PCR Technique Applied to the Ecological Study of Paulownia Witches’-Broom Disease in Taiwan
Meng-Ling Wu,
1)Ling-Mu Jaung,
1)Cheng-Kuen Ho,
2)Chih-Yun Lee,
1)Ting-Hsuan Hung
3,4)【 Summary】
With the characteristics of insect-borne transmission and systemic infection, Paulownia witches’-broom (PaWB) disease has been considered a serious epidemic disease in Taiwan. PaWB phytoplasma (PaWBP) seriously weakens paulownia (Paulownia taiwaniana) plants, and has an enormous influence on paulownia cultivation. To accurately diagnose PaWB, a rapid and reliable detection method, a polymerase chain reaction (PCR)-based analysis using primers derived from PaWBP 16S ribosomal (r)DNA sequences, was previously established. We used the PCR assay to trace the incubation periods of PaWBP, symptom expression stages of infected paulownia, distribu- tion and concentration of PaWBP in paulownia trees, and insect vectors. The incubation period of PaWBP-infected paulownia (10 cm in height) was about 3 mo; distributions of PaWBP in the mes- ophyll, petioles, veins, and bark of paulownia plants were from high to low, respectively according to the PCR assay. We also found that it took about 3 mo for PaWBP to move from the top grafting site to the basal root in 30-cm-tall paulownia plants. In this study, it was proven that Halyomorpha mista could successfully transmit PaWBP from diseased paulownia to healthy paulownia seedlings, and detection of PaWBP in H. mista was confirmed by the PCR as well. Field tracking showed 2 population peaks of H. mista during the year: March to April and August to October. These results may be helpful in insect vector control in the future. In resistance experiments, paulownia hybrids also showed higher resistance than purebred plants.
Key words: paulownia witches’-broom (PaWB), phytoplasma, polymerase chain reaction (PCR), ecology, insect vector.
Wu ML, Jaung LM, Ho CK, Lee CY, Hung TH. 2012. PCR technique applied to the ecological study of paulownia witches’-broom disease in Taiwan. Taiwan J For Sci 27(4):345-56.
1)Forest Protection Division, Taiwan Forestry Research Institute, 53 Nanhai Rd., Taipei 10066, Taiwan. 林業試驗所森林保護組,10066台北市南海路53號。
2)Silviculture Division, Taiwan Forestry Research Institute, 53 Nanhai Rd., Taipei 10066, Taiwan. 林 業試驗所育林組,10066台北市南海路53號。
3)Department of Plant Pathology and Microbiology, National Taiwan Univ. 1 Roosevelt Rd., Sec. 4, Taipei 10617, Taiwan. 國立台灣大學植物病理與微生物學系,10617台北市羅斯福路四段1號。
4)Corresponding author, e-mail:thhung@ntu.edu.tw 通訊作者。
Received September 2012, Accepted October 2012. 2012年9月送審 2012年10月通過。
研究報告
PCR技術應用於台灣泡桐簇葉病之發病生態
與媒介昆蟲研究吳孟玲
1)莊鈴木
1)何政坤
2)李芷芸
1)洪挺軒
3,4)摘 要
由植物菌質體(phytoplasma)所引起之泡桐簇葉病(paulownia witches´-broom, PaWB),是一種蟲 媒系統性病害,在臺灣為泡桐重要流行病害。受泡桐簇葉病菌質體(PaWB phytoplamsa, PaWBP)感染 之泡桐樹勢衰弱,嚴重影響泡桐之栽種。為準確檢測植株是否遭受感染,已針對PaWBP 16S rDNA開 發之專一性引子對,高敏感度、快速且正確的PCR偵測技術,所增幅之PaWBP專一性PCR片段大小為 510 bp。本研究即以此專一性引子對追蹤PaWBP在泡桐寄主中之消長與發病關係,初步發現以人工嫁 接病芽感染10公分株高之台灣泡桐,其病原菌潛伏期(incubation period)平均為三個月;PaWBP在病 株分佈(distribution),以葉脈含量最高,其次為莖部、葉肉及皮層含量最低;同時亦發現PaWBP移動 情形(movement)並不快速,以PCR法追蹤發現約3個月後PaWBP才由頂部嫁接感染點移至基部。在媒 介昆蟲(vector insect)的調查上,經人工傳播試驗及PCR證實媒介昆蟲為常山黃斑樁象(Halyomorpha mista),由田間媒介昆蟲族群消長動態的調查發現,一年之中有兩個高峰期,分別出現在3至4月及8 至10月間。本研究亦進行雜交種抗病試驗,其中貴州與四川泡桐雜交種潛伏期較長,表現弱病徵型抗 病性。
