energy of the deposited species at the high surface temperature, resulting in a denser stack. Besides, the etching reaction is also favored at a higher temperature. However, for the 25 °C deposited films, the deposition rate slightly increases while adding CF4, when the CF4flow rate
is below 6 sccm. This phenomenon is due to the swelling of the oxide network caused by incorporation of fluorine.3,4 Therefore, strictly speaking, the deposition rate of FxSiOy films is determined by the competition among the deposition, etching reaction, and the swelling of the oxide due to F in-corporation. Figure 2 also reveals that adding of Ar causes the deposition rate of films deposited at room temperature to monotonically decrease with an increasing CF4flow rate, in contrast to those without Ar. Moreover, adding Ar enhances the etching reaction and reduces the incorporated fluorine concentration, both screening the phenomenon of the oxide network expansion due to F incorporation, as the increase of the deposition temperature does as well.
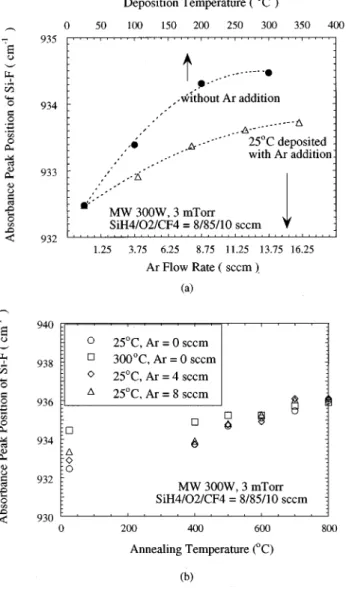
The variation of the FTIR peak position of Si–F bonds also accounts for the change in the thermal stability under different deposition conditions. Figure 3~a! depicts the Si–F
FIG. 1. Changes of~a! FTIR spectrum, and ~b! Si–F bond absorbance peak intensity ~normalized with the of Si–O peak intensity, stretching mode,
;1070 cm21! with different annealing temperatures, for F
xSiOyfilms
de-posited at 25 °C with various Ar flow rates.
FIG. 2. Deposition rate of FxSiOyfilm as a function of CF4flow rate, with
different deposition temperatures and Ar flow rates.
FIG. 3. Variations of FTIR Si–F bond absorbance peak position in the~a! as-deposited, and ~b! annealed ~400–800 °C! FxSiOyfilms, with different
deposition temperatures and Ar flow rates.
2557
Appl. Phys. Lett., Vol. 70, No. 19, 12 May 1997 Changet al.
This article is copyrighted as indicated in the article. Reuse of AIP content is subject to the terms at: http://scitation.aip.org/termsconditions. Downloaded to IP: 140.113.38.11 On: Fri, 03 Oct 2014 05:55:25
peak position of as-deposited FxSiOy films with various pro-cess temperatures and Ar flow rates. Notably, that Si–F peak position shifts upward with an increasing deposition tem-perature and Ar flow rate. This finding suggests that the Si–F bonds with higher vibration frequency possess higher ther-mal stability. Figure 3~b! presents the peak position of the remaining Si–F bonds after annealing at different tempera-tures. The Si–F bonds, capable of withstanding a high tem-perature without outgassing, tend to have the same vibration frequency around 936 cm21. By simulating the vibration of the Si–F bond simply with Hooke’s law, the higher vibration frequency corresponds to a higher force constant and, hence, a higher bonding strength of Si–F. Besides, the strength of the Si–F bond in the O42xSiFx local structure tends to de-crease with a higher x, due to the repulsive force of fluorine. Consequently, the most stable fluorinated oxide structure ~re-maining after high-temperature annealing! is in the form of a single fluoride.
In summary, this work demonstrates that the thermal sta-bility of FxSiOy films can be improved by adding Ar gas during deposition, when using CF4as the fluorinating
precur-sor. The stability can be raised from 400 to 600 °C for films deposited at room temperature. Such a feature is due to
en-hancing the removal of volatile fluorides on the oxide surface by Ar ion sputtering, as the increase in deposition tempera-ture does as well. However, for both of these methods, im-proving the thermal stability by depleting the excess~weakly bonded! fluorine, yields a higher k value ~e.g., k;3.41 for 16 sccm of Ar addition, in contrast to k;3.17 for that with-out Ar addition!. Besides, adding Ar does not significantly improve the moisture resistance of FxSiOy films. A high-temperature (;300 °C) deposition to derive a denser stack or a capped ECR–oxide layer is still necessary to block moisture absorption.
This work is supported under the Taiwan Republic of China National Science Council Contract No. NSC 86-2215-E-009-047.
1R. K. Laxman, Semicond. Int. 5, 71~1995!. 2
S. W. Lim, Y. Shimogaki, Y. Nakano, K. Tada, and H. Komiyama, Appl. Phys. Lett. 68, 832~1996!.
3K. M. Chang, S. W. Wang, C. J. Wu, C. H. Li, T. H. Yeh, and J. Y. Yang,
First International Symposium on Low and High Dielectric Constant Ma-terials and Technology, Los Angeles, May 1996.
4
K. M. Chang, S. W. Wang, C. J. Wu, T. H. Yeh, C. H. Li, and J. Y. Yang, Appl. Phys. Lett. 69, 1238~1996!.
5P. Singer, Semicond. Int. 5, 88~1996!.
2558 Appl. Phys. Lett., Vol. 70, No. 19, 12 May 1997 Changet al.
This article is copyrighted as indicated in the article. Reuse of AIP content is subject to the terms at: http://scitation.aip.org/termsconditions. Downloaded to IP: 140.113.38.11 On: Fri, 03 Oct 2014 05:55:25