I
國立台灣大學醫學院微生物學研究所 碩士論文
Department of Microbiology College of Medicine
National Taiwan University Master Thesis
以小鼠模式探討年齡和腸道菌因子以及肝臟巨噬細胞 對 B 型肝炎病毒容忍性之影響
The role of liver macrophages in age and gut microbiota dependent HBV-persistent C3H/HeN mice
鄭吉宏
Chi-Hung Cheng
指導教授: 陳培哲 博士 王弘毅 博士
Advisor: Pei-Jer Chen, Ph.D Hurng-Yi Wang, Ph.D 中華民國一百零四年七月
July, 2015
I
Index
致謝 ... 1
國立臺灣大學碩士學位論文口試委員會審定書 ... 2
中文摘要 ... 3
ABSTRACT ... 5
CHAPTER 1: INTRODUCTION ... 8
1.1Nature history of hepatitis B infection ... 8
1.2 Animal models of hepatitis B virus infection ... 8
1.3 Mouse gut microbiota participated in liver immunity against HBV ... 10
1.4 The role of Kupffer cells in hepatitis B ... 12
1.4.1 The characteristics of Kupffer cells ... 12
1.4.2 The role of KCs during HBV infection ... 13
1.5 Hypothesis and specific aim ... 15
CHAPTER 2: MATERIAL AND METHODS ... 17
2.1 Materials ... 17
2.1.1 Antibodies ... 17
2.1.2 Reagents ... 18
2.2 Methods ... 18
2.2.1 Hydrodynamic HBV transfection mouse model ... 18
2.2.2 Method for isolation and enrichment of liver NPC ... 19
2.2.3 Flow cytometry analysis ... 20
2.2.4 Kupffer cell depletion ... 21
2.2.5 Sterilization of mouse gut microbiota ... 21
CHAPTER 3: RESULTS ... 22
3.1 The depletion of KC promoted HBV clearance of young C3H/HeN mice ... 22
II
3.2 Kupffer cell population of adult C3H/HeN mice dramatically decreased after transfection
of HBV ... 23
3.3 The delayed recovery of KCs in antibiotics-treated adult C3H/HeN mice after exposure to HBV ... 25
3.4 TLR4 contributed to the immune tolerance to HBV in young C3H ... 26
3.5 Kupffer cell population of young C3H/HeJ mice decreased after transfection of HBV ... 28
CHAPTER 4: DISCUSSIONS ... 29
CHAPTER 5: FIGURES ... 34
Figure 1. Map of HBV construct ... 34
Figure 2. Flowchart of KC depletion experiment. ... 35
Figure 3. The efficiency of KCs depletion by using clodronate liposome ... 37
Figure 4. HBV persistent rate of young C3H/HeN mice was reduced after the depletion of KCs ... 40
Figure 5. HDI time course of young C3H/HeN mice ... 44
Figure 6. HDI time course of adult C3H/HeN mice ... 47
Figure 7. Flowchart of antibiotic experiment ... 49
Figure 8. KCs of antibiotics-treated adult C3H/HeN mice decreased at day 3 after the exposure to HBV ... 52
Figure 9. The loss of TLR4 signaling resulted in dramatic reduction of HBV persistent rate. ... 54
Figure 10. Kupffer cell population of young C3H/HeJ mice decreased after the transfection of HBV ... 57
Figure 11. Gating strategy of liver macrophages ... 60
REFERENCE ... 61
1
致謝
首先我要感謝陳培哲老師,老師總是能精準地指出我真正遇到的問題並且引 導我遠離錯誤的道路,不僅在認識科學上、對我的人生以及生活態度真正地成長 和進步,很感謝老師當初願意選擇並且指導我。我也要感謝王弘毅老師這兩年半 的照顧,王老師總是能充滿熱誠地和學生討論實驗,其對科學追求的態度總是能 讓學生充滿動力。感謝謬希椿老師、楊宏志老師、葉秀慧老師在這段路途中給予 的指導。
很感謝我的研究生生活有佳宏學長、友瑜學長、示崙學姐以及維弘的陪伴,
他們是我重要的精神支柱和學習對像,在我經歷茫然且無知時刻給我方向,也感 謝和我負責同一個題目的莉玲學姐和佩瑄在實驗上的幫助,我們在學習分離和分 析肝臟巨噬細胞的時候遭遇許多困難和挫折,特別是在流式細胞儀的應用上投入 大量的時間和心力,最後也成功解決許多問題。特別感謝昭皓學長,學長總是很 有耐心地讓我問問題,並且給予我指導。感謝我的家人支持我念研究所,感謝所 有的同學和支持我的朋友。最後感謝這段過程犧牲無數的小生命。
2
國立臺灣大學碩士學位論文口試委員會審定書
3
中文摘要
慢性 B 型肝炎在全球約有 3 億的慢性帶原者,患者發生感染時的年齡與慢性 B 肝型肝炎的發展有很大的關聯性,其中有高於 90%的新生兒會發展成慢性帶原 者,而 5 歲以後成為帶原者的機率只有 5~7%。在過去的研究中,我們發現利用 高壓尾靜脈注射使 C3H/HeN 小鼠模擬 B 型肝炎病毒感染,可以依據小鼠年齡產 生兩種不同的免疫反應。6 周歲的小鼠對 B 型肝炎病毒產生免疫容忍,無法產生 有效的免疫反應清除病毒,12 周歲小鼠則可在 6 周內清除病毒,然而 12 周歲小 鼠清除 B 型肝炎病毒的能力會因為使用抗生素去除腸道菌而降低。觀察小鼠的腸 道菌發現,腸道菌的菌相會隨著年齡變化,到 12 周歲時會達到穩定。許多研究 顯示,肝臟的免疫環境會根據外來抗原產生變化,特別是從腸道來的細菌代謝產 物會使肝臟成為免疫容忍狀態,降低外來抗原引起的免疫反應,因此我們推論在 C3H/HeN 小鼠模型中,6 周歲小鼠因為當時的腸道菌抗原造成較強的免疫容忍使 其無法有效清除 B 型肝炎病毒,而 12 周歲小鼠的腸道菌因為組成和 6 周歲小鼠 不同,其產生的抗原組成也有差異,進而造成肝臟的免疫環境足以產生有效的免 疫反應來清除病毒。庫佛氏細胞在許多的研究中被認為是造成肝臟免疫容忍的重 要免疫細胞,它會過濾外來抗原並產生免疫調控因子來調節免疫反應。我們將 6 周歲 C3H/HeN 小鼠的庫佛氏細胞剃除後,發現小鼠有效地清除病毒。接著我們 觀察 6 周歲和 12 周歲小鼠在接觸 B 型肝炎後肝臟巨噬細胞族群的變化情形,發 現在 12 周歲小鼠以高壓尾靜脈注射打入 B 型肝炎病毒質體後第二天,庫佛氏細 胞會大量消失,而 6 周歲小鼠仍保有許多庫佛氏細胞。6 周歲 C3H/HeJ 小鼠為 4 號類鐸受體突變並且失去功能的品系,我們推測其可能具有較差的肝臟免疫容 忍,並證實其的有快速清除 HBV 的能力。同時我們也觀察到 6 周歲 C3H/HeJ 小 鼠在高壓尾靜脈注射 B 型肝炎病毒質體後的 18 小時以及第 2 天,庫佛氏細胞也 大量消失。由此結果推論,小鼠會藉由剔除庫佛氏細胞來破壞肝臟免疫容忍,進 而使肝臟的環境易於產生較強的免疫反應來對抗病毒。最後我們發現腸道菌剃除
4
的 12 周歲 C3H/HeN 小鼠,其庫佛氏細胞在高壓尾靜脈注射 B 型肝炎病毒質體後 的第三天大量消失,此時間點在 6 周和 12 周歲 C3H/HeN 小鼠以及 6 周歲
C3H/HeJ 小鼠,庫佛氏細胞族群已經回復大多數。因此我們推論高壓尾靜脈注射 B 型肝炎病毒質體後的第三天,新增的庫佛氏細胞可能和原庫佛氏細胞具有不同 的功能,進而幫助清除 B 型肝炎病毒。
綜合以上結果,我們發現庫佛氏細胞的消失對於小鼠清除 B 型肝炎病毒可能 為必要的過程。B 型肝炎病毒透過何種抗原引起庫佛氏細胞的消失,以及庫佛氏 細胞是如何接收到 B 型肝炎病毒的訊息是未來重要的研究方向。
5
ABSTRACT
Approximately 300 million people are HBV carrier. Mechanisms leading to
hepatitis B virus (HBV) persistence is still unclear. One of important factor is the age of
HBV infection. More than 90% of newborns or infants who acquire HBV infection
become HBV carriers. The risk is reduced to 25~30% in 0~5 years old child and 5~7%
after 5 years old. In our previous studies, we established age-related HBV clearance
mice model on C3H/HeN mice and indicated that gut microbiota might be an important
factor in HBV clearance. It is known that the composition of gut microbiota can change
with age and become stable in adult C3H/HeN mice. We suppose that foreign antigens
especially derived from gut microbiota can shape liver immune environment into
immune tolerance to HBV in young C3H/HeN mice. Whereas, Gut microbiota in adult
C3H/HeN provides difference signals from young C3H/HeN mice to influence liver
environment and contribute to interrupt liver tolerance at the exposure to HBV. Kupffer
cell (KC) is known for its immune regulatory effect in liver tolerance. Therefore we
depleted KCs of young and adult C3H/HeN mice before transfection of HBV and found
that the depletion of KCs can promote HBV clearance in young C3H/HeN mice. It
indicates that the interrupt of liver tolerance may be an important pathway to clear HBV.