關鍵詞:泡桐簇葉病、PCR技術、發病生態、媒介昆蟲。
吳孟玲、莊鈴木、何政坤、李芷芸、洪挺軒。2012。PCR技術應用於台灣泡桐簇葉病之發病生態與媒 介昆蟲研究。台灣林業科學27(4):345-56。
INTRODUCTION
Paulownia witches’-broom (PaWB) is one of the first plant diseases reported to be caused by mycoplasma-like organisms (Doi et al. 1967), which now are known as phytoplas- mas, belonging to the class Mollicutes (Tully 1993). With characteristics of insect-borne transmission and systemic infection, PaWB disease is considered a serious epidemic dis- ease in Taiwan. PaWB phytoplasma (PaWBP) seriously weakens paulownia plants and enormously influences the forestry industry.
PaWBP is reportedly transmitted by the stink- bugs Halyomorpha mista Uhler and H. holys Stal (Tsai et al. 1988, Namba et al. 1993).
Infected paulownia trees are characterized by the proliferation of branches with very small yellowish leaves, i.e., witches’-brooms, followed by dieback of the branches (Tsai et al. 1988). Currently, there is no chemical available to effectively control phytoplasma diseases. Cultivation of pathogen-free pau- lownia seedlings and control of insect vectors are feasible for PaWB control. It is usually difficult to diagnose whether paulownia trees are infected by PaWBP due to its latent infec- tion. To accurately diagnose PaWB, a rapid and reliable detection method for PaWBP was needed. Although oligonucleotide prim-
ers derived from 16S ribosomal (r)RNA sequences have been widely used for poly- merase chain reaction (PCR) amplification in plant-pathogenic phytoplasma detection (Lee et al. 1994, Chen and Lin. 1997, Hung et al. 1999a, 1999b), major PCR products ob- tained were nonspecific in an electrophoretic analysis.
To establish a more-reliable detection method for PaWBP, we previously developed a highly specific PCR-based detection assay (Wu et al. 2002). This PCR-based assay pro- vides a specific and sensitive method suitable for the rapid diagnosis of PaWB. This assay will be helpful for management of PaWBP in the future, and can be applied to monitor the distribution and concentration of PaWBP in infected paulownia plants for further studies.
In this study, we used this PCR assay to trace the incubation periods of PaWBP, symptom expression stages of PaWBP-infected pau- lownia trees, distribution and movement of PaWBP in paulownia, and insect vectors. It was proven that H. mista could successfully transmit PaWBP from diseased paulownia plants to healthy paulownia seedlings in this study. Highlighting the population peaks of H.
mista would be helpful in insect vector con- trol. Paulownia hybrids exhibited in higher resistance than purebred plants in resistance experiment for PaWB disease control in the future.
