行政院國家科學委員會專題研究計畫 期中進度報告
利用精準開環複分解聚合(ROMP)之技術合成新型功能性高 分子材料(1/3)
計畫類別: 個別型計畫
計畫編號: NSC91-2216-E-011-025-
執行期間: 91 年 08 月 01 日至 92 年 07 月 31 日 執行單位: 國立臺灣科技大學化學工程系
計畫主持人: 廖德章
計畫參與人員: 黃慶成、陳文祥、陳藏斌、鄭宗涵、劉俊宏、劉志峯;
報告類型: 精簡報告
處理方式: 本計畫涉及專利或其他智慧財產權,2 年後可公開查詢
中 華 民 國 92 年 6 月 2 日
行政院國家科學委員會專題研究計畫成果報告
計畫名稱: 利用精準開環複分解聚合(ROMP)之技術合成新型 功能性高分子材料(1/3)
計畫編號: NSC-91-2216E-011-025 執行期限: 91/08/01 ~ 92/07/31
計畫主持人: 廖德章 執行機構: 國立台灣科技大學化工系
計畫參與人員:
黃慶成、陳文祥、陳藏斌、鄭宗涵、劉俊宏、劉志?A. Abstract
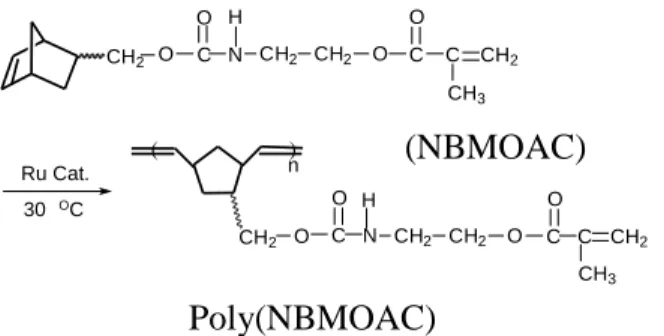
A novel cross-linkable monomer, 5-(methacryloyloxyethylamino carboxylmethyl)bicyclo[2.2.1]hept-2-ene (NBMOAC), was prepared. Ring-opening metathesis polymerization (ROMP) of NBMOAC was carried out to prepare polymers with cross-linkable side chains using {RuCl
2(CHPh)[P(C
6H
11)
3]
2} [Ru catalyst(II)] as catalyst. No gel formation occurred during ROMP of NBMOAC. Two methylene units between urethane group and ester group of NBMOAC and/or poly(NBMOAC) disturbed the hydrogen bonding and enhanced the solubility. Poly(NBMOAC) could be considered as an organo-soluble polymeric material. Free radical polymerization of NBMOAC could be carried out because it contains a methacryloyl group and poly(MMANB) could be obtained. Cross-linking was observed because of the norbornene-containing side chains of the poly(MMANB). New hybrid materials from dual cure could be obtained.
Keywords: norbornene, ROMP.
中文摘要
本 計 劃 製 備 了 一 種 新 穎 的 可 交 鏈 單 體 (NBMOAC) 。 在 使 用 {RuCl
2(CHPh)[P(C
6H
11)
3]
2}觸媒的條件下,此單體 NBMOAC 可進行開環複分解 聚合反應,並得到具有側鏈可交鏈基團的具原冰片烯衍生物。在 NBMOAC 進行 開環複分解聚合反應時,並不會有交鏈發生。NBMOAC 單體及/或其聚合物結構 中,在酯基與? 基之間存在有伸乙基,而此基團將可以破壞氫鍵,並大大提高溶 解性。Poly(NBMOAC) 聚合物因而可視為是一種新穎的有機可溶性高分子材 料。NBMOAC 因其具有丙烯酸基,亦可進行自由基聚合反應,而得到具有原冰 片烯側鏈的聚合物 poly(MMANB) 。Poly(MMANB)聚合物,並可進一步進行交 鏈反應,得到新穎的混成材料。
關鍵詞:原冰片烯,開環複分解聚合
B. Introduction
There is an ever growing application for cross-linkable polymers in the domain of
interpenetrating polymer networks, nonlinear optical materials, macro- and
microlithography, and in the formation of thermally and chemically more resistant
materials. Interests have been developed in recent years in ROMP of norbornene
derivatives containing functional groups with the aim of obtaining polymer structures
with attractive properties. Grubbs et al. reported the polymerization of 5-methacryloyl-1-cyclooctene(MCO) having cross-linked side chain with ruthenium catalyst (I)
1). Recently, we reported the ROMP of 5-(methyl methacryloyl isocyanate)bicyclo[2.2.1]hept-2-ene (NBMMAI) by catalyst (II).
2)The high functional group tolerance of the catalyst (II) has prompted an investigation of the use of ROMP in the formation of cross-linked polymers.