Next, we investigated the population of liver macrophages in C3H/HeN mice. The
population of liver macrophages can be divided into two subset, infiltrating
6
macrophages and KCs, by CD11b and F4/80. We found that KCs dramatically
decreased and massive macrophage infiltration after hydrodynamic injection (HDI) of
pAAV/HBV1.2 in adult C3H/HeN mice. The change of liver macrophage population
was completely restored at day 9 after HDI. However, the reduction of KC in young
C3H/HeN mice was not notable than adult C3H/HeN mice and mainly caused by the
effect of HDI. These results support our hypothesis that adult C3H/HeN mice have the
ability to interrupt liver tolerance by reduction of KC at the exposure to HBV. We have
the similar result on C3H/HeJ mice which are TLR4-deficient strand. Young C3H/HeJ
mice have the ability to clear HBV that may be due to the weak liver tolerance causing
by the loss of TLR4 signaling. As our previous study, the ability of HBV clearance can
be partially disrupted by antibiotic treatment in adult C3H/HeN mice. We further
investigated the population of liver macrophages in antibiotic-treated adult C3H/HeN
mice and found that the population of KCs was significantly reduced compared with young and adult C3H/HeN mice which didn’t received antibiotic treatment. It indicates
that the function of KCs at day 3 after the exposure of HBV are opposite to the original
KCs before HDI and may promote HBV clearance.
In conclusion, our results suggest that the reduction of KCs is important to promote
HBV clearance. It may be due to the interruption of liver tolerance that make liver
environment trend to induce stronger innate immune response to against HBV. In the
7
future, to find out what antigens of HBV promote the reduction of KCs is a top priority.
That will provide a clue to further investigate the mechanism of age-related immune
response to HBV.
8
CHAPTER 1: INTRODUCTION
1.1 Nature history of hepatitis B infection
Hepatitis B is one of the most common infectious disease and linked to increased
risks of liver cirrhosis and hepatocellular carcinoma. According to the report of World
Health Organization (El Kasmi et al.), more than 2 billion people have been infected
with HBV, and 240 million people among them are chronic carriers (Cowie, Carville, &
Maclachlan, 2013). These chronic HBV carrier have higher probability of developing
liver cirrhosis and hepatocellular carcinoma. The mechanism leading to HBV
persistence is unclear. So far as we known, despite the diversity of host genetics, a more
important and non-genetic factor has been the age of the HBV exposures (Chen, 1993).
More than 90% of infants who acquire HBV infection becoming chronic HBV carrier.
The risk was reduced to 25~30% in 0~5 years old child and 5~7% after 5 years old
(Hyams, 1995). It is postulated that the higher risk of developing chronic HBV infection in young age is due to the “immune system immaturity” and “neonate tolerance”.
However, the mechanisms of age-dependent HBV clearance have not yet been
identified.
1.2 Animal models of hepatitis B virus infection
The nature host of HBV include human and great apes including chimpanzee, gibbon,
gorilla and orangutan (Lyons et al., 2012). Some surrogate animal such as woodchuck for
9
WHV, Pekin duck for DHBV, tamarins for GB virus A and B, ground squirrel for GSHV
also used in HBV study. These animal models mostly don’t have inbred strain and lack
commercially immunological reagents to maintain the quality of studies. Therefore, these
models are full of limitation to investigate the mechanisms of pathogenesis of viral
hepatitis. Small rodent models are more practical in studies, but they can’t naturally be
infected by HBV. HBV transgenic mice can be an option to investigate the replication,
gene expression, and immunopathogenesis of HBV, but there still are many limitations in
HBV transgenic mice. HBV in HBV transgenic mice lack the early event of their life
cycle including viral entry and cccDNA formation. Most transgenic mouse models are
tolerant to viral anti gens and fail to induce immune response to HBV (Guha, Mohan,
Roy-Chowdhury, & Roy-Chowdhury, 2004). Hydrodynamic tail vein injection (HDI) is
a simple, easy to manipulated technique for deliver naked DNA to rodent hepatocyte.
Hydrodynamic injection of approximately 8% of body weight by volume in a short period
of time causes the enhanced intravascular pressure and the force of fluids induces
structural changes in the liver. The structural change includes enlargement of liver
fenestration and generation of transient pores in the plasma membrane, facilitates nucleic
acids delivery into hepatocytes (Suda, Gao, Stolz, & Liu, 2007). In previous studies, the
non-transgenic immunocompetent mouse model for long-term expression of HBV by
HDI was established (L.-R. Huang, H.-L. Wu, P.-J. Chen, & D.-S. Chen, 2006). This
10
model allows HBV expression in certain mouse for over 1 year and produces detectable
HBV specific cellular and humoral immune response. Base on HDI, our previous studies
established an age-related HBV clearance C3H/HeN mouse model. Young (6 weeks old)
C3H/HeN mice can express detectable Hepatitis B surface antigen (HBsAg) and HBV
genome over 25 weeks, whereas adult (12 weeks old) C3H/HeN mice clear HBV within
6 weeks after HDI (Chou et al., 2015). This age-dependent HBV clearance pattern of
C3H/HeN mice provides us an opportunity to investigate why human child have more
chance to become HBV carrier after exposure to HBV.
1.3 Mouse gut microbiota participated in liver immunity against HBV
Growing evidences support that gut microbiota can regulate host immune response.
The direct exposure of liver to the metabolites and PAMPs from gut microbiota is likely
to shape the liver immunity and regulate the immune response. For instance, dietary or
genetic obesity induces alterations of gut microbiota, thereby increasing the levels of
deoxycholic acid, a gut bacterial metabolite known to cause DNA damage. The
enterohepatic circulation of DCA provokes senescence-associated secretory phenotype in
hepatic stellate cells, which in turn secretes various inflammatory and tumour-promoting
factors in the liver, thus facilitating HCC development in mice after exposure to chemical
carcinogen (Yoshimoto et al., 2013). For another example, liver sinusoidal endothelial
11
cells are organ-resident, non-myeloid APC capable of cross-presenting soluble exogenous
antigen to CD8+ T cells. As uptake of circulating antigens into LSEC is likely cross-
presentation and contributes to CD8+ T cell tolerance observed in situations where
soluble antigen is present in the circulation (Limmer et al., 2000). It was reported that the
composition of gut microbiota can influence liver immunity. A study found that aside
from dysbiosis of the gut microbiota, gut microbiota-mediated inflammation of the
intestinal mucosa and the related impairment in mucosal immune function play an
important role in the pathogenesis of NAFLD (Jiang et al., 2015).