MATERIALS AND METHODS Plant materials
PaWBP-infected paulownia (P. taiwani- ana) tissues with characteristic symptoms of PaWB were collected from the Lienhwachih Branch of the Taiwan Forestry Research Institute and used in this study. Twenty-one paulownia hybrids and 3 purebred plants (Table 1) were used. These experimental
hybrids were obtained from pathogen-free seedlings through shoot-tip grafting tech- niques (Murashige et al. 1972) and kept in an insect-proof greenhouse. PaWBP- infected paulownia buds were side-grafted onto healthy paulownia plants as inoculum sources. Healthy paulownia seeedlings were aseptically germinated in a growth chamber and used as negative controls. Monitoring of PaWBP was conducted using a PCR assay with PaWBP-specific primers, and symptoms were recorded.
Specific primers for PaWBP
Two opposing primers for the PCR- based detection of PaWBP were chosen from the sequence of cloned PaWBP-specific DNA fragments (Wu et al. 2002). The primer pair, composed of the forward primer (5’-TCC ATG TCT CTT TCA ATT CCT-3’) and re- verse primer (5’-AAA AGC TAT TCA AAA TTA TGC C-3’), were designed to amplify a PaWBP-specific DNA fragment by PCR.
Preparations of DNA samples for PCR For rapid mass-screening, mini-prepara- tions of total nucleic acid extracts of PaWBP- infected samples were used as templates for the PCR. Nucleic acid samples were prepared using a method described by Lee and Davis (1988), Lee et al. (1988), and Lee et al. (1990) with minor modifications Mesophyll (ca.
500 mg) was powdered in liquid nitrogen, and each sample was suspended in 1.5 mL of DNA extraction buffer (0.1 M Tris-HCl [pH 8.0], 0.05 M EDTA, 0.5 M NaCl, and 1% N- lauroylsarcosine), and transferred to a 1.5-mL Eppendorf tube. After incubation at 55℃ for 1 h, the sample was centrifuged at 4000 xg for 5 min. After the supernatant (ca. 800 μL) was collected, 100 μL of 5 M NaCl and 100 μL of 10% hexadecyl-trimethyl-ammonium- bromide (CTAB) in 0.7 M NaCl were added,
and the mixture was incubated at 65℃ for 10 min. The sample was subjected to 1 cycle of chloroform/isoamyl alcohol (24: 1) extrac- tion, and the aqueous supernatant was then re- extracted with an additional cycle of phenol/
chloroform/isoamyl alcohol (25: 24: 1). The
nucleic acids were precipitated by mixing 600 μL of the supernatant and 360 μL of isopro- panol followed by centrifugation at 12,000 xg for 10 min. The pellets were washed with 70% ethanol, dried, and resuspended in 150 μL of TE buffer.
Table 1. Different species of paulownia purebred plants and hybrids used in the paulownia witches’-broom phytoplasma (PaWBP) resistance experiment
Inoculation number No. of paulownia plants Inoculation source1) Paulowina spp. 2)
1 5 PaWB-1 st3)
2 3 PaWB-2 tg
3 1 PaWB-1 t4)
4 1 PaWB-1 kg
5 3 PaWB-1 kg×f5)
6 5 PaWB-2 gt
7 3 PaWB-2 tf
8 4 PaWB-1 tk
9 5 PaWB-1 ft
10 2 PaWB-1 ft×tk6)
11 6 PaWB-1 ft×ts
12 3 PaWB-2 ft×tg
13 1 PaWB-2 ft×ts
14 5 PaWB-1 ft×t
15 4 PaWB-2 ft×t
16 5 PaWB-1 ft×tg
17 4 PaWB-1 ft×ts
18 5 PaWB-1 ft×tf
19 1 PaWB-2 ft×tg
20 5 PaWB-1 ft×ts
21 4 PaWB-2 ft×tk
22 3 PaWB-2 k7)
23 2 PaWB-2 ft×tf
24 1 PaWB-1 t8)
1)Two PaWBP-infected paulownia plants confirmed by the PCR were used as inocula sources for grafting.
2)s, P. shensiensis; t, P. taiwaniana; g, P. glabrata; k, P. kawakamii; f, P. fortunei.
3)Paulownia hybrid of P. shensiensis and P. taiwaniana.
