Open Access
Research article
The trend of susceptibilities to amphotericin B and fluconazole of
Candida species from 1999 to 2002 in Taiwan
Yun-Liang Yang
1, Shu-Ying Li
2, Hsiao-Hsu Cheng
3, Hsiu-Jung Lo*
3and
TSARY Hospitals
Address: 1Department of Biological Science and Technology, National Chiao Tung University, Hsinchu, Taiwan, China, 2Laboratory for
Mycopathogen, Chlamydia and Mycoplasma, Division of Laboratory Research and Development, Center for Disease Control, Taipei, Taiwan, China and 3Division of Clinical Research, National Health Research Institutes, Miaoli, Taiwan, China
Email: Yun-Liang Yang - yyang@mail.nctu.edu.tw; Shu-Ying Li - syl@cdc.gov.tw; Hsiao-Hsu Cheng - shallman@tensall.com.tw; Hsiu-Jung Lo* - hjlo@nhri.org.tw; TSARY Hospitals - hjlo@nhri.org.tw
* Corresponding author
Abstract
Background: Candida species have various degrees of susceptibility to common antifungal drugs.
The extent of resistance to amphotericin B and fluconazole of Candida glabrata isolates causing candidemia has been reported. Active surveillance may help us to monitor the trend of susceptibility to antifungal drugs and to determine if there is an emerging co-resistance to both drugs of Candida species, specifically, of C. glabrata in Taiwan.
Methods: The susceptibilities to amphotericin B and fluconazole of Candida species collected in
1999 and 2002 of the Taiwan Surveillance of Antimicrobial Resistance of Yeasts (TSARY) were determined by the microdilution method.
Results: The antifungal susceptibilities of 342 and 456 isolates collected from 11 hospitals
participating in both TSARY 1999 and TSARY 2002, respectively, have been determined. The resistance rate to amphotericin B has increased from 0.3% in the TSARY1999 to 2.2% in the TSARY 2002. In contrast, the resistance rate to fluconazole has decreased from 8.8% to 2.2%. Nevertheless, significantly more C. glabrata isolates were not susceptible to fluconazole in the TSARY 2002 (47.4%) than that in the TSARY 1999 (20.8%). There were 9.8% and 11% of C. glabrata isolates having susceptible-dose dependent and resistant phenotype to fluconazole in the TSARY 1999, verse 45.3% and 2.1% in the TSARY 2002.
Conclusion: There was an increase of resistance rate to amphotericin B in C. glabrata. On the
other hand, although the resistance rate to fluconazole has decreased, almost half of C. glabrata isolates were not susceptible to this drug. Hence, continuous monitoring the emerging of co-resistance to both amphotericin B and fluconazole of Candida species, specifically, of C. glabrata, will be an important early-warning system.
Background
In the past decade, nosocomial yeast infections have
increased globally. In Taiwan, the prevalence of nosoco-mial candidemia increased 16-fold from 1981 through Published: 03 November 2005
BMC Infectious Diseases 2005, 5:99 doi:10.1186/1471-2334-5-99
Received: 08 March 2005 Accepted: 03 November 2005 This article is available from: http://www.biomedcentral.com/1471-2334/5/99
© 2005 Yang et al; licensee BioMed Central Ltd.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1993 [1,2]. In the United States, yeast infections rank as the fourth most common cause of nosocomial blood-stream infection [3,4]. Furthermore, candidemia contrib-ute considerable mortality (31% to 38%), extend the length of hospital stay [5,6], and increase social cost due to lost productivity and disabling complications [7]. Con-sequently, the Taiwan Surveillance of Antimicrobial Resistance of Yeasts (TSARY) was initiated in 1999 for epi-demiological study of yeast infections in Taiwan [8,9]
Candida species have various degrees of susceptibility to
common antifungal agents. Candida lusitaniae is less sus-ceptible to amphotericin B [10] while Candida krusei and
Candida glabrata are less susceptible to fluconazole than
other Candida species [11-14]. The extent of fluconazole resistance of C. glabrata isolates causing candidemia has been reported throughout the United States [15]. Further-more, C. glabrata exhibits variable cross-resistance to the other triazoles, such as voriconazole and posaconazole [13,16-18] and amphotericin B became the next choice. The aim of this study is to investigate the trend of suscep-tibility to amphotericin B and fluconazole of Candida spe-cies in Taiwan from 1999 to 2002. Especially, we would like to determine if there is an emerging co-resistance to amphotericin B and fluconazole of Candida species, spe-cifically, of C. glabrata, in Taiwan.