The correlation between gut microbiota and HBV infection is rare to report. In our
previous study, we found that the profile of gut microbiota dynamically changed during
6 to 12 weeks old in C3H/HeN mice, and became stable after 12 weeks old. The adult
C3H/HeN mice which gastrointestinal tracts were sterilized by antibiotic treatment failed
to clear HBV, whereas the control group without antibiotic treatment cleared HBV
approximately in 6 weeks after HDI. These result inspired us to try to find out how gut
microbiota involved in the process of HBV clearance or persistence. It might be an
important clue to unravel the age-dependent immune response to HBV.
12
1.4 The role of Kupffer cells in hepatitis B 1.4.1 The characteristics of Kupffer cells
Kupffer cells (KCs) are tissue-resident macrophages residing in the liver and
located in the liver sinusoids. They are the largest population of innate immune cell in
the body. Functionally, KCs are well known for their scavenging ability, thereby
removing protein complexes, small particles, and apoptotic cells from circulation. The
constant exposure of liver cells to various gut-derived commensal microbes and food-
borne antigens from the portal circulation has led to the concept that the liver is
immunologically tolerant under steady-state conditions in the absence of pathogenic
challenge (Gorczynski, 1992). To initiate and maintain these tolerant responses, Kupffer
cells are considered to play an important role by creating an immunosuppressive local
environment in the liver. Under steady-state conditions, the spontaneous or induced
production by Kupffer cells of IL-10, TGF-β and prostaglandins has been suggested to
suppress immunity (You, Cheng, Kedl, & Ju, 2008). But with the advance of flow
cytometry, mouse liver macrophages were divided into two subsets, cytokine-producing
CD11b high F4/80 low cells and phagocytic and ROS-producing CD11blow F4/80high cells.
Based on their properties, the CD11b high F4/80 low macrophages are identified as
circulating macrophage which supplied from blood circulation and enhanced infiltration
and express inflammatory cytokines when encountering pathogen or the damage of
13
liver. CD11blow F4/80high macrophages are identified as liver resident macrophages,
Kupffer cells. Under steady-state conditions KCs are specialized as phagocytes to clear
and degrade particulates and play a limited immunoregulatory role via the release of
soluble mediators, such as IL-10 (Ikarashi et al., 2013; Movita et al., 2012). KCs and
most other tissue-resident macrophages are derived from erythro-myeloid progenitors
which develop in yolk sac. Most circulating blood cell including circulating
macrophages are derived from haematopoietic stem cells distinct from erythro-myeloid
progenitors which occur earlier (Gomez Perdiguero et al., 2015).
1.4.2 The role of KCs during HBV infection
Evidence for productive HBV infection of cells other than hepatocytes is lacking.
Also, detailed information on the presence of HBV or HBV derived protein in KC in
vivo or the uptake of HBV or its proteins by human KC ex vivo has not been reported.
Some studies indicate that HBV antigens can interact with KC by the cross-presentation
of other innate immune, such as dendritic cell. HBV antigens can also be recognized by
surface receptor of KCs. For instance, HBsAg can interact with human blood
monocytes in a CD14-dependent fashion, and with dendritic cells via the mannose
receptor, which are both receptors known to be also expressed on KC (Op den Brouw,
Binda, Geijtenbeek, Janssen, & Woltman, 2009; Vanlandschoot et al., 2002).
Pretreatment of non-parenchymal cells including KC, with HBV-Met cell-derived
14
supernatants, HBsAg, HBeAg, or hepatitis B virions almost completely abrogated TLR-
induced anti-viral activity, i.e., IFNβ production, interferon-stimulated gene induction,
IRF3, NF-κB, and ERK1/2 expression (Wu et al., 2009). In vivo, TLR2 expression by
KC and peripheral blood monocytes in HBeAg-positive chronic HBV-infected
individuals was lower than that in HBeAg-negative patients and controls. TLR2 ligation
induced less IL-6 and TNF in those HBeAg-positive patients (Visvanathan et al., 2007).
KCs are constantly exposed to pathogen-derived products from the gut. To prevent excessive inflammation and pathology of the liver, continuous activation of KC is
avoided as these cells become refractory to subsequent endotoxin challenge, a
phenomenon known as endotoxin-tolerance. This contributes to the well-described
tolerogenic phenotype in the liver (Biswas & Lopez-Collazo, 2009; Saeed et al., 2014).
In HBV-carrier mice, KCs expressed more IL-10 and mediated the systemic tolerance
induction in an IL-10-dependent manner (Xu, Yin, Sun, Wei, & Tian, 2014). However
little information about how KCs interact with HBV in HBV clearable model. In some
studies, KCs can be depletion induced by pathogen derived antigen to change the
environment of liver immunity. For instance, the infection by Listeria monocytogenes
induced the early necroptotic death of Kupffer cells, which was followed by monocyte
recruitment and an anti-bacterial type 1 inflammatory response. The death of KCs is
therefore a key signal orchestrating type 1 microbicidal inflammation and type-2-
15
mediated liver repair upon infection (Bleriot et al., 2015). It’s a hint to clarify how KCs
work in HBV persistence/clearance model.
1.5 Hypothesis and specific aim
We focus on innate immune response to HBV that determine the development of
adaptive immunity against HBV. With the advantage of age-related HBV C3H/HeN
mice model, we can investigate the early stage of HBV exposure in young and adult
C3H/HeN mice. As mentioned above that foreign antigens derived from gut microbiota
can contact and prime KCs into immune-tolerant phenotype through portal vein.
Immune-tolerant phenotype KCs can suppress innate immune response by expressing
immune regulatory cytokine when encountering foreign antigens and shape the liver
immunity into immune-tolerant environment. But some foreign antigens derived from
gut microbiota have the ability to help liver immunity to induce strong innate immunity
through unknown pathway. The environment of liver immunity are determined by the
dominative signal of foreign antigens which decide by the composition of gut
microbiota. In young C3H/HeN mice, we suppose KCs can suppress the innate
immunity against HBV. As the composition of gut microbiota changing and becoming
stable in adult C3H/HeN mice, the environment of liver immunity can suppress
immune-tolerant KCs in unknown pathway when encountering HBV and induce
16
effective innate immune response to trigger adaptive immunity to clear HBV. Our
specific aim is to investigate how KCs involve in liver immunity at the exposure to
HBV.
17
CHAPTER 2: MATERIAL AND METHODS
2.1 Materials
2.1.1 Antibodies
Antibody Source
anti-mouse Ly-6c- APC-Cy7 (AL-21) BD PharMingen, San Diego, CA, USA
anti-mouse F4/80-PerCP/Cy5.5 (BM8) Biolegend, San Diego, CA, USA
anti-mouse/human CD11b-FITC (M1/70) Biolegend, San Diego, CA, USA
anti-mouse Ki-67-PE (16A8) Biolegend, San Diego, CA, USA
rat anti-mouse CD45-APC (30F-11) BD PharMingen, San Diego, CA, USA
anti-mouse I-A/I-E-PE/Cy7 (M5/114.15.2) Biolegend, San Diego, CA, USA
18
2.1.2 Reagents
Reagent source
HBSS (14175) Invitrogen, Carlsbad, USA
HBSS (14025) Invitrogen, Carlsbad, USA
Type IV collagenase (C5138) Sigma, St.Louis, USA
Percoll GE Healthcare
Ampicillin sodium salt (A9518) Sigma, St.Louis, USA
Vancomycin hydrochloride (V2002) Sigma, St.Louis, USA
Neomycin trisulfate salt hydrate (N1876) Sigma, St.Louis, USA
Metronidazole (M3761) Sigma, St.Louis, USA
Bovine Serum Albumin (A9430) Sigma, St.Louis, USA
2.2 Methods
2.2.1 Hydrodynamic HBV transfection mouse model
Male C3H/HeN mice were purchased from National Laboratory Animal Center (5~6
weeks young mice and 12~14 weeks adult mice), and Male C3H/HeN mice were
purchased from Jackson Laboratory. Animals were kept in National Taiwan University
College of Medicine Laboratory of Animal Center under specific pathogen-free
conditions. All mice were used according to the guidelines for experimental animal use
19
from the National Taiwan University College of Medicine. HBV expression plasmid
pAAV/HBV1.2 (genotype A) was described previously (L. R. Huang, H. L. Wu, P. J.