4)Purebred paulownia of P. taiwaniana.
5)Double cross: a hybrid of P. kawakamii and P. glabrata crossbred with a P. fortunei purebred.
6)Double cross: a hybrid of P. fortunei and P. taiwaniana crossbred with another hybrid of P. tai- waniana and P. kawakamii.
7)Purebred paulownia of P. kawakamii.
8)Purebred paulownia of P. taiwaniana from a greenhouse of TFRI.
PCR conditions
The PCR was performed using 25 μL of a reaction mixture containing 20 mM Tris- HCl (pH 8.4), 50 mM KCl, 4 mM MgCl2, 0.2 mM each of dATP, dTTP, dCTP, and dGTP, 10 pmole of the forward primer, 10 pmole of the reverse primer, 0.75 units of Taq DNA polymerase (Gibco, Big Cabin, OK, USA), and 200 ng of template from the nucleic acid preparation. The thermal cycle conditions were: 1 cycle at 94℃ for 3 min; 30 cycles at 94℃ for 1 min, 54℃ for 1 min, and 72℃
for 2 min; followed by a 72℃ extension for 10 min. Reactions were carried out in a DNA Thermal Cycler 9600 (Applied Biosystems, Foster, CA, USA).
Analysis of PCR products by electropho- resis
PCR products were identified by gel electrophoresis using 1.5% agarose (Boeh- ringer Mannheim, Pleasanton, CA, USA) in TAE buffer (40 mM Tris-acetate [pH 8.0] and 1 mM EDTA). After electrophoresis, the gel was stained with ethidium bromide (0.5 μg/
mL) and photographed. A 100-bp DNA ladder set (Promega, Madison, WI, USA) was in- cluded as a size marker. Electrophoresis was run for 30~40 min at a high voltage (100 V).
Insect vector
PaWBP-free bugs (H. mista) were ini- tiated from single females collected from PaWBP-free paulownia (P. taiwaniana) plants. These bugs were added to caged pods of PaWBP-free paulownia plants at 25~28℃, under a 16-h light and 8-h dark cycle. After starvation for 30 min, the adults were put in a Petri dish for a 24-h acquisition feeding period of paulownia infected with PaWBP.
These viruliferous adults were transferred to healthy paulownia seedlings for a 24-h inocu- lation access period and then eliminated with
an insecticide. Inoculated paulownia plants were cultivated at 25~28℃. Transmission was determined according to the results of a PCR analysis and symptom development.
For the study of bug-transmission, adult bugs were moved to a PaWBP-infected pau- lownia plant for a 24-h acquisition access peri- od (AAP). These viruliferous adults were then collected and divided into 5 groups including single, 5, 10, 20, and 50 adults, respectively.
PaWBP-free paulownia plants (1 mo old and 10 cm tall) were individually inoculated with single, 5, 10, 20, and 50 viruliferous adults for the inoculation access period (IAP).
RESULTS
PaWBP detection in diseased paulownia plants
The PaWBP-specific primer pair de- signed in this study was used to detect PaWB-infected paulownia plants in the field.