3)In this study, a new monomer, 5-(methacryloyloxyethyl amino carboxyl methyl)bicyclo[2.2.1]hept-2-ene (NBMOAC), was prepared. It was interesting to investigate the reactivity of ROMP of NBMOAC having methacryloyl and cyclic norbornene groups. The structure of poly(NBMOAC) was identified by IR,
1H NMR and
13C NMR.
Ph=phenyl Cy=cyclohexyl8
(II)55 (I)55
Ph Cl C H Ph Cl Ru
PCy3
PCy3 PCy3
Ph
PCy3 Cl Ru
C H Cl
C. Experimental
Preparation and polymerization of NBMOAC using catalyst (II)
5-(Hydroxymethyl)bicyclo[2.2.1]hept-2-ene was reacted with methacryloyl oxyethyl isocyanate (MOI) and then NBMOAC was obtained(exo/endo=28/72).
ANAL. Calculated for C
15H
21O
4N: C, 64.52 %; H, 7.52 %; N, 5.02 %; found: C, 64.07 %; H, 7.50 %; N, 4.98 %.
1H NMR spectrum of NBMOAC is shown in Figure 1.
13C NMR(CDCl
3):δ(ppm) = 18.53 (C
10), 29.20 (C
n3), 29.78 (C
x3), 38.46 (C
x2), 39.59 (C
n2), 40.39 (C
12), 41.88 (C
13), 42.50 (C
n4), 42.49 (C
x4), 44.16 (C
n1), 43.90 (C
x1), 45.25 (C
x7), 49.66 (C
n7), 64.01 (C
n8), 68.69 (C
x8), 126.20 (C
11), 132.50 (C
n6), 136.46-136.51 (C
x5,C
x6), 137.21 (C
n5), 137.77 (C
14), 157.09 (C
15), 167.57 (C
16).
IR (KBr pellet, cm
-1): 3360 (υ
N-H), 3050 (υ
C-H, olefinic C-H stretch), 2960 (υ
C-H, C-H stretch), 1715 (υ
C=O), 1640 (υ
C=C), 1540 (υ
C-H, aliphatic C-H), 1300 (υ
C-O).
In DSC thermogram of NBMOAC, an endotherm at 63 ℃ corresponding to the melting point and an exothermal peak at 178℃ were observed. The exothermal peak (ca. 178℃) was due to the thermally initiated cross-linking reaction of the methacryloyl group.
The catalyst (II) was injected into NBMOAC solution and then the solution was stirred at 30 ℃ for 2 hrs and terminated by the addition of ethyl vinyl ether.
Poly(NBMOAC) was obtained.
1H NMR spectrum of poly(NBMOAC) is shown in Figure 2.
13C NMR (CDCl
3):δ(ppm) = 18.15 (C
10), 35.67-44.04 (C
1-4, C
7), 63.56 (C
8), 66.84-67.02 (C
12,C
13), 125.72 (C
11), 129.14-134.60 (C
5, C
6), 135.84 (C
14), 156.56 (C
15), 167.13 (C
16). IR (KBr pellet, cm
-1): 3340 (υ
N-H), 3020 (υ
C-H, olefinic C-H stretch), 2955 (υ
C-H, C-H stretch), 1700 (υ
C=O), 1630
(υ
C=C), 1525 (υ
C-H, aliphatic C-H), 1295 (υ
C-O).
D. Results and discussion
A new cross-linkable monomer (NBMOAC) was prepared. ROMP of
NBMOAC was carried out as shown in scheme I. Gel formation was not observed
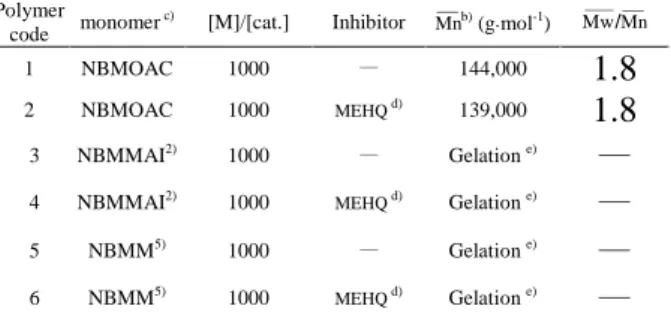
during ROMP of NBMOAC whether MEHQ was added or not(Table 1). A relatively
high molecular weight and narrow molecular weight distribution (
Mw/
Mn= 1.6~1.8)
was obtained (Tables 1 and 2). These behaviors are quite different from the results
O O C n5 n4
n7s n7a H
H H H
H
HH H H
n3n n3x n8
n2 n1 n6
CH2
11
10 13
9 12
CH2 CH2 O N
H
C C CH2 CH3 O 14
15 16
of ROMP of MCO, NBMMAI and NBMM
1, 2, 5, 6). Gel formation was observed during the polymerization of NBMMAI and NBMM without ligand exchange
2, 5, 6). Poly(MCO) with monomodal molecular weight distributions was formed in moderate yields without gel formation when 18 mol % MEHQ was used
1). When 20 mol % MEHQ was used in the polymerization of NBMMAI, gel formation was still observed for 10 min at 30 ℃
2).
Ru Cat.
O CH2
CH3 CH2 C C H
N C O O
n 30
O CH2
CH2
OC
( )
O CH2 CH2
CH3 CH2
C C H
N O C CH2
O O
Scheme I. Polymerization of NBMOAC using catalyst (II)
Figure 1 1H NMR spectrum of NBMOAC monomer (taken in CDCl3)
7 1
H N C C
H H
H H O C
O
CH3 CH2 C O
C O H H C
9
8 6 5
4
3 2
11
12 13 10
( )
16 15
14 exo endo
x3x x3nH H
CH2
x6 x1
x2 x8
H H H
H H H H x7s x7a
x5 x4
O
CH3 CH2 C C H
N C O
O CH2 CH2 O
9 12 13
10 11 16 15
14
(NBMOAC)
Poly(NBMOAC)
Figure 2 1H NMR spectrum of poly(NBMOAC) (taken in CDCl3)
The effect of the monomer to catalyst ([M]/[cat.]) ratio on polymer yield, molecular weight and molecular weight distribution is shown in Table 2. The results showed that low [M]/[cat.] ratios resulted in a relatively narrow molecular weight distribution. Increasing the [M]/[cat.] ratio resulted in the formation of higher molecular weight polymers. Gel formation was not observed during the ROMP of NBMOAC with low [M]/[cat.] ratio. This phenomenon is different from the result of poly(NBMMAI)
2).
Table 1. Effect of various monomers on molecular weight and molecular weight distribution in the ring-opening metathesis polymerization a)
Polymer
code monomer c) [M]/[cat.] Inhibitor Mnb) (g.mol-1) Mw/Mn
1 NBMOAC 1000 — 144,000
1.8
2 NBMOAC 1000 MEHQ d) 139,000
1.8
3 NBMMAI2) 1000 — Gelation e)
—
4 NBMMAI2) 1000 MEHQ d) Gelation e)
—
5 NBMM5) 1000 — Gelation e)
—
6 NBMM5) 1000 MEHQ d) Gelation e)
—
(a)Experiment conditions: monomer; catalyst II; [M]/[cat.] = 1000; CH2Cl2; temperature (30℃). (b) Molecular weight determined by GPC (polystyrene was used as a standard). (c) 5-(methacryloyloxyethyl amino carboxyl methyl)bicyclo[2.2.1]hept-2-ene (NBMOAC); 5-(methyl methacryloyl isocyanate)bicyclo [2.2.1]hept-2-ene (NBMMAI); 5-(methyl methacryloyl)bicyclo[2.2.1]hept-2-ene (NBMM). (d) MEHQ (p-methoxy phenol) = 20 mol%. (e) Gel formation during the polymerization.
( ) O
O
C C CH2
CH3 CH2 NCH2
H C O 2O
NBMOAC
O C H N CH2
CH3 CH2 C C O
O
NBMMAI
CH2 CH3
CH2 C C O O
NBMM
Characterization of the poly(NBMOAC)
With respect to the
1H NMR spectrum of poly(NBMOAC), as the vinylic proton
peaks of norbornene at 5.98 and 6.04 ppm disappeared, the
1H NMR of
poly(NBMOAC) showed new vinylic protons as broad signal between 5.10 and 5.40
ppm corresponding to the cis and trans double bond of poly(NBMOAC), respectively.
The vinylic signals of cross-linkable side chain were observed at 5.55 ppm and 6.08 ppm. In the
13C NMR spectrum, the signals at 5.67 ~ 44.04 ppm are ascribed to the cyclic structure of the polymer. The various vinylic carbon peaks of the ring-opened polymer backbone appeared between 129.13 and 134.60 ppm. The signals at 125.71 and 135.84 ppm are due to the methacryloyl group in the side chains.
13C NMR was complex with many overlapping multiples or broad unresolved peaks. Considerable micro structural variety is possible in these systems owing to the possibility of the head-tail additions, cis and trans vinylene units. NBMOAC and poly(NBMOAC) are also characterized by IR analysis. The difference is that there is a strong band at 719 cm
-1in the monomer and near 965 cm
-1in the polymer, indicating that polynorbornene main chain has predominant trans double bond.
Table 2. Molecular weight and molecular weight distribution of polymers
Polymer code [M]/[cat.] Mna) (g.mol-1) Mw/Mna)
1 75 18,000 1.6
2 150 37,000 1.6
3 300 78,000 1.6
4 500 103,000 1.7
5 750 126,000 1.7
(a)As determined by GPC in THF using polystyrene as a calibration standard.
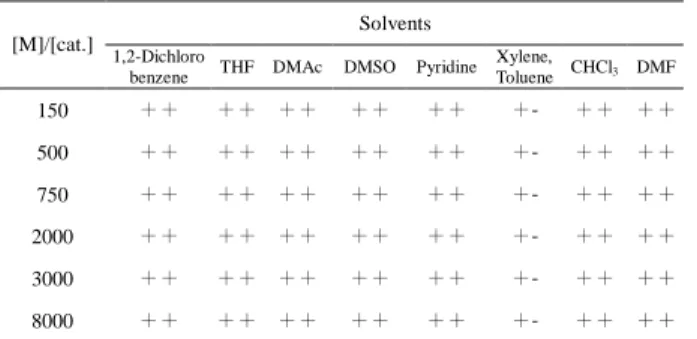
Solubilities of poly(NBMOAC)s are shown in Table 3. Poly(NBMOAC)s are soluble in N, N-dimethylsulfoxide (DMSO), N, N-dimethylformamide (DMF), N, N-dimethylacetamide (DMAc), tetrahydrofuran (THF), pyridine, methylene chloride, chloroform and dichlorbenzene, (Table 3).
Table 3. Solubility of polymers in various solvents
Solubility: + + : soluble at room temperature; +: soluble at a temperature of 60 ℃; +-: partially soluble at 60 ℃.
Synthesis of AB cross-linked systems of poly(NBMOAC) and polyMMA
Incorporation of the poly(NBMOAC) which was obtained through chemical cross-links in polyMMA could be considered to improve thermal stability and chemical resistance of polyMMA.
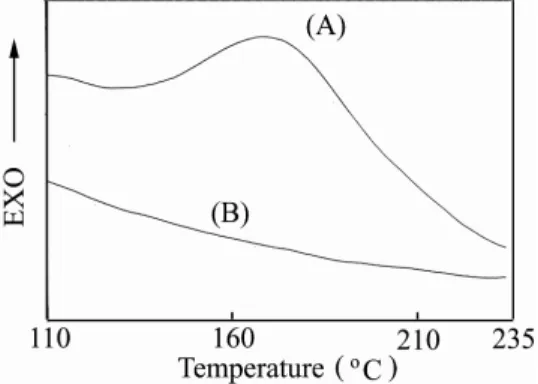
2~4)Reaction of this multifunctionalized poly(NBMOAC) with methacryloyl side chains under free radical polymerization conditions led to the formation of AB crosslinked system of polyMMA. The resulting material was found to be insoluble in benzene, toluene, chloroform and methylene chloride, but polyMMA was soluble in these organic solvents. Further evidence for the cross-linking reaction was provided through thermal analysis using differential scanning calorimetry (DSC). As shown in Figure 3(A), the exothermal peak (ca. 176 ℃ ) indicates that the thermally initiated cross-linking reaction of the methacryloyl group occurred. Upon cross-linking system of poly(NBMOAC) and MMA, the exothermal peak disappeared [Figure 3(B)], and no other distinctive phase transition was observed. Since cross-linking generally
Solvents [M]/[cat.]
1,2-Dichloro
benzene THF DMAc DMSO Pyridine Xylene,
Toluene CHCl3 DMF
150 ++ ++ ++ ++ ++ +- ++ ++
500 ++ ++ ++ ++ ++ +- ++ ++
750 ++ ++ ++ ++ ++ +- ++ ++
2000 ++ ++ ++ ++ ++ +- ++ ++
3000 ++ ++ ++ ++ ++ +- ++ ++
8000 ++ ++ ++ ++ ++ +- ++ ++
enhances thermal stability, this should be reflected in the decomposition temperature (T
d), the temperature at which 10% weight loss occurs, as determined by TGA. The TGA results clearly demonstrated the difference in thermal stability among these materials. In the comparison of thermal stability among polyMMA, AB cross-linked materials containing poly(NBMOAC), the thermal stability of interpenetrating (IPN) polymer of polyMMA and poly(NBMOAC) was higher than that of pure polyMMA.
Increase in thermal stability of 13.5℃ [1% poly(NBMOAC)] and 20.5℃ [5%
poly(NBMOAC)] was observed for the new materials relative to pure polyMMA. In conclusion, more thermally and chemically resistant materials of interpenetrating (IPN) polymer of polyMMA and poly(NBMOAC) were obtained.
Figure 3. Typical thermograms of (A) poly(NBMOAC) and (B) AB cross-linking material of poly(NBMOAC) and MMA. Data were obtained via DSC at a scan rate of 20 ℃‧min-1 .