Chen, & D. S. Chen, 2006) and known to express vigorously in mouse hepatocyte. Before
HDI, mice were anesthetized with a mixture of ketamine (Merial, France) and Rompun
(Bayer AG, Germany). 10 μg of HBV plasmid was injected into the tail vein of mouse in
a volume of phosphate buffered saline (PBS) equivalent to 8% of the mouse body weight.
The injection time was approximately controlled in 5 seconds. Serum HBsAg level was
determined by using the Roche Cobas e system kit (Roche Diagnostics, USA). The HBV
clearance was determined when the S/N ratio of HBsAg in serum was less than 10.
2.2.2 Method for isolation and enrichment of liver NPC
Mice were anesthetized with ketamine and Rompun before invasive process. The
chest and abdomen were open. Portal vein was cannulated with intravenous catheter and
then gripped by micro-clips. Inferior vena cava (IVC) above the liver was clamped by
micro-clips. First, after cutting the IVC below the liver, liver was perfused with 50mL
calcium-magnesium-free Hank’s balanced salt solution (HBSS) and following 45mL
HBSS containing 0.04% collagenase IV. The flow rate was 5 mL/min. Second, whole
liver was excised and disrupted in HBSS until the liver cell was fully suspended in the
solution. The liver cell suspension was collected with HBSS and filtered through 70μm
20
nylon mesh to final volume 50mL. Next, cell suspension was centrifuged at 50xg for 5
minutes at 4℃. Since most of the cells in the pellet are heparocyte, the supernatant
containing non-parenchymal cells (NPCs) was collected and centrifuged at 500xg for 10
minutes at 4℃. The pellet containing NPC was resuspended in 4mL HBSS and gently
layered on top of discontinuous percoll gradient (25% / 50%) and centrifuged at 1000xg
for 20 minutes at 25℃. NPCs were collected from the interphase of 25% / 50% percoll.
Cells were collected, washed with 15mL HBSS, and centrifuged at 500xg for 10
minutes at 4℃. Pelleted NPCs were counted and resuspended in HBSS prior to
experiment.
2.2.3 Flow cytometry analysis
For surface antigen analysis by flow cytometry, cells was incubated with anti-
mouse CD16/CD32 (BD mouse Fc block) for 5 minutes at 4℃ to prevent Fc receptor
mediated non-specific binding. Next, cells were stained with appropriate amount of
fluorochrome-conjugated antibodies (according to recommendation of antibody
datasheet) and incubated for 30 minutes 4℃. Cells were washed twice by 2 mL wash
buffer (1 % BSA in PBS) then centrifugation at 500xg for 3 minutes at 4℃. After last
wash step, the cells were fixed with 4% paraformaldehyde in PBS for 10 minutes. Data
were acquired by BD FACSVERSE and analyzed by FlowJo. KC-enriched NPC were
21
divided into two populations, infiltrating macrophage and KCs, by CD11b and F4/80
according to previous study (Fig. 12) (Ikarashi et al., 2013).
2.2.4 Kupffer cell depletion
Kupffer cells were depleted by one intravenous injection of 200μL (6-week-old mice) or 240μL (12-week-old mice) clodronate-containing liposomes (5 mg/mL
clodronate; Encapsula Nano Sciences) or control liposomes (Encapsula Nano Sciences)
2 day before HDI. The efficiency of Kupffer cell depletion were evaluated by flow
cytometry 2 days after the injection of clodronate liposomes or control liposomes.
2.2.5 Sterilization of mouse gut microbiota
The mouse gastrointestinal tract was sterilized by a well-established antibiotic
cocktail protocol from 5- to 12-week-old. The antibiotic cocktail comprised ampicillin
sodium salt (1g/L), neomycin trisulfate salt hydrate (1g/L), vancomycin hydrochloride
from Streptomyces orientalis (0.5g/L) and metronidazole (1g/L) dissolved in drinking
water (Rakoff-Nahoum, Paglino, Eslami-Varzaneh, Edberg, & Medzhitov, 2004).
22
CHAPTER 3: RESULTS
3.1 The depletion of KC promoted HBV clearance of young C3H/HeN mice
In our hypothesis, we supposed that KCs play the important role in immune
tolerance to HBV in young C3H/HeN mice. Therefore, we designed the KC depletion
experiment on young (6 weeks old) C3H/HeN mice and Adult (12 weeks old) C3H/HeN
mice to test our hypothesis. First, the KCs were depleted by one intravenous injection of
clodronate liposome two days before HDI of pAAV/HBV1.2 (Fig. 2). The percentage of
KCs were reduced from 33% to 9% 2 days after injection of 200μL clodronate liposome
in young C3H/HeN mice. The cell number of KCs was reduced from 1.25 x 106 to 2.59
x 104. In adult C3H/HeN mice, 240μL clodronate liposome were injected and resulted in
the decrease of KCs from 60.1% to 4.5%. The cell number was reduced from 1.7 x 106
to 3.1 x 104 (Fig. 3). Next, the mice were transfected pAAV/HBV1.2 by HDI at second
day after depletion of KCs. HBsAg in serum of mice was detected to acquire the HBV
persistent rate. We found that the HBsAg of KC-depleted young C3H/HeN mice were
almost cleared 20 weeks after HDI, whereas the persistent rate of HBsAg in control
groups were still have more than 70% (Fig. 4AB). In adult C3H/HeN mice, there is no
difference between the KC-depleted group and control group (Fig. 4CD).
23
3.2 Kupffer cell population of adult C3H/HeN mice dramatically decreased after transfection of HBV
As previous study, the M2-like phenotype of KCs underwent necroptosis after
Listeria Monocytogenes infection. M1-like Circulating monocytes and macrophages were
recruited to liver and replenished KCs. The new KCs replenished from circulating
macrophages were M1-like and developed into M2-like KCs along with the Listeria
Monocytogenes clearance. This dynamic change of liver macrophages is necessary for
Listeria Monocytogenes clearance (Bleriot et al., 2015). In view of this concept, we
supposed that adult C3H/HeN mice might have the ability to induce the reduction of M2-
like KCs after exposure to HBV. The reduction of M2-like KCs suggested the decrease
of immune-tolerant effect to HBV and was similar to the KC depletion experiment
resulting in the HBV clearance. Whereas the population of KCs in young C3H/HeN mice
might not have the HBV-specific change since they were immune-tolerant to HBV. We
isolated KC-enriched NPCs from C3H/HeN mice and analyzed the populations of liver
macrophages by flow cytometry after HDI. To discriminate the results from the effect of
HDI and (or) foreign antigen (pAAV/HBV1.2), we investigated liver macrophages after
the HDI of pAAV/HBV1.2, empty plasmid or PBS, respectively. After gated on CD45+,
liver macrophages were divided into two populations, CD11bhigh F4/80low and CD11blow
F4/80high. According to previous studies, KCs belonged to the CD11blow F4/80high
24
population, and the infiltrating macrophages were the CD11bhigh F4/80low population
(Movita et al., 2012). In baseline condition of young C3H/HeN mice, KC, the major
population of liver macrophage, was about 65.5% of the CD45+ cells and infiltrating
macrophage was 5.3%. At the 18 hours after HDI, the population of KCs decreased to
32.3% and the population of infiltrating macrophages increased to 53.3%. The dramatic
raise of infiltrating macrophages suggested the damage that caused by HDI since there
was no significant difference in any group (Fig. 5AB). The populations of liver
macrophages at day 2, day 3 and day 9 after HDI showed that the KCs started to regain
and infiltrating macrophages reduced (Fig. 5CDE). The percentage were close to the
baseline at day 9. This result suggested that the dynamic change of liver macrophages
was caused by HDI and there is no HBV-specific effect in young C3H/HeN mice since
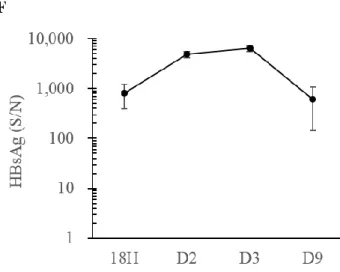
the titers of HBsAg were still high at day 9 (Fig. 5F). In adult C3H/HeN mice, the
population of KCs dramatically decreased to 1.04% at day 2 after HDI of pAAV/HBV1.2,
whereas the vector and PBS control groups still had 18.4% and 55.1% respectively (Fig.