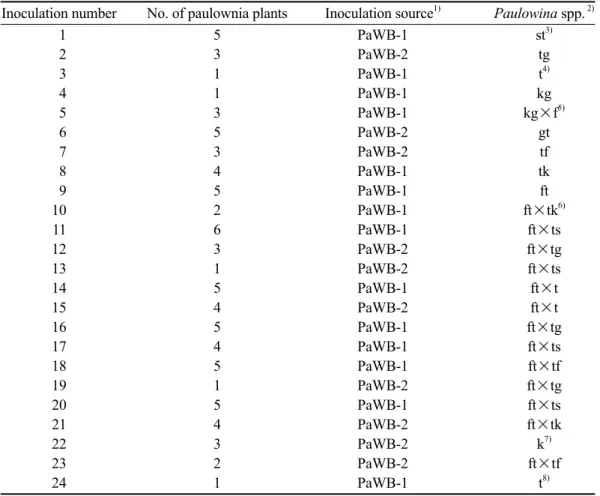
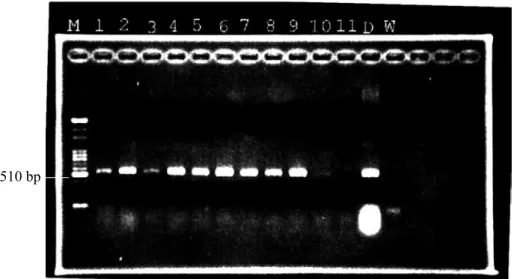
A specific 510-bp product was amplified in the PCR detection (Fig. 1) which showed the high specificity and sensitivity (Fig. 2) of this primer pair. Grafted paulownia plants as de- scribed above were also examined by PCR af- ter 3 mo of grafting assuring successful graft- inoculation (Fig. 3).
Phytoplasma concentration distribution of PaWBP in paulownia plants
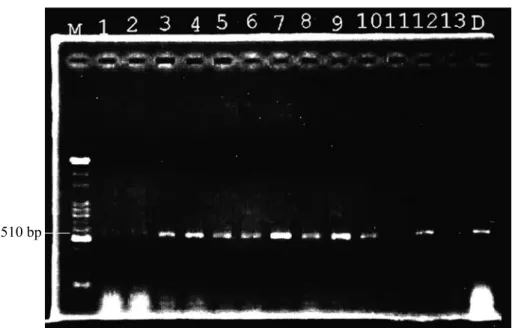
Different tissue parts of PaWBP-infected paulownia were collected, and these showed positive results in PCR detection. Compari- sons of the PaWBP concentration distribution in paulownia tissues including the mesophyll, veins, petioles, and bark by the PCR assay showed higher concentrations in mesophyll tissues of diseased paulownia. Distributions of PaWBP in the mesophyll, veins, petioles, and bark of paulownia were from high to low, respectively (Fig. 4).
Fig. 1. Paulownia witches’-broom phytoplasma (PaWBP)-specific primer pair designed in this study was used for the field detection of paulownia. An electrophoretic analysis showed a specific 510-bp product of PaWBP implying high specificity of this primer pair. M, 100-bp ladder marker; DNA templates of lanes 1~10 were extracted from field-grown paulownia;
lane 11, healthy control; lane D, disease control; lane W, double-distilled H2O.
Fig. 2. Primer pair sensitivity test resulted in high sensitivity for paulownia witches’-broom phytoplasma (PaWBP) detection. M, 100-bp ladder marker; DNA template extracted from diseased paulownia were serially diluted, and concentrations of DNA were 1000, 500, 250, 100, 50, 10, 5, 1, and 0.5 ng from lanes 1~9, respectively; lane 10, double-distilled H2O.
Movement of PaWBP in paulownia plants PaWB disease progress in grafted plants showed that PaWBP first moved down to the
lower leaves and roots from the graft point, then proliferated and moved through the phlo- em to the upper leaves, and finally resulted in
witches’ broom (Fig. 5A). Higher concentra- tions of PaWBP in lower leaves than upper ones were also observed in the PCR assay (Fig. 5B).
Symptom expression in different pau- lownia hybrids
Paulownia hybrids of 10 cm high were grafted with PaWBP-infected paulownia
Fig. 3. Examination of grafted paulownia by a PCR to ensure successful grafting before symptom expression. M, 100-bp ladder marker; lanes 1~13, DNA templates extracted from different artificially grafted paulownia seedlings; lane D, disease control.
Fig. 4. Distributions of paulownia witches’-broom phytoplasma (PaWBP) in grafted paulownia. Lanes 1~4 and lanes 5~8 are mesophyll, veins, petioles, and bark of paulownia, respectively; M, 100-bp ladder marker.
by bud-graft inoculation. Symptom expres- sions of different paulownia hybrids were recorded after 4 mo of grafting and showed different levels of susceptibility or resistance to PaWBP. Nine hybrids showed typical witches’ broom symptoms limited from the graft point to lower leaves (Fig. 6A); 7 hy- brids showed stunted and shortened internode symptoms instead of typical witches’ broom symptoms (Fig. 6B); and 5 hybrids showed
no symptoms, but the PCR showed positive results (Fig. 6C).
Examination of insect vectors of PaWB disease
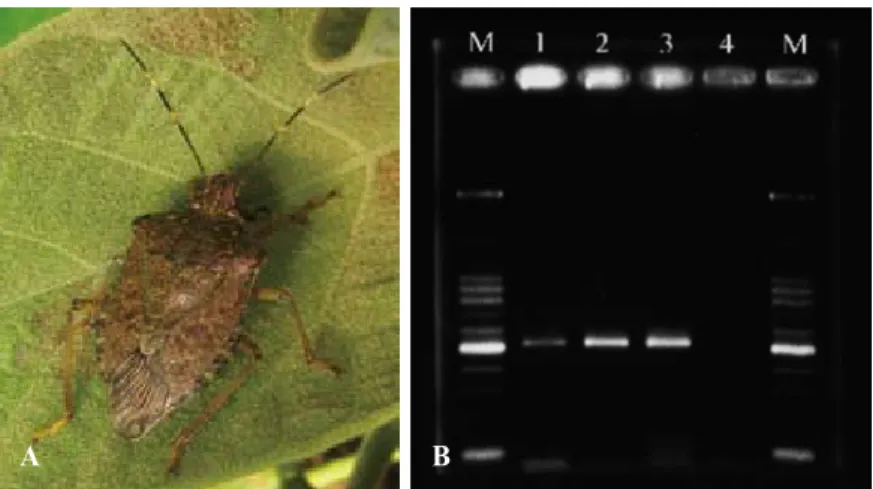
PaWBP-free bugs (Fig. 7A) were fed PaWBP-infected paulownia plants and ex- amined by the PCR after a 24-h acquisition period. A PaWBP-specific fragment (510 bp) was amplified for PCR detection (Fig. 7B).
Fig. 5. Paulownia witches’-broom phytoplasma (PaWBP) was oberved to first move down to lower leaves and roots from the graft point rather than move up to top leaves in paulownia (A). The same result was obtained by PCR detection (B) Lanes 1~12 were DNA templates collected from the first top leaf to the 12th leaf of grafted paulownia, respectively; lane 13, dobule-distilled H2O; M, 100-bp ladder marker.
The same fragment was also obtained from paulownia seedlings (Fig. 8), which had pre- viously been PaWBP-free but were inoculated by viruliferous bugs.
DISCUSSION
PCR amplification of the 16S rRNA gene using oligonucleotide primers was used to
Fig. 6. Some resistant paulownia hybrids showed typical witches’-broom symptoms limited from the graft point to lower leaves (A); some showed stunted and shortened internode symptoms instead of typical witches’-broom symptoms. The 2 paulownia on the left side were grafted, while a healthy one is on the right side (B). All grafted hybrids were detected by a PCR and resulted in amplifying the specific 510-bp product of paulownia witches’-broom phytoplasma (PaWBP) DNA (C). M, 100-bp ladder marker; DNA templates of lanes 1~3 were extracted from symptomless paulownia hybrids; lanes 4~6, hybrids with limited witches’
broom from the graft point; lanes 7~9, hybrids with stunted and shortened internodes; lane 10, diseased paulownia with typical symptoms; lane 11, healthy paulownia plant.
detect several plant-pathogenic phytoplasmas (Lee et al. 1994). In our preliminary experi- ments, amplification of nonspecific fragments from healthy plants by a PCR using primers for the 16S rRNA gene caused a confound- ing problem in the detection of PaWBP. This might have been caused by amplification of chloroplast DNA in host plants or the pres- ence of contaminating Mollicutes from plant
A B
C
D D H
Fig. 7. Insect vector, Halyomorpha mista (A), of paulownia witches’-broom phytoplasma (PaWBP) was confirmed in this study by its feeding on PaWBP-infected paulownia and then being checked using a PCR, and a specific 510-bp product was amplified (B) confirming that H. mista carried PaWBP within its body. M, 100-bp ladder marker; DNA templates of lanes 1~3 were extracted from PaWBP-fed H. mista; lane 4, PaWBP-free H. mista.
Fig. 8. Viruliferous adults used to transmit paulownia witches’-broom phytoplasma (PaWBP) to healthy paulownia and detection by a PCR. M, 100-bp ladder marker; DNA templates of lanes 1~7 were extracted from paulownia infected by viruliferous adults; lane 8, healthy control; lane D, disease control; lane W, double-distilled H2O.
surfaces as discussed elsewhere (Deng and Hiruki 1991, Ahrens and Seemuller 1992).
Our previous study showed that PaWBP is a stable and useful diagnostic DNA probe for detecting PaWBP. However it takes at least 2 d to detect a PaWBP infection by dot hybrid-
ization with biotinylated DNA probes (Wu et al. 2002). As demonstrated in this study, PCR assay features higher sensitivity and is known to be an excellent method for pathogen detec- tion. Detailed procedures of this approach are summarized in Fig. 4. This protocol consists
A B
of 3 major steps: (1) extraction of template DNA from paulownia tissues; (2) PCR ampli- fication; and (3) analysis of the PCR products.
All procedures can be completed within 6 h:
2 h for DNA extraction; 3 h for the PCR; and 1 h for analysis of PCR products by agarose gel electrophoresis.
This PCR-based assay showed high sensitivity, and requirements for diseased tis- sue were minimized to about 1 ng DNA (Fig.
2). This method has approximately 75-fold higher sensitivity than dot hybridization, the sensitivity of which was 375 ng minimum as recorded in a previous study (Wu et al. 2002).
Some artificially grafted paulownia plants and resistant hybrids showed symptomless disease progress; however, the disease was still detectable by the PCR showing a specific PCR fragment (510 bp) in the electrophoretic analysis. In resistant hybrid testing (Table 1), hybrids nos, 1, 6, 7, 11, 13, 23, and 25 showed high resistance (without typical symptoms or dying), although PaWBP were still detectable.
These results show that the PCR-based assay established in this study is more sensitive than traditional dot hybridization in PaWBP detec- tion and is able to overcome difficulties, such as a low concentration and uneven distribution, for monitoring symptomless or infected plants.
Typically, PaWBP clusters in the phlo- em, but this study found high concentrations of PaWBP in the mesophyll, which is quite unusual. Moreover, it was also noted that PaWBP in paulownia first moved down to lower leaves and roots from the graft point and then to upper leaves (Fig. 3a). Different concentrations and movements of PaWBP in paulownia found in this study provide infor- mation which allow for a better PaWB dis- ease sampling strategy. We recommend tak- ing lower leaves rather than upper leaves for higher detection accuracy, especially in those plants with latent infections.
An insect vector was also examined and confirmed in this study using the PCR as- say (Fig. 5). PaWBP-free H. mista was used to acquire PaWBP by feeding on PaWBP- infected paulownia plants. Positive results with high sensitivity were obtained, show- ing that PaWBP was acquired via H. mista.
After PaWBP was confirmed in H. mista, these viruliferous bugs were then allowed to feed on PaWBP-free paulownia seedlings.
Paulownia plants that contacted viruliferous bugs were examined by the PCR and showed positive results (Fig. 5b) and witches’ broom symptoms 3 mo after grafting, proving that H. mista is able to carry and transmit PaWBP.
Although PaWBP-free seedlings derived from tissue culture are widely planted in the field, transmission caused by H. mista can still oc- cur in the field in the future. So a comprehen- sive paulownia health management program is recommended. Since paulownia hybrids showed higher resistance than purebred plants, this can be applied to PaWB disease control. Two major population peaks of H.
mista in the year, March to April and August to October, obtained by field tracking are im- portant information to consider in insect vec- tor control in the future as well.
LITERATURE CITED
Ahrens U, Seemuller E. 1992. Detection of DNA of plant pathogenic mycoplasma-like organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene.
Phytopathology 82:828-32.
Chen MF, Lin CP. 1997. DNA probes and PCR primers for the detection of a phytoplas- ma associated with peanut witches’-broom.
Eur J Plant Pathol 103:137-45.
Deng SL, Hiruki C. 1991. Amplification of 16S rRNA genes from culturable and noncul- turable Mollicutes. J Microbiol Meth 14:53-
61.
Doi Y, Teranaka M, Yora K, Asuyama H.
1967. Mycoplasma or P.T.L. group-like mi- croorganisms found in the phloem elements of plants infected with mulberry dwarf, potato witches’-broom, aster yellows or paulownia witches’-broom. Ann Phytopathol Soc Jpn 33:259-66.
Hung TH, Wu ML, Su HJ. 1999a. Detection of fastidious bacteria causing citrus greening disease by nonradioactive DNA probes. Ann Phytopathol Soc Jpn 65(2):93-100.
Hung TH, Wu ML, Su HJ. 1999b. Develop- ment of a rapid method for the diagnosis of citrus greening disease using the polymerase chain reaction. J Phytopathol 147:599-604.
Lee IM, Davis RE. 1988. Detection and in- vestigation of genetic relatedness among aster yellows and other mycoplasma-like organisms by using clone DNA and RNA probes. Mol Plant Microbe Interact 1:303-10.
Lee IM, Davis RE, DeWitt ND. 1990. Non- radioactive screening method for isolation of disease-specific probe to diagnose plant dis- eases caused by mycoplasma-like organisms.
Appl Environ Microb 56:1471-5.
Lee IM, Davis RE, Hammond R, Kirkpat- rick B. 1988. Cloned riboprobe for detection of a mycoplasmalike organism (MLO). Bio- chem Bioph Res Commun 155:443-8.
Lee IM, Gundersen DE, Hammond RW,
Davis RE. 1994. Use of mycoplasma-like organism (MLO) group-specific oligonucle- otide primers for nested-PCR assays to detect mixed-MLO infections in a single host plant.
Phytopathology 84:559-66.
Murashige T, Bitters WP, Rangan TS, Nau- er EM, Roistachek CN, Holliday PB. 1972.
A technique of shoot apex grafting and its utilization towards recovering virus-free citrus clones. HortScience 7:118-9.
Namba S, Kato S, Iwanami S, Oyaizu H, Shiozawa H, Tsuchizaki T. 1993. Detection and differentiation of plant-pathogenic myco- plasma-like organisms using polymerase chain reaction. Phytopathology 83:786-91.
Tsai JH, Chen XY, Shen CY, Jin KX. 1988.
Mycoplasmas and fastidious vascular prokary- otes associated with tree diseases in China. In:
Hiruk C, editor. Tree mycoplasmas and my- coplasma diseases. Alberta, Canada: Univ. of Alberta Press. p 69-96.
Tully JG. 1993. International Committee on Systematic Bacteriology Subcommittee on the Taxonomy of Mollicutes. Minutes of the In- terim Meetings; 1-2 Aug 1992, Ames, IA. Int J Syst Bacteriol 43:394-7.
Wu ML, Chang TT, Wang LC, Fu CH. 2002.
Rapid detection of phytoplasma associated with paulownia witches’-broom using a nonra- dioactive DNA probe and a PCR-based assay.
Taiwan J For Sci 17(2):123-33.