Methods
Organisms and media
Yeast isolates were collected from 11 hospitals participat-ing in both TSARY 1999 and TSARY 2002 [9,19]. Isolates were stored frozen at -70°C in bead containing Micro-bank cryovials (PRO-LAB Diagnostics, Austin, TX, USA). At the end of the collection period, isolates were kept fro-zen and transported by an express delivery company to the laboratory at National Health Research Institutes (NHRI) within 24 hours. After their arrival, the isolates were first sub-cultured on to sabouraud dextrose agar (SDA, BBL, Becton Dickinson Cockeysville, MD, USA) to check for purity and identifications. Pure isolates were labeled and stored in vials containing 50% glycerol at -70°C for subsequent analyses.
Identification
The identification procedure of yeast isolates in the NHRI laboratory was performed as described previously [8]. In general, isolates identified as C. albicans by hospitals were first subjected to the germ tube assay in brain heart infu-sion (BHI, BBL) medium containing 10% fetal bovine serum (JR12003, JRH Biosciences, Australia) at 37°C for 2–3 hours [20]. Isolates positive in germ tube assay were checked for growth at 42°C to differentiate C. albicans from C. dubliniensis [21]. The VITEK Yeast Biochemical
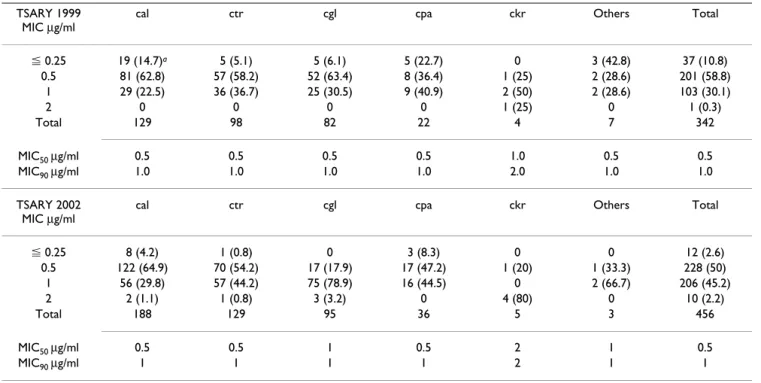
Table 1: The Susceptibilities of Candida Species to Amphotericin B
TSARY 1999 MIC µg/ml
cal ctr cgl cpa ckr Others Total
⬉ 0.25 19 (14.7)a 5 (5.1) 5 (6.1) 5 (22.7) 0 3 (42.8) 37 (10.8) 0.5 81 (62.8) 57 (58.2) 52 (63.4) 8 (36.4) 1 (25) 2 (28.6) 201 (58.8) 1 29 (22.5) 36 (36.7) 25 (30.5) 9 (40.9) 2 (50) 2 (28.6) 103 (30.1) 2 0 0 0 0 1 (25) 0 1 (0.3) Total 129 98 82 22 4 7 342 MIC50 µg/ml 0.5 0.5 0.5 0.5 1.0 0.5 0.5 MIC90 µg/ml 1.0 1.0 1.0 1.0 2.0 1.0 1.0 TSARY 2002 MIC µg/ml
cal ctr cgl cpa ckr Others Total
⬉ 0.25 8 (4.2) 1 (0.8) 0 3 (8.3) 0 0 12 (2.6) 0.5 122 (64.9) 70 (54.2) 17 (17.9) 17 (47.2) 1 (20) 1 (33.3) 228 (50) 1 56 (29.8) 57 (44.2) 75 (78.9) 16 (44.5) 0 2 (66.7) 206 (45.2) 2 2 (1.1) 1 (0.8) 3 (3.2) 0 4 (80) 0 10 (2.2) Total 188 129 95 36 5 3 456 MIC50 µg/ml 0.5 0.5 1 0.5 2 1 0.5 MIC90 µg/ml 1 1 1 1 2 1 1
cal, C. albicans; ctr, C. tropicalis; cgl, C. glabrata; cpa, C. parapsilosis; ckr, C. krusei
Card (YBC, bioMerieux, St. Louis, MI, USA) was then used to analyze isolates appearing to be negative by the germ tube assay in the NHRI laboratory and isolates identified as non-albicans Candida species by the hospitals. API-32C (bioMerieux) was used to assess the NHRI result when the VITEK-YBC showed less than 90% confidence. Antifungal susceptibility testing
The minimum inhibitory concentration (MIC) to ampho-tericin B or fluconazole of each yeast isolate was deter-mined by in vitro antifungal susceptibility testing according to the guidelines by the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) [22]. The RPMI medium 1640 (31800-022, Invitrogen Corpora-tion, Carlsbad, CA, USA) was used for dilution. Several strains from American Type Culture Collection, namely, ATCC 14053 C. albicans, ATCC 9003 C. glabrata, ATCC 6258 C. krusei, and ATCC20019 Candida parapsilosis were used as controls. The growth of each isolate was measured by a Spectra MAX Plus (Molecular Devices Cop. Sunnyvale, California, USA) after 48-hour incubation at 35°C. We also measured the MICs of some randomly-sampled isolates by Etest (AB Biodisk Solna, Sweden) to confirm our results by microdilution.