6AC). This reduction of KCs was transient and recovered partially at day 3 after HDI (Fig.
6D). At day 9, the populations of liver macrophages totally recovered and were similar to
the baseline (Fig. 6E). These results indicated that adult C3H/HeN mice could induced
the reduction of KCs after the exposure to HBV, whereas young C3H/HeN mice failed to.
The massive infiltration of circulating macrophages into liver might not be the critical
25
part that lead to the clearance of HBV because there were still a lot of infiltrating
macrophages in young C3H/HeN after HDI. The dramatic decrease of KCs might be the
important step that promoted HBV clearance since more and more evident indicated the
immune-tolerant role of KC. After the loss of vast KCs, infiltrating macrophages must
differentiate and replenish KCs. These newly KCs might also be one of the candidates
that contributed to HBV clearance. However, the different fates of KCs between HBV-
transfected adult and young C3H/HeN mice could be the clue to unravel the age-
dependent immune response against HBV.
3.3 The delayed recovery of KCs in antibiotics-treated adult C3H/HeN mice after exposure to HBV
The previous study showed that the gut microbiota-depleted adult C3H/HeN mice
increased the HBV persistent rate to approximately 50% (Chou et al., 2015). In other
words, about half of the adult C3H/HeN mice which had the ability to clear HBV
efficiently became immune-tolerant to HBV after the sterilization of gut microbiota by
antibiotic treatment. This consequence inspired us to investigate whether there was any
correlation between gut microbiota and KCs that interfere in the immune response to
HBV. We prepared the antibiotics-treated C3H/HeN mice based on the previous
research to investigate the populations of liver macrophages (Fig. 7A). The populations
26
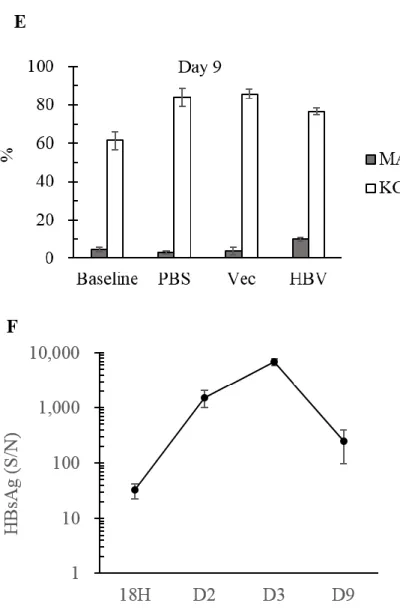
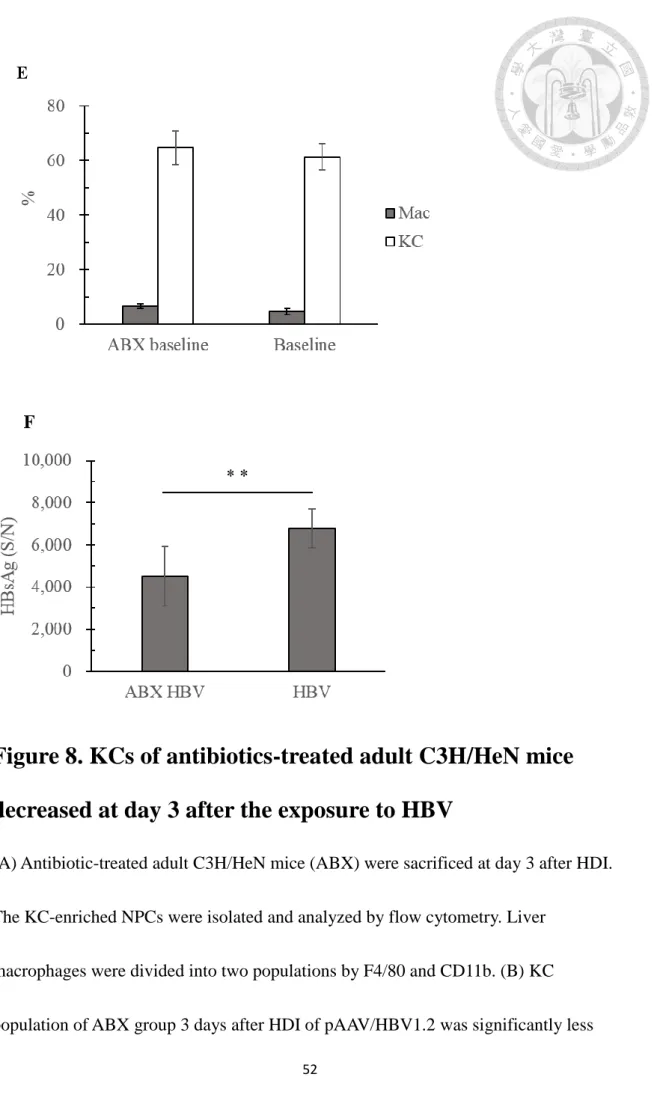
of liver macrophages day 3 after HDI were acquired (Fig. 8AB). The populations of
liver macrophages from adult C3H/HeN mice with or without antibiotic treatment were
not significantly different (Fig. 8E). However, the population of KCs from antibiotics-
treated mice was significantly reduced after transfection of HBV compared with young
and adult C3H/HeN groups that didn’t receive antibiotic treatment. Its population of
infiltrating macrophages was higher than the others (Fig. 8CD). In young and adult
C3H/HeN groups, we observed that the populations of liver macrophages were partially
recovered at day 3 compared with the population at 18 hours and day 2. This result
indicate that the antibiotic treatment might interfere infiltrating macrophages in
differentiation into KCs after the reduction of KCs caused by HBV. However, we didn’t
confirm this consequences were caused by the direct effect of antibiotics or the
depletion of gut microbiota. According to the previous study, before becoming M2-like
macrophages, KCs could transiently express pro-inflammatory cytokines after the
differentiation from infiltrating macrophages (Bleriot et al., 2015). It is unclear how
KCs involved in the innate immunity upon exposure to HBV at this vague stage of
differentiation. Antibiotic treatment could influence vast aspects. It might be an
important clue to link the gut microbiota and the innate immunity against HBV.
3.4 TLR4 contributed to the immune tolerance to HBV in
27
young C3H
According to the previous study, prior exposure of monocytes/macrophages to
minute amounts of endotoxin cause them to become refractory to subsequent endotoxin
or other antigen, such as tripalmitoyl glyceryl cysteine, challenge. They produced less
proinflammatory mediators, such as TNF-α and interleukin-6 than unprimed naïve
macrophages (Biswas & Lopez-Collazo, 2009; Saeed et al., 2014). Liver is a special
organ that can be directly exposed to LPS and other foreign antigens from gut
microbiota. Furthermore, the liver has a large number of macrophage lineage cells,
Kupffer cells, which make up 80% of the total body macrophages in human (Doherty &
O'Farrelly, 2000). We next asked whether TLR4 signaling was a factor that caused
immune tolerance to HBV in young C3H mice. The 6-week-old C3H/HeJ (TLR4
signaling deficient) mice were transfected HBV by HDI. The follow-up of HBsAg in
serum showed the significant improvement of HBV clearance compared to young
C3H/HeN mice (Fig. 9). The HBV persistent rate of young C3H/HeJ mice was less than
10% 8 weeks after HDI. This result suggested that TLR4 signaling might be a
dominative factor that shaped the liver immunity of young C3H/HeN mice toward
immune tolerance to HBV. However, it could be merely another immune activated
pathway. The loss of TLR4 signaling which seemed to have immune-tolerant effect on
macrophages might strengthen the innate immune response to promote HBV clearance.
28
That could veiled the real factors that caused the age-related immune response against
HBV.
3.5 Kupffer cell population of young C3H/HeJ mice decreased after transfection of HBV
Based on the efficient clearance of HBV in C3H/HeJ mice, we next asked whether
the decrease of KCs after HBV transfection would occur in C3H/HeJ mice. First, we
compared the populations of liver macrophages in the baseline of young C3H/HeJ with
young C3H/HeN (Fig. 10A). The population of KCs from young C3H/HeJ was slightly
less but significant than young C3H/HeN. The population of infiltrating macrophages
from C3H/HeJ were higher than C3H/HeN. We found that KCs dramatically reduced to
less than 5% two days after HDI of pAAV/HBV1.2 and then partially restored at day 3
(Fig. 10B). This phenomenon was similar to what we previously found in adult
C3H/HeN mice. The most distinguishable discrepancies between adult C3H/HeN,
young C3H/HeJ and young C3H/HeN were at 18 hours and day 2 (Fig. 10CD). The
dynamical change of liver macrophage supported the result of KC depletion experiment
which we supposed KCs played the immune-tolerant role in our HBV mice model.
29
CHAPTER 4: DISCUSSIONS
In our study, we tried to find out the mechanism of age-related immune response
against HBV by using HBV-transfected C3H/HeN mouse model because of the age-
dependent HBV clearance of C3H/HeN mice. KC is a specialized tissue resident
macrophage that contributes to liver tolerance which has the ability to decrease the
inflammation causing by foreign antigen from portal vein or hepatic artery. In our
previous studies, we found that the dynamically changed composition of gut microbiota
was correlated with the age of C3H/HeN mice and the age-related HBV clearance in
C3H/HeN mice. We further supposed that gut microbiota which was suggested to shape
liver immunity in many references might involve in the process of immune response
against HBV. Based on these, we speculated whether the interaction between KCs and
gut microbiota could influence the outcome of HBV transfection in C3H/HeN mice.
However, to investigate innate immunity about HBV in our mouse model, there are
many disturbances caused by hydrodynamic injection. For this reason, we must examine
our data carefully and rule out the artificial factors.
It’s difficult and complicated to confirm the role of KCs in the process of immune
response during the HBV exposure. To begin with, we indicate that KCs may cause the
immune-tolerant effect in young C3H/HeN mice during the exposure of HBV in the
KCs depletion experiment. But the depletion of KCs could mere be a therapeutic
30
treatment that indirectly compensate the immune-tolerant pathway from HBV but not
directly disrupt it. We didn’t know how KCs involved in HBV clearance in adult
C3H/HeN mice. Therefore, we tried to investigate whether the population of liver
macrophages changed after the exposure of HBV. We supposed that HBV couldn’t
induce notable immune response in young C3H/HeN mice because of the HBV
tolerance. The dynamic change of liver macrophage populations can be referred to the
effect of hydrodynamic injection. The results of control groups also support this
concept. Compared with the young C3H/HeN mice, the adult C3H/HeN and young
C3H/HeJ mice can be induced the reduction of KCs especially 2 days after HBV
transfection. These results correspond with the experiment of KC depletion. We
consider the reduction of KCs is an immune activated process that led to the clearance
of HBV by adaptive immunity ultimately. The next question is how does HBV induce
the reduction of KCs. It can be through direct or indirect contact between KC and HBV.
To further investigate the correlation between KC and HBV, the mutant HBV can be
applied. For instance, the core-null HBV or HBCY132A mutant HBV which are known
for the high HBV-persistent rate in mouse model. The intact core antigen may be a
candidate that induces KCs reduction. We also need to further investigate whether there
is any difference which may cause distinguishable fates of KCs between the steady-state
KCs in HBV clearable group and HBV tolerant group.
31
In our previous study, after antibiotic treatment, about 50% of adult C3H/HeN
mice became tolerance to HBV. We investigated the populations of liver macrophages
from ABX treated mice and found the significant reduction of KCs at day 3 after HBV transfection compared to other group that didn’t receive ABX treatment. This result
indicates that the restoration of KCs at day 3 after HBV transfection may be an
important step to HBV clearance. KCs that differentiate from infiltrating macrophages
can transiently express inflammatory cytokines before becoming to M2-like
macrophages (Bleriot et al., 2015). The function of KC at this vague stage of
differentiation is unclear. To confirm this question, we can investigate the population of
KCs in the experiment of KC depletion. The restoration of KCs at day 3 after HBV
transfection should be observed if it is important for HBV clearance.
In young C3H/HeJ mice, we found that TLR4 signaling was important for HBV
persistence. This result may support our hypothesis that we assumed some foreign
antigens, such as LPS, primed KCs into immune-tolerant phenotype that caused HBV
tolerance and some antigens promoted immune activation against HBV. The TLR4
signaling may be the immune-tolerant and dominative signal that results in immune
tolerance to HBV in young C3H/HeN mice. In adult C3H/HeN mice, we suppose KC
still contributes immune-tolerant effect because of its important role in liver tolerance
but the immune-tolerant effect of KC can be disrupted during exposure to HBV. As our
32
previous study, the composition of gut microbiota in C3H/HeN mice dynamically
changed with age and became stable at 12 weeks old. Liver receives signals trigger by
foreign antigen derived from gut microbiota can also change with age. We suppose that
the composition of gut microbiota in adult C3H/HeN mice can provide liver immune
environment with the ability to suppress the immune-tolerant effect of KC during
exposure to HBV. The reduction of KCs can be one of the pathway to suppress the
immune-tolerant effect. In view of this concept, TLR4 may be the rescue signal for KCs
and can be verified by treating LPS before HBV transfection on adult C3H/HeN mice.
If treating LPS on adult C3H/HeN mice can rescue KCs, the immune response against
HBV may will be changed. However, there is still an important point that we should
consider. The loss of TLR4 signaling can be an immune-activated process that
strengthen the innate immune response and make the liver immunity trend to clear
pathogen, such as HBV.
In our study, we found an age-dependent response of KCs reduction in C3H/HeN
mice after HBV transfection. We have not further investigated the correlation between
this age-dependent fates of KCs and the composition of gut microbiota. To find out
whether gut microbiota that involve in HBV clearance in our C3H/HeN mice model
participate in the age-dependent reduction of KCs, we need to investigate the population
of KCs in germ-free environment. It can be mere an age-dependent phenomena, if the
33
result of age-dependent reduction of KCs can be reproduced in germ-free C3H/HeN
mice. We also need to confirm whether our study is strand specific since our study all
based on C3H/HeN mice.
34
CHAPTER 5: FIGURES
Figure 1. Map of HBV construct
The pAAV/HBV1.2 plasmid was constructed by L.R. Huang. An over-length HBV
whole genome DNA was cloned into a pAAV vector. The plasmid is able to express
HBV transcripts and proteins in mouse hepatocytes and to secrete HBsAg, HBeAg, and
HBcAg.
35
Figure 2. Flowchart of KC depletion experiment.
6-week-old (or 12-week-old) male C3H/HeN mice were divided into three groups
which were intravenously injected clodronate liposome, control liposome or PBS,
respetively, at D-2. 2 days later (D0), all mice were proceeded hydrodynamic injection
of 10μg pAAV/HBV1.2 resolved in 8% body weight of PBS. HBV persistent rate was
evaluated by tracking the level of HBsAg in serum every week.
36
37
Figure 3. The efficiency of KCs depletion by using clodronate liposome
(A) Young and (B) adult C3H/HeN mice were proceeded KCs depletion treatment by
using clodronate liposome. The efficiency of KCs depletion was evaluated by flow
cytometry 2 days after the intravenous injection of clodronate liposome. The
populations of liver macrophages from young / adult C3H/HeN mice were acquired and
showed that vast KCs were depleted. The population of infiltrating macrophages
(MACs) increased after the depletion of KCs. (C) (D) (E) (F) The statistics showed that
infiltrating macrophages of adult C3H/HeN mice increased after the depletion of KCs.
38
(*) P < 0.05, (**) P < 0.01, (***) P <0.001. (n = 3~5).
39
40
Figure 4. HBV persistent rate of young C3H/HeN mice was reduced after the depletion of KCs
(A) The body weight of young C3H/HeN mice. (B) Young male C3H/HeN mice were
divided into three groups, one experimental group treated clodronate liposome (CLD, n
41
= 9)20 and two control group which were PBS control (PBS, n = 8) and vehicle control
(VEH, n = 12). CLD group showed significant decrease of HBV persistent rate
compared with control groups at 20 weeks after HDI of pAAV/HBV1.2. (C) The body
weight of adult C3H/HeN mice. (D) Adult male C3H/HeN mice were divided into
experimental group treated clodronate liposome (CLD, n = 10) and vehicle control
group (VEH, n = 5). (*) P < 0.05, (**) P < 0.01, (***) P <0.001. The follow-up of serum
and body weight were assisted by Dr. Wu and Pei Hsuan.
42
43
44
Figure 5. HDI time course of young C3H/HeN mice
(A) Young C3H/HeN mice were sacrificed 18 hours, 2 days, 3 days or 9 days after HDI.
The KC-enriched NPCs were isolated and analyzed by flow cytometry. Liver
macrophages were divided into two populations by F4/80 and CD11b. (B) (C) (D) (E)
MACs became dominate population after HDI and then gradually decreased in all
groups. The populations of liver macrophages were restored to baseline at day 9. (F)
The levels of HBsAg in serum were detected at 18 hours, 2 days, 3days and 9 days after
HDI. (n = 3~5). The isolation of KCs was assisted by Dr. Wu and Pei Hsuan.
45
46
47
Figure 6. HDI time course of adult C3H/HeN mice
(A) Adult C3H/HeN mice were sacrificed 18 hours, 2 days, 3 days or 9 days after HDI.
The KC-enriched NPCs were isolated and analyzed by flow cytometry. Liver
macrophages were divided into two populations by F4/80 and CD11b. (B) (C) (D) (E)
MACs became dominate population after HDI and then gradually decreased in all
groups. KCs were dramatically reduced day 2 after transfected HBV. The populations of
liver macrophages were restored to baseline at day 9 in all groups. (F) The levels of
48
HBsAg in serum were detected at 18 hours, 2 days, 3days and 9 days after HDI. (*) P <
0.05, (**) P < 0.01, (***) P <0.001. (n = 3~5). The isolation of KCs was assisted by Dr.
Wu and Pei Hsuan.
49
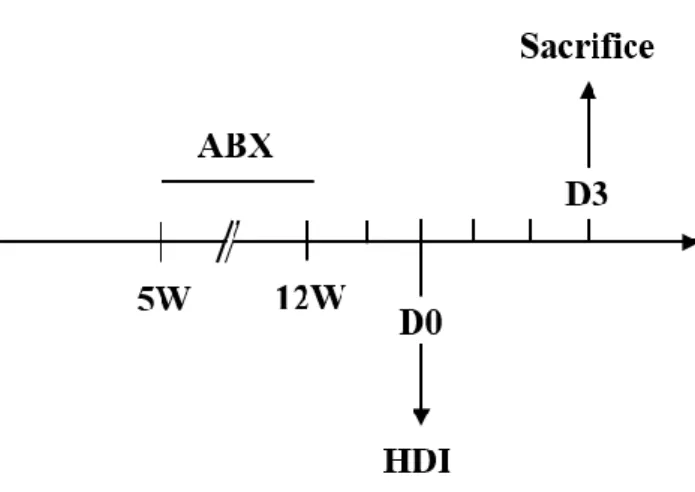
Figure 7. Flowchart of antibiotic experiment
C3H/HeN mice were treated antibiotic cocktail (ABX) in drinking water from 5- to 12-
week-old. Mice were transfected HBV by HDI 2 days after withdrawal of antibiotic
cocktail and sacrificed 3 days after HDI.
50
51
52
Figure 8. KCs of antibiotics-treated adult C3H/HeN mice decreased at day 3 after the exposure to HBV
(A) Antibiotic-treated adult C3H/HeN mice (ABX) were sacrificed at day 3 after HDI.
The KC-enriched NPCs were isolated and analyzed by flow cytometry. Liver
macrophages were divided into two populations by F4/80 and CD11b. (B) KC
population of ABX group 3 days after HDI of pAAV/HBV1.2 was significantly less
53
than the control groups which received HDI of PBS or empty vector. (C) The
population of KCs from ABX group was significantly less than adult and young C3H/HeN mice that didn’t received antibiotic treatment. (D) The population of
infiltrating macrophages from ABX group was significantly higher than adult and young C3H/HeN mice that didn’t received antibiotic treatment. (E) The populations of liver
macrophages was similar in the baseline of adult C3H/HeN mice with or without
antibiotic treatment. (F) The comparison of the HBsAg level between antibiotics-treated
C3H/HeN mice and adult C3H/HeN mice. (*) P < 0.05, (**) P < 0.01, (***) P <0.001.
(n = 5~7). The isolation of KCs was assisted by Dr. Wu and Pei Hsuan.
54
Figure 9. The loss of TLR4 signaling resulted in dramatic reduction of HBV persistent rate.
6-week-old C3H/HeN (n = 8) and C3H/HeJ (n = 17) mice were transfected HBV by
HDI. The HBV persistent rate was acquired by tracking HBsAg in serum every week.
HBsAg persistent rate was less than 10% at 9th week after HDI in C3H/HeJ mice,
whereas it was 100% in C3H/HeN mice. (*) P < 0.05, (**) P < 0.01, (***) P <0.001.
55
56
57
Figure 10. Kupffer cell population of young C3H/HeJ mice decreased after the transfection of HBV
(A) KC-enriched NPCs were isolated from 6-week-old naive C3H/HeN and C3H/HeJ
mice and analyzed by flow cytometry. (B) KC-enriched NPCs were isolated from 6-
week-old C3H/HeN and C3H/HeJ mice 18 hours, 2 days and 3 days after transfection of
HBV and analyzed by flow cytometry. Infiltrating macrophages and KCs were divided
58
by CD11b and F4/80. (C) The comparison of infiltrating macrophage population
between among young C3H/HeN, adult C3H/HeN and young C3H/HeJ. (D) The
comparison of KC population between among young C3H/HeN, adult C3H/HeN and
young C3H/HeJ. (*) P < 0.05, (**) P < 0.01, (***) P <0.001. (n = 3~5). The isolation of
KCs was assisted by Dr. Wu and Pei Hsuan.
59
60
Figure 11. Gating strategy of liver macrophages
KC-enriched NPCs isolated from mouse liver were analyzed by flow cytometry. After
gating out the debris of NPCs on light scatter, we selected CD45+ cell and divided
NPCs into KCs and infiltrating macrophages by CD11b and F4/80. The CD11bhigh
F4/80low population is infiltrating macrophages (Mac) and CD11blow F4/80high
population is KCs.
61
REFERENCES
Biswas, S. K., & Lopez-Collazo, E. (2009). Endotoxin tolerance: new mechanisms,
molecules and clinical significance. Trends Immunol, 30(10), 475-487. doi:
10.1016/j.it.2009.07.009
Bleriot, C., Dupuis, T., Jouvion, G., Eberl, G., Disson, O., & Lecuit, M. (2015). Liver-
resident macrophage necroptosis orchestrates type 1 microbicidal inflammation
and type-2-mediated tissue repair during bacterial infection. Immunity, 42(1), 145-
158. doi: 10.1016/j.immuni.2014.12.020
Chen, D. S. (1993). Natural history of chronic hepatitis B virus infection: new light on an
old story. J Gastroenterol Hepatol, 8(5), 470-475.
Chou, H. H., Chien, W. H., Wu, L. L., Cheng, C. H., Chung, C. H., Horng, J. H., . . . Chen,
D. S. (2015). Age-related immune clearance of hepatitis B virus infection requires
the establishment of gut microbiota. Proc Natl Acad Sci U S A, 112(7), 2175-2180.
doi: 10.1073/pnas.1424775112
Cowie, B. C., Carville, K. S., & Maclachlan, J. H. (2013). Mortality due to viral hepatitis
in the Global Burden of Disease Study 2010: new evidence of an urgent global
public health priority demanding action. Antivir Ther. doi: 10.3851/IMP2654
Doherty, D. G., & O'Farrelly, C. (2000). Innate and adaptive lymphoid cells in the human
liver. Immunological Reviews, 174(1), 5-20. doi: 10.1034/j.1600-
62
0528.2002.017416.x
El Kasmi, K. C., Anderson, A. L., Devereaux, M. W., Fillon, S. A., Harris, J. K., Lovell,
M. A., . . . Sokol, R. J. (2012). Toll-like receptor 4-dependent Kupffer cell
activation and liver injury in a novel mouse model of parenteral nutrition and
intestinal injury. Hepatology, 55(5), 1518-1528. doi: 10.1002/hep.25500
Gomez Perdiguero, E., Klapproth, K., Schulz, C., Busch, K., Azzoni, E., Crozet, L., . . .
Rodewald, H. R. (2015). Tissue-resident macrophages originate from yolk-sac-
derived erythro-myeloid progenitors. Nature, 518(7540), 547-551. doi:
10.1038/nature13989
Gorczynski, R. M. (1992). Immunosuppression induced by hepatic portal venous
immunization spares reactivity in IL-4 producing T lymphocytes. Immunol Lett,
33(1), 67-77.
Guha, C., Mohan, S., Roy-Chowdhury, N., & Roy-Chowdhury, J. (2004). Cell culture and
animal models of viral hepatitis. Part I: hepatitis B. Lab Anim (NY), 33(7), 37-46.
doi: 10.1038/laban0704-37
Huang, L.-R., Wu, H.-L., Chen, P.-J., & Chen, D.-S. (2006). An immunocompetent mouse
model for the tolerance of human chronic hepatitis B virus infection. Proc Natl
Acad Sci U S A, 103(47), 17862-17867. doi: 10.1073/pnas.0608578103
Huang, L. R., Wu, H. L., Chen, P. J., & Chen, D. S. (2006). An immunocompetent mouse
63
model for the tolerance of human chronic hepatitis B virus infection. Proc Natl
Acad Sci U S A, 103(47), 17862-17867. doi: 10.1073/pnas.0608578103
Hyams, K. C. (1995). Risks of chronicity following acute hepatitis B virus infection: a
review. Clin Infect Dis, 20(4), 992-1000.
Ikarashi, M., Nakashima, H., Kinoshita, M., Sato, A., Nakashima, M., Miyazaki, H., . . .
Seki, S. (2013). Distinct development and functions of resident and recruited liver
Kupffer cells/macrophages. J Leukoc Biol, 94(6), 1325-1336. doi:
10.1189/jlb.0313144
Jiang, W., Wu, N., Wang, X., Chi, Y., Zhang, Y., Qiu, X., . . . Liu, Y. (2015). Dysbiosis
gut microbiota associated with inflammation and impaired mucosal immune
function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep., 5.
Limmer, A., Ohl, J., Kurts, C., Ljunggren, H. G., Reiss, Y., Groettrup, M., . . . Knolle, P.
A. (2000). Efficient presentation of exogenous antigen by liver endothelial cells
to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med, 6(12), 1348-
1354. doi: 10.1038/82161
Lyons, S., Sharp, C., LeBreton, M., Djoko, C. F., Kiyang, J. A., Lankester, F., . . .
Simmonds, P. (2012). Species association of hepatitis B virus (HBV) in non-
human apes; evidence for recombination between gorilla and chimpanzee variants.
PLoS One, 7(3), e33430. doi: 10.1371/journal.pone.0033430
64
Movita, D., Kreefft, K., Biesta, P., van Oudenaren, A., Leenen, P. J., Janssen, H. L., &
Boonstra, A. (2012). Kupffer cells express a unique combination of phenotypic
and functional characteristics compared with splenic and peritoneal macrophages.
J Leukoc Biol, 92(4), 723-733. doi: 10.1189/jlb.1111566
Op den Brouw, M. L., Binda, R. S., Geijtenbeek, T. B., Janssen, H. L., & Woltman, A. M.
(2009). The mannose receptor acts as hepatitis B virus surface antigen receptor
mediating interaction with intrahepatic dendritic cells. Virology, 393(1), 84-90.
doi: 10.1016/j.virol.2009.07.015
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S., & Medzhitov, R. (2004).
Recognition of Commensal Microflora by Toll-Like Receptors Is Required for
Intestinal Homeostasis. Cell, 118(2), 229-241. doi:
http://dx.doi.org/10.1016/j.cell.2004.07.002
Saeed, S., Quintin, J., Kerstens, H. H., Rao, N. A., Aghajanirefah, A., Matarese, F., . . .
Stunnenberg, H. G. (2014). Epigenetic programming of monocyte-to-macrophage
differentiation and trained innate immunity. Science, 345(6204), 1251086. doi:
10.1126/science.1251086
Suda, T., Gao, X., Stolz, D. B., & Liu, D. (2007). Structural impact of hydrodynamic
injection on mouse liver. Gene Ther, 14(2), 129-137. doi: 10.1038/sj.gt.3302865
Vanlandschoot, P., Van Houtte, F., Roobrouck, A., Farhoudi, A., Stelter, F., Peterson, D.
65
L., . . . Leroux-Roels, G. (2002). LPS-binding protein and CD14-dependent
attachment of hepatitis B surface antigen to monocytes is determined by the
phospholipid moiety of the particles. J Gen Virol, 83(Pt 9), 2279-2289.
Visvanathan, K., Skinner, N. A., Thompson, A. J., Riordan, S. M., Sozzi, V., Edwards,
R., . . . Locarnini, S. (2007). Regulation of Toll-like receptor-2 expression in
chronic hepatitis B by the precore protein. Hepatology, 45(1), 102-110. doi:
10.1002/hep.21482
Wu, J., Meng, Z., Jiang, M., Pei, R., Trippler, M., Broering, R., . . . Schlaak, J. F. (2009).
Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses
in murine parenchymal and nonparenchymal liver cells. Hepatology, 49(4), 1132-
1140. doi: 10.1002/hep.22751
Xu, L., Yin, W., Sun, R., Wei, H., & Tian, Z. (2014). Kupffer cell-derived IL-10 plays a
key role in maintaining humoral immune tolerance in hepatitis B virus-persistent
mice. Hepatology, 59(2), 443-452. doi: 10.1002/hep.26668
Yoshimoto, S., Loo, T. M., Atarashi, K., Kanda, H., Sato, S., Oyadomari, S., . . . Ohtani,
N. (2013). Obesity-induced gut microbial metabolite promotes liver cancer
through senescence secretome. Nature, 499(7456), 97-101. doi:
10.1038/nature12347
You, Q., Cheng, L., Kedl, R. M., & Ju, C. (2008). Mechanism of T cell tolerance induction
66
by murine hepatic Kupffer cells. Hepatology, 48(3), 978-990. doi:
10.1002/hep.22395