國立臺灣大學公共衛生學院職業醫學與工業衛生研究所 碩士論文
Institute of Occupational Medicine and Industrial Hygiene Collage of Public Health
National Taiwan University Master thesis
一、母親手機使用與孩童神經認知行為發展
Exposure to maternal mobile phone use and children’s neurocognitive development
二、臍帶血中酚類化合物與胎兒生長發育之相關性
Association between phenolic compounds in umbilical cord blood and child growth
吳佩璇 Pei-Hsuan Wu
指導教授:陳保中 博士 Advisor: Pau-Chung Chen, Ph.D.
中華民國 101 年 6 月
June, 2012
致謝
「終於要開始寫致謝了!」,這大概是這時候最貼切也最想大喊的一句話。這
個部分終於可以比較輕鬆的書寫,也不用再為了內容而想破頭,唯一要擔心的反 而是漏掉任何一位我要感謝的人。碩班這兩年過得很充實也很忙碌,很快地,兩 年的碩班生活即將告一段落,真的要感謝很多鼓勵和幫助我的人,因為你們的陪 伴,讓我的碩班生活很精彩。
這兩年最要感謝的當然是我的指導教授─陳保中老師。保中老師平常就讓我 有許多獨立思考和自由發揮的空間,在這樣的訓練過程中,難免有些不知所措和 遇到瓶頸的時候,但老師總是先耐心地聽完我的想法,再從中點出我的盲點並給 予很多值得參考的建議。也因為老師鼓勵我們接受各種挑戰,從中我也得到了更 多學習和成長的機會。除了學術研究之外,老師所分享的人生哲學也是這兩年收 穫最多的部分。另外也感謝所上每一位老師的教導,這兩年從各位老師們身上學 習到很多專業的知識。要特別感謝我的口試委員們,謝武勳主任、陳家揚老師、
陳美蓮老師和吳明蒼老師,謝謝您們願意給予指導並提供許多寶貴的意見。
保中家的學生是最常讓大家找不到的,也的確有很多事情要學習,從收案到 資料分析和實驗分析,這些都是我們必須參與的過程,正因為有這麼多事情要完 成,在實驗室待了兩年之後也練就了一種隨時待命和使命必達的好功夫。忙碌的 生活,還好有一大群實驗室夥伴們的陪伴,永遠不會忘記大家的默契「再忙,也
要忙裡偷閒」。謝謝保中家的行動database 佳容學姊,每次總是耐心地幫忙處理收
案和統計的問題;謝謝實驗幫我最多的珖彣學姊,每次討論就像替我打了強心針,
不只對實驗安心不少也更有信心;謝謝辦公室的總管們,靜君學姊、哩哩學姊和 阿鼻,任何疑難雜症似乎都難不倒你們,謝謝你們對我的照顧和生活上的幫忙;
謝謝全能的美惠學姊,學姊忙著處理一堆計畫事務之餘,還能適時地幫我解決資
料分析問題也提供重要的建議;謝謝雪倫學姊,10 樓有你的陪伴讓我做實驗一點
你們願意聽我發牢騷和耐心地解開我所有的疑惑。佳嫺和孟吟也是在我最忙碌的 碩二生活幫我很多的可愛學妹們,謝謝你們的體諒與幫忙,偶爾跟你們聊聊真的 能替緊湊的碩二生活舒緩不少。永遠不會忘記我們是最忙碌但也是最歡樂的保中
家,也不會忘記733 一直都具有吸引人的魔力,不在裡面待個一個小時是捨不得
離開的。
感謝北醫薛玉梅老師的啟蒙與教導,讓我在大學已奠定基礎,也更能適應碩 士班的學習。當然也要感謝一起在碩班努力的北醫夥伴們,映君、仰真、雅婷、
大毛和小蟲,從大學就培養出的革命情感,在碩士班更是發揮的淋漓盡致。尤其
謝謝映君和大毛,完全沒怨言的陪我一起奮鬥。謝謝99 級的同學們,很開心我們
可以一起在努力,也謝謝你們平時的加油打氣和cover。謝謝假日做實驗的好夥伴
小綠,也謝謝你的鼓勵。還要謝謝超熱心的瓊文,感謝你在我無助的面對儀器時 總是願意仔細的教我確認儀器狀況,也願意幫我解決一堆疑難雜症。
透過網路社團的聯繫,我們這群認識將近十年的高中死黨們能夠一起分享學 校或工作上的酸甜苦辣,有你們陪著一起開心或抱怨,的確讓有點苦悶的碩班生 活有個發洩的空間,謝謝你們。
最後要感謝最親愛也最不會跟我計較的家人們,謝謝你們體諒我因為忙於實 驗或收案無法每次假期都回家跟你們團聚;謝謝你們在我最無助或最沮喪的時候 豪不吝嗇地給我安慰和溫暖;每次跟你們分享喜悅的時候也謝謝你們替我高興。
能順利完成碩士班這兩年的學習,家人總是給我最大的動力,謝謝你們:)
佩璇 民國 101 年 6 月
Part I
一、母親手機使用與孩童神經認知行為發展 Exposure to maternal mobile phone use and children’s
neurocognitive development
中文摘要
研究背景與目的:現今社會中手機使用已越趨頻繁,然而手機之電磁波暴露對於 孩童健康發展之不良影響仍有爭議。少數研究已探討手機暴露與孩童神經行為發 展間之相關性,但目前仍未有研究探討手機暴露與孩童智力之影響。本研究之目 的為,先描述從妊娠期到產後一年之母親手機使用情形,並進一步探討其手機使 用量和孩童神經行為發展與智力間之影響。
材料與方法:自 2004 年 5 月至 2005 年 2 月間,收集來自台灣北部地區不同醫療 院所的產婦及其新生兒為研究對象,最終納入 133 對的產婦及其新生兒進行分析。
我們使用「嬰幼兒綜合發展測驗」(Comprehensive Developmental Inventory for Infants and Toddlers, 簡稱 CDIIT) 以及「魏氏兒童智力量表第四版」(Wechsler Intelligence Scale for Children-Fourth edition, 簡稱 WISC-IV) 評估孩童之 神經行為發展與智力分數。另外,我們也使用「瑞文氏圖形推理測驗」(Standard Progressive Matrices, 簡稱 SPM)評估母親的智力。藉由自填式問卷評估母親的 手機使用量,再使用迴歸模式(regression model)進行統計分析。
結果:本研究發現,從妊娠期至產後一年,多數的母親每天手機的接聽通數皆少 於 3 通,且每通電話之通話時間皆少於 3 分鐘。研究結果並未發現手機暴露對於 孩童的神經行為發展有不良之影響。
結論:目前仍未有明確的證據足以證明手機之暴露會造成孩童神經行為之不良影 響,未來仍需要更多的研究針對此議題進行探討。
關鍵字:手機,孩童神經行為發展,孩童智力
Abstract
Background and objective: In today’s world, mobile phone use has been commonly
and increased rapidly in many countries. Health effects of exposure to mobile phone use on the children are controversial. An association between exposure to mobile phone use and children’s neurodevelopment was reported but there are still no studies investigating the effect on children’s intelligence. We described the maternal mobile phone use from pregnancy to 12 months, and examined the association between exposure to mobile phone use and children’s neurocognitive development in young children from the general population in Taipei, Taiwan.
Methods: The study was a part of the Taiwan Birth Panel Study. A total of 133 pairs of
parents and their singleton child were recruited into this study. We used the Comprehensive Developmental Inventory for Infants and Toddlers (CDIIT) and Wechsler Intelligence Scale for Children-Fourth edition (WISC-IV) to assess child neurocognitive development. We also assessed the intelligence of the mothers using the Standard Progressive Matrices Plus (SPM+). Mothers completed the questionnaires to report their mobile phone use. Regression model was used to estimate the association
was less than 3 minutes in each phone call from pregnancy to 12 months. In this study, we found no significant association between maternal mobile phone use and neurocognitive development in young children.
Conclusions: There is no convincing evidence that maternal mobile phone use had an
adverse effect on the neurocognitive development of children. Further studies are needed to explore the association of mobile phone use and children’s neurocognitive development.
Key words: prenatal exposure, mobile phone, neurocognitive development, young
children
Contents
中文摘要 ... i
Abstract ... ii
Table of contents ... v
Introduction ... 1
Materials and Methods ... 3
Study population ... 3
Mobile phone exposure ... 4
Child neurodevelopment ... 5
Children’s and Maternal Intelligence ... 6
Home Observation for Measurement of the Environment Inventory (HOME Inventory) ... 7
Statistical Analysis ... 7
Results ... 9
Discussion ... 10
Conclusion ... 12
References ... 14
Appendix 1 ... 20
Appendix 2 ... 21
Table of contents
Table 1. Characteristics of the study population ... 16 Table 2. Distribution of maternal mobile phone use from pregnancy to 12 months ... 17 Table 3. Linear regression models of the child neurodevelopment and mobile phone use ... 18 Table 4. Logistic regression models of the child neurodevelopment and mobile phone use ... 19
Introduction
In today’s world, mobile phone use has been commonly and increased rapidly in many countries. In globally, there were nearly 5 billion mobile phone subscribers in 2010 (International Telecommunication Union 2010). And then in Taiwan, there were more than 90% people have mobile phone (Directorate-General of Budget, Accounting and Statistics, Executive Yuan 2010). Therefore, the risk of exposure to radiofrequency fields is increasingly, especially in children. Children are more susceptible to radiofrequency fields, and may be more vulnerable than adults because of their developing nervous systems. Furthermore, children expose the radiofrequency fields at earlier age, and they will have longer lifetime of exposure than adults (Divan et al., 2010; Kheifets et al., 2005).
The World Health Organization emphasized that the studies which investigate the effects of children mobile phone use on cognitive effect are high priority and necessary (World Health Organization 2007). Only few studies evaluated the association between mobile phone use and child neurodevelopment, but the results were not consistent.
Divan et al. (2008) showed that prenatal and postnatal exposure to the mobile phone
neurodevelopment at 14 months (Vrijheid et al., 2010). On the other hand, some studies didn’t find specific evidence for adverse effect of maternal mobile phone use on the early neurodevelopment of children (Divan et al., 2011; Divan et al., 2010).
The purpose of this study is to describe the maternal mobile phone use during pregnancy, and at postnatal ages up to 12 months. Furthermore, the adverse developmental effects of mobile phone use on the children are scanty and contentious.
Another purpose of this study is to examine the association between prenatal and postnatal mobile phone use and early-childhood neurodevelopment and intelligence in young children.
Materials and Methods
Study population
This study was based on the Taiwan Birth Panel Study (TBPS), a prospective cohort study, which was conducted between April 2004 and January 2005 (Hsieh et al., 2011). Informed consents were obtained from study participants before delivery to collect maternal blood, umbilical cord blood, and questionnaires. After delivery, all subjects were interviewed by trained interviewers to obtain the information during prenatal life style. Then, we followed up these children at 2, 5 and 7 years old. The postnatal questionnaires were completed by main caregivers in each follow-up.
Moreover, the neurodevelopment and intelligence test were conducted at the age of 2 and 7 years, respectively. The protocols used in this study were approved by the Ethical Committee of National Taiwan University Hospital.
In this study, we only included singleton infants and there were no active smoking mothers in these participants. We also excluded preterm infants, subjects who didn’t complete the neurodevelopment and intelligence test at 2 and 7 years of age and
Mobile phone exposure
At 5 years of age, we modified the questionnaires for mobile phone exposure of the Danish National Birth Cohort (DNBC) study (Danish National Birth Cohort 2010). The questionnaires focused on mobile phone use among mothers during pregnancy (three trimesters of pregnancy), and at postnatal ages up to 12 months. Mothers reported their use of mobile phone (average daily phone calls, average times spoken per phone calls), and use of hands-free equipment from pregnancy to 12 months, and the location of mobile phone during pregnancy (clothing pocket or bag). In the questionnaires, daily number of calls were divided into three groups (below 3 calls, 4 to 10 calls, and over 10 calls) and times spoken per phone calls were divided into four groups (below 1 minute, 1 to 3 minutes, 4 to 10 minutes, and over 10 minutes). In order to estimate the cumulative dose of mobile phone use, we used the median to represent each exposure group, and multiplied daily phone calls and times spoken per phone together. The dose of pregnancy was sum of three trimesters and the postnatal dose at 1-year-old was sum of 12 months per year. Therefore, the cumulate dose was used for estimating the prenatal and postnatal mobile phone exposure.
Child neurodevelopment
Child neurodevelopment was assessed at 2 years of age using the Comprehensive Developmental Inventory for Infants and Toddlers (CDIIT) (Liao and Pan, 2005). The CDIIT was designed to assess development in the area of cognitive, motor, gross motor, fine motor, language, social, and self-help. And the available age range of the CDIIT is from 3 to 71 months. The standardization used 3703 infants of aged 3 to 71 months who were randomly selected in Taiwan and the CDIIT has good test-retest reliabilities (Liao and Pan, 2005). Also, the CDIIT has the acceptable concurrent validity with the Bayley scales of Infant Development-II (BSID-II) which is commonly used in the international (Liao et al., 2005).
The assessment of the CDIIT was divided into two parts, the physical therapists and questionnaires. The cognitive and motor subtests, and part of the language subtest were individually and directly evaluated by the testers. In addition, the social and self-help subtests and some items of the language subtest were scored from the questionnaires which completed by the main caregivers. The results of the CDIIT test, developmental quotients (DQ) were obtained for the whole test (whole DQ), five
DQ is 100 (Liao and Pan, 2005). According to the manual of CDIIT, a DQ of 85 or above is within normal limits, and the children whose DQ below 70 and within 70-84 were classified as delay and borderline, respectively (Liao et al., 2005).
Children’s and Maternal Intelligence
We used the Chinese version of the Wechsler Intelligence Scale for Children-Fourth edition (WISC-IV) (David, 2007), the universally acknowledged tools, to evaluate the children’s intelligence in 6-7 years old children. The WISC-IV has five domains of Full Scale (FSIQ), Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Working Memory Index (WMI), and Processing Speed Index (PSI). The assessment of WISC-IV was tested by trained testers, and we used the age-corrected scores of 5 domains. In the norm, the mean score of the WISC-IV is 100 and the standard deviation is 15.
Mother’s intelligence was assessed by the Standard Progressive Matrices Plus (SPM+) (Raven et al., 2006). There are 60 questions in the SPM+ test, and the subjects have to do their best to answer the most numbers of the questions in 30 minutes. The available age of SPM+ is from 12 to adults, and full score of the SPM+ test is 60. In the regression models, we adjusted the total score of the mother’s intelligence.
Home Observation for Measurement of the Environment Inventory (HOME
Inventory)
At 2 years of age, we also took the HOME Inventory to measure the caregiving environment. The Home Inventory is designed to measure the quality and quantity of stimulation and support available to a child in the home environment (Caldwell and Robert, 2003). The Infant/Toddler HOME Inventory (IT-HOME) is used for birth to age three children, and comprises 45 items clustered into six subscales: parental responsivity, acceptance of child, organization of the environment, learning materials, parental involvement, and variety of experience. For the HOME Inventory, the subjects were observed and the main caregivers were interviewed by the trained visitors. The missing data were substituted by the mode of the HOME scores in this study.
Statistical Analysis
We used linear regression models and logistic regression models to estimate the association between mobile phone use and child neurodevelopment. The maternal
quartile of the cumulative dose, and low exposure was defined as all other quartiles. In the logistic regression models, the cut-points of the 8 areas of the CDIIT DQ and the 5 domains of the WISC-IV test were the first quartile of the distribution.
We adjusted some potential confounders in the models, including maternal age, maternal education, family income, infant gender, lead levels in the cord blood, HOME score, prenatal ETS exposure as reported on a questionnaire. The models of postnatal mobile phone exposure, we also adjusted postnatal ETS exposure and the hours that mothers accompany with child per day. Moreover, we adjusted the maternal intelligence in the models of mobile phone use and children’s intelligence. All statistical analysis was conducted with SAS 9.2.
Results
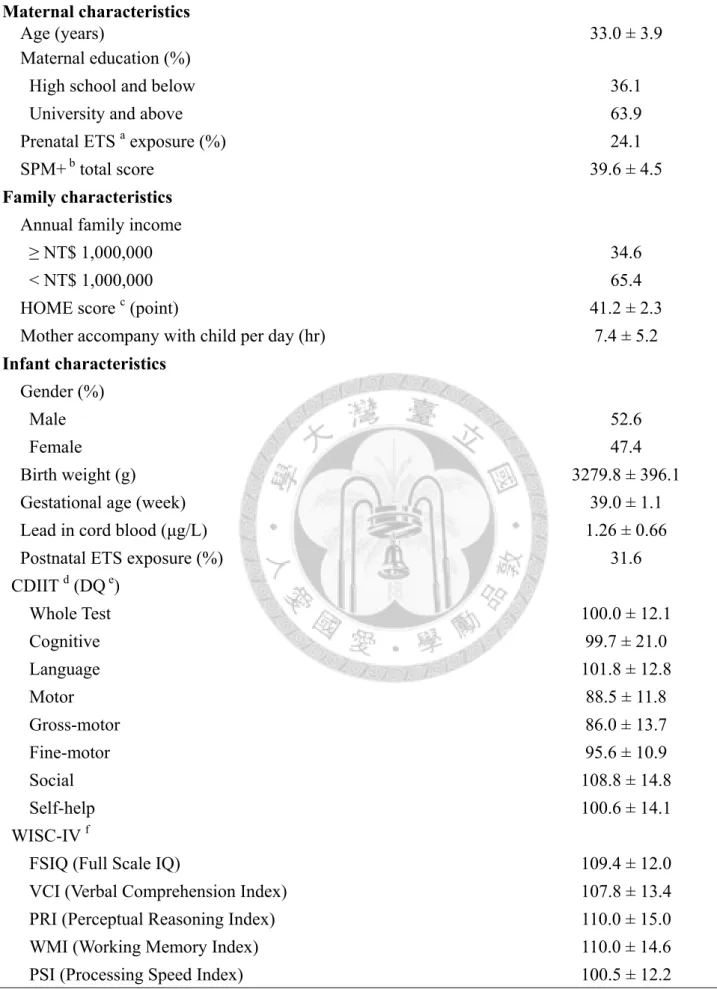
In this analysis, only 133 pairs of mothers and children had complete mobile phone use data and finished the CDIIT and the WISC-IV test. Most mothers have higher education level, and only 5% mothers consumed alcohol during pregnancy. In addition, all the infants are full-term birth and only 2 infants’ birth weight below the normal limits. These subjects were from general population, the mean DQ of 8 tests of the CDIIT and the mean score of the WISC-IV are all within normal limits (Table 1, p16).
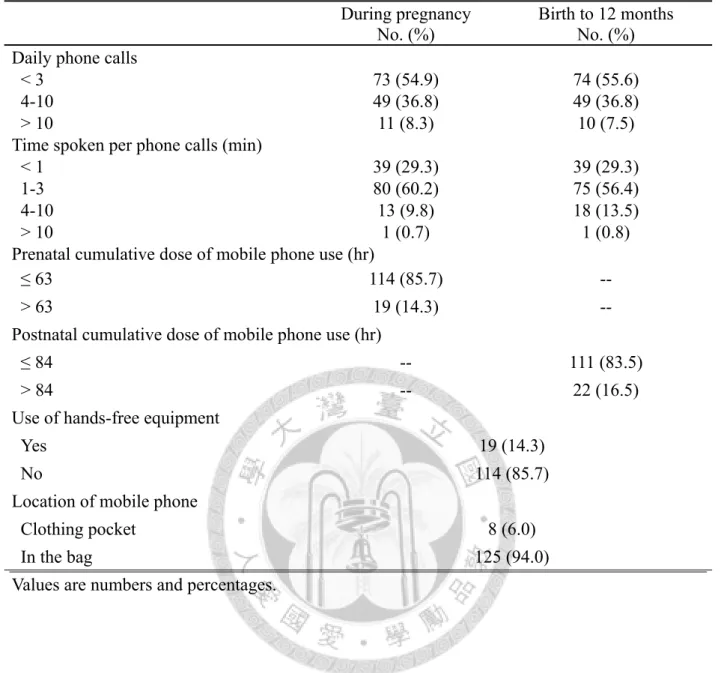
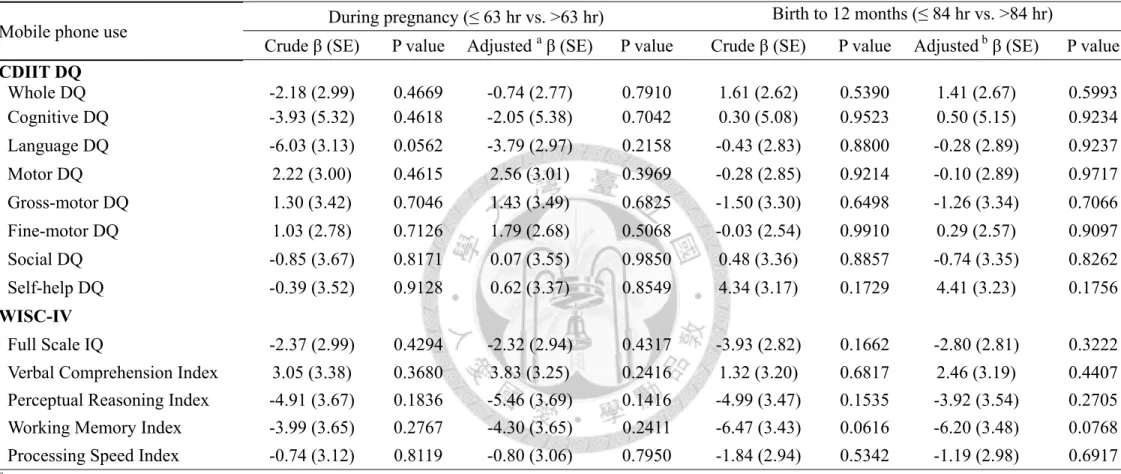