The interpretation of MICs was conducted according to the guidelines of the CLSI. The MICs to amphotericin B and fluconazole were defined as the lowest concentration of amphotericin B and fluconazole to reduce the turbidity of cells to greater than 95% and 50%, respectively. For amphotericin B, isolates with MIC ⭌ 2 µg/ml were
consid-ered to be resistant, whereas those with MIC ⬉ 1 µg/ml were susceptible. For fluconazole, isolates with MIC ⭌ 64 µg/ml were considered resistant, while those with MIC ⬉ 8 µg/ml were susceptible. Isolates with MICs between 16 and 32 µg/ml were susceptible-dose dependent. The MICs of 50% and 90% of the total population were defined as MIC50 and MIC90. For any species with less than ten, the MIC50 and MIC90 were not showed.
Database and analysis
The database for this study contained the following char-acteristic information of each submitted isolate: hospital origin, location and type of the hospital, identification and source of the isolate. The statistic significance of the differences in frequencies and proportions was deter-mined by the chi-square test with Yates' correction. A p value of ⬉ 0.05 was considered statistically significant.
Results
Distribution of Candida species
The distribution of Candida species was similar in both surveys. Candida albicans was the most common species consisting 37.7% of the total isolates in the TSARY 1999 and 41.2% in the TSARY 2002. Candida tropicalis (28.7% in 1999 vs. 28.3% in 2002) and C. glabrata (24% in 1999 vs. 20.8% in 2002) were the two most common non-albi-cans Candida species, followed by C. parapsilosis (6.4% in 1999 vs. 7.9% in 2002), C. krusei (1.2% in 1999 vs. 1.1% in 2002), and others (2% in 1999 vs. 0.7% in 2002). When classified according to the sources, isolates from urine, sputum, blood, wound, and others were 143
Table 2: The Susceptibilities of Candida Species to Fluconazole
TSARY 1999 MIC µg/ml
cal ctr cgl cpa ckr Others Total
S 121 (93.8)a 77 (78.6) 65 (79.2) 21 (95.5) 0 5 (71.4) 289 (84.5) SDD 3 (2.3) 9 (9.2) 8 (9.8) 1 (4.5) 0 2 (28.6) 23 (6.7) R 5 (3.9) 12 (12.2) 9 (11) 0 4 (100) 0 30 (8.8) Total 129 98 82 22 4 7 342 MIC50 µg/ml 0.25 2 4 1 ND ND 2 MIC90 µg/ml 4 64 64 4 ND ND 16 TSARY 2002
MIC µg/ml cal ctr cgl cpa ckr Others Total
S 178 (94.7) 124 (96.1) 50 (52.6) 36 (100) 1 (20) 2 (66.7) 391 (85.8) SDD 6 (3.2) 5 (3.9) 43 (45.3) 0 1 (20) 0 55 (12) R 4 (2.1) 0 2 (2.1) 0 3 (60) 1 (33.3) 10 (2.2) Toal 188 129 95 36 5 3 456 MIC50 µg/ml 0.25 1 8 1 ND ND 1 MIC90 µg/ml 1 4 32 2 ND ND 16
cal, C. albicans; ctr, C. tropicalis; cgl, C. glabrata; cpa, C. parapsilosis; ckr, C. krusei
(41.8%), 101 (29.5%), 30 (8.8%), 26 (7.6%), and 42 (12.3%), respectively, in the TSARY 1999 verse 186 (40.8%), 111 (24.3%), 50 (11%), 20 (4.4%), and 89 (19.5%), respectively, in the TSARY 2002.
Susceptibilities to amphotericin B
The susceptibilities to amphotericin B are shown in Table 1. A total of 10 isolates (2.2%) were resistant to ampho-tericin B in the TSARY 2002, whereas only one (0.3%) in the TSARY 1999 (p < 0.05). Of these 11 amphotericin B resistant isolates, 9 were non-albicans Candida species, including 5 C. krusei, 3 C. glabrata, and 1 C. tropicalis. In general, C. krusei was less susceptible to amphotericin B than other species.
Susceptibilities to fluconazole
The susceptibilities to fluconazole of Candida species are shown in Table 2. In the TSARY 1999, a total of 289 (84.5%), 23 (6.7%), and 30 (8.8%) isolates were suscep-tible, susceptible-dose dependent, and resistant to fluco-nazole, respectively, whereas in the TSARY 2002, there were 391 (85.5%), 55 (12%), and 10 (2.2%). The MIC50 and MIC90 of these isolates in the TSARY1999 were 2 µg/ ml and 16 µg/ml, respectively, and in the TSARY 2002, they were 1 µg/ml and 16 µg/ml. In the TSARY 1999, 12 (12.2%) C. tropicalis, 9 (11%) C. glabrata, 5 (3.9%) C. albicans, and 4 (100%) C. krusei, while in the TSARY 2002, 4 (2.1%) C. albicans, 3 (60%) C. krusei, and 2 (2.1%) C. glabrata were resistant to fluconazole. Fewer isolates in the TSARY 2002 were resistant to fluconazole than that in the TSARY 1999 (p < 0.05). In contrast, more isolates from the TSARY 2002 were susceptible-dose dependent than that in the TSARY 1999 (p < 0.05). Con-sequently, there were similar portions of isolates suscepti-ble to fluconazole in both surveys. Nevertheless, there were less isolates with MICs ⬉ 2 µg/ml to fluconazole in the TSARY 1999 (71.6%, 207/289) than in the TSARY 2002 (81.8%, 320/391) (p < 0.05). Finally, in the TSARY 1999, 82 (24%) of isolates had MICs between 4 and 8 µg/ ml to fluconazole. It was down to 71 (15.6%) in the TSARY 2002.
Discussion
The trend of susceptibilities to antifungal drugs of Candida species from 1999 to 2002 has been determined in this study. As expected, C. krusei had the highest resistance rate to fluconazole among Candida species tested, which is consistent with previous reports [9,11]. In contrast, all C.
parapsilosis isolates were susceptible to fluconazole, which
is also consistent with previous reports that C. parapsilosis is the most susceptible species to fluconazole [9,18,23,24]. Though the overall resistance rate to fluco-nazole has decreased from 8.8% to 2.2%, there were sig-nificantly more C. glabrata isolates not susceptible to fluconazole in the TSARY 2002 than that in the TSARY
1999. Overexpression of CgCDR1, CgCDR2, and
CgSNQ2-encoded efflux pumps has been shown to be a
major mechanism contributing to the drug resistance [25-27]. It would be interesting to investigate the molecular mechanisms of drug resistance of those clinical resistant isolates.
Recently, triazoles have been developed as the new savior to the issue of drug resistance in Candida infection. Nev-ertheless, C. glabrata exhibits variable cross-resistance among triazoles [9,18,23]. Thus, amphotericin B appears to be the choice for treating systemic infections caused by this species. However, along with the increased use of amphotericin B, 20% and 36% of C. glabrata isolates from North America and Latin America, respectively, were reported to be resistant [23]. These data suggest that co-resistance to amphotericin B and fluconazole of C.
gla-brata species may become a problem for clinical therapy
worldwide. In our study, we found only three C. glabrata isolates resistant to amphotericin B, which is lower than what has been reported. In that study, 20% of C. glabrata causing candidemia collected in Taiwan in 2003 were resistant to amphotericin B [16]. Coincidently, more C.
glabrata isolates in the TSARY 2002 (78.9%) had the MICs
of amphotericin B at 1 µg/ml than that in the TSARY 1999 (30.5%). Hence, periodic surveillance is needed to closely monitor the trends of susceptibility to antifungal drugs and for early detection of the newly emerging co-resist-ance to amphotericin B and fluconazole of Candida spe-cies, especially, of C. glabrata.
Abbreviations used
TSARY, Taiwan Surveillance of Antimicrobial Resistance of Yeasts; NHRI, National Health Research Institutes; SDA, sabouraud dextrose agar; BHI, brain heart infusion; YBC, Yeast Biochemical Card; MIC, minimum inhibitory concentration; NCCLS, National Committee of Clinical Laboratory Standards; CLSI, Clinical and Laboratory Standards Institute.
Competing interests
The author(s) declare that they have no competing inter-ests.
Authors' contributions
YLY and HJL design the study and drafted the manuscript. HHC conduct the experiments with contribution with SYL. TSARY Hospitals provided isolates.
Acknowledgements
We would like to thank Bristol Myers Squibb and Pfizer for supplying the amphotericin B and fluconazole, respectively. We also wish to thank the 11 participating hospitals for providing clinical isolates and information regard-ing to those isolates. They are Buddhist Tzu-Chi General Hospital, Hua-Lien Hospital, DOH, the Executive Yuan, Kaohsiung Military Hospital, Kaohsiung Medical College Chung-Ho Memorial Hospital, Kuan-Tien
Gen-Publish with BioMed Central and every scientist can read your work free of charge "BioMed Central will be the most significant development for disseminating the results of biomedical researc h in our lifetime."
Sir Paul Nurse, Cancer Research UK Your research papers will be:
available free of charge to the entire biomedical community peer reviewed and published immediately upon acceptance cited in PubMed and archived on PubMed Central yours — you keep the copyright
Submit your manuscript here:
http://www.biomedcentral.com/info/publishing_adv.asp
BioMedcentral eral Hospital, Lo-Hsu Foundation Inc. Lo-Tung Poh Ai Hospital, St. Mary
Hospital, Tri Service General Hospital, Veterans General Hospital-Taic-hung, Veterans General Hospital-Kaohsiung, Zen Ai General Hospital. This work was in part supported by the grants DOH93-DC-1101, and DOH94-DC-1102 from Center for Disease Control and CL-93-PP-06 from National Health Research Institutes.
References
1. Hung CC, Chen YC, Chang SC, Luh KT, Hsieh WC: Nosocomial can-didemia in a university hospital in Taiwan. J Formos Med Assoc 1996, 95:19-28.
2. Chen YC, Chang SC, Sun CC, Yang LS, Hsieh WC, Luh KT: Secular
trends in the epidemiology of nosocomial fungal infections at a teaching hospital in Taiwan, 1981 to 1993. Infect Control Hosp
Epidemiol 1997, 18:369-375.
3. Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP: National
surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Pro-gram. SCOPE Participant Group. Surveillance and Control of Pathogens of Epidemiologic. Diagn Microbiol Infect Dis 1998, 30:121-129.
4. Beck-Sague C, Jarvis WR: Secular trends in the epidemiology of
nosocomial fungal infections in the United States, 1980– 1990. National Nosocomial Infections Surveillance System. J
Infect Dis 1993, 167:1247-1251.
5. Leleu G, Aegerter P, Guidet B: Systemic candidiasis in intensive
care units: a multicenter, matched-cohort study. J Crit Care
2002, 17:168-175.
6. Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP:
Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med 1988, 148:2642-2645.
7. Tortorano AM, Caspani L, Rigoni AL, Biraghi E, Sicignano A, Viviani MA: Candidosis in the intensive care unit: a 20-year survey. J
Hosp Infect 2004, 57:8-13.
8. Lo HJ, Ho AH, Ho M: Factors accounting for mis-identification
of Candida species. J Microbiol Immunol Infect 2001, 34:171-177.
9. Yang YL, Ho YA, Cheng HH, Ho M, Lo HJ: Susceptibilities of Can-dida species to amphotericin B and fluconazole: the
emer-gence of fluconazole resistance in Candida tropicalis. Infect
Control Hosp Epidemiol 2004, 25:60-64.
10. Hadfield TL, Smith MB, Winn RE, Rinaldi MG, Guerra C: Mycoses
caused by Candida lusitaniae. Rev Infect Dis 1987, 9:1006-1012.
11. Akova M, Akalin HE, Uzun O, Gur D: Emergence of Candida kru-sei infections after therapy of oropharyngeal candidiasis with
fluconazole. Eur J Clin Microbiol Infect Dis 1991, 10:598-599.
12. Orozco AS, Higginbotham LM, Hitchcock CA, Parkinson T, Falconer D, Ibrahim AS, et al.: Mechanism of fluconazole resistance in Candida krusei. Antimicrob Agents Chemother 1998, 42:2645-2649. 13. Yang YL, Cheng HH, Lo HJ: In vitro activity of voriconazole
against Candida species isolated in Taiwan. Int J Antimicrob
Agents 2004, 24:294-296.
14. Piemonte P, Conte G, Flores C, Barahona O, Araos D, Alfaro J, et al.:
Emergency of fluconazole-resistant infections by Candida
krusei and Candida glabrata in neutropenic patients. Rev Med
Chil 1996, 124:1149.
15. Pfaller MA, Diekema DJ, Messer SA, Boyken L, Hollis RJ: Activities
of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by Broth microdilu-tion, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J
Clin Microbiol 2003, 41:1440-1446.
16. Hsueh PR, Lau YJ, Chuang YC, Wan JH, Huang WK, Shyr JM, et al.:
Antifungal susceptibilities of clinical isolates of Candida spe-cies, Cryptococcus neoformans, and Aspergillus species from Taiwan: surveillance of multicenter antimicrobial resistance in Taiwan program data from 2003. Antimicrob Agents Chemother
2005, 49:512-517.
17. Pfaller MA, Messer SA, Boyken L, Hollis RJ, Rice C, Tendolkar S, et al.:
In vitro activities of voriconazole, posaconazole, and flucona-zole against 4,169 clinical isolates of Candida spp. and
Cryp-tococcus neoformans collected during 2001 and 2002 in the
ARTEMIS global antifungal surveillance program. Diagn
Microbiol Infect Dis 2004, 48:201-205.
18. Pfaller MA, Diekema DJ, Jones RN, Sader HS, Fluit AC, Hollis RJ, et
al.: International surveillance of bloodstream infections due
to Candida species: frequency of occurrence and in vitro sus-ceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol 2001, 39:3254-3259.
19. Yang YL, Li SY, Cheng HH, Lo HJ: Susceptibilities to
amphoter-icin B and fluconazole of Candida species in TSARY 2002.
Diagn Microbiol Infect Dis 2005, 51:179-183.
20. Larone DH: Laboratory Procedures. In Medically Important Fungi:
A Guide to Identification New York: ASM Press; 1995:209-224.
21. Sullivan D, Coleman D: Candida dubliniensis: characteristics
and identification. J Clin Microbiol 1998, 36:329-334.
22. Clinical and Laboratory Standards Institute (formly NCCLS):
Refer-ence method for broth dilution antifungal susceptibility test-ing of yeasts; approved standard. M27A, Wayne, PA 1997.
23. Pfaller MA, Espinel-Ingroff A, Jones RN: Clinical evaluation of the
Sensititre YeastOne colorimetric antifungal plate for anti-fungal susceptibility testing of the new triazoles voricona-zole, posaconavoricona-zole, and ravuconazole. J Clin Microbiol 2004, 42:4577-4580.
24. Yang YL, Cheng HH, Ho YA, Hsiao CF, Lo HJ: Fluconazole
resist-ance rate of Candida species from different regions and hos-pital types in Taiwan. J Microbiol Immunol Infect 2003, 36:187-191.
25. Bennett JE, Izumikawa K, Marr KA: Mechanism of increased
flu-conazole resistance in Candida glabrata during prophylaxis.
Antimicrob Agents Chemother 2004, 48:1773-1777.
26. Sanglard D, Ischer F, Calabrese D, Majcherczyk PA, Bille J: The ATP
binding cassette transporter gene CgCDR1 from Candida
gla-brata is involved in the resistance of clinical isolates to azole
antifungal agents. Antimicrob Agents Chemother 1999,
43:2753-2765.
27. Sanguinetti M, Posteraro B, Fiori B, Ranno S, Torelli R, Fadda G:
Mechanisms of azole resistance in clinical isolates of Candida
glabrata collected during a hospital survey of antifungal
resistance. Antimicrob Agents Chemother 2005, 49:668-679.
Pre-publication history
The pre-publication history for this paper can be accessed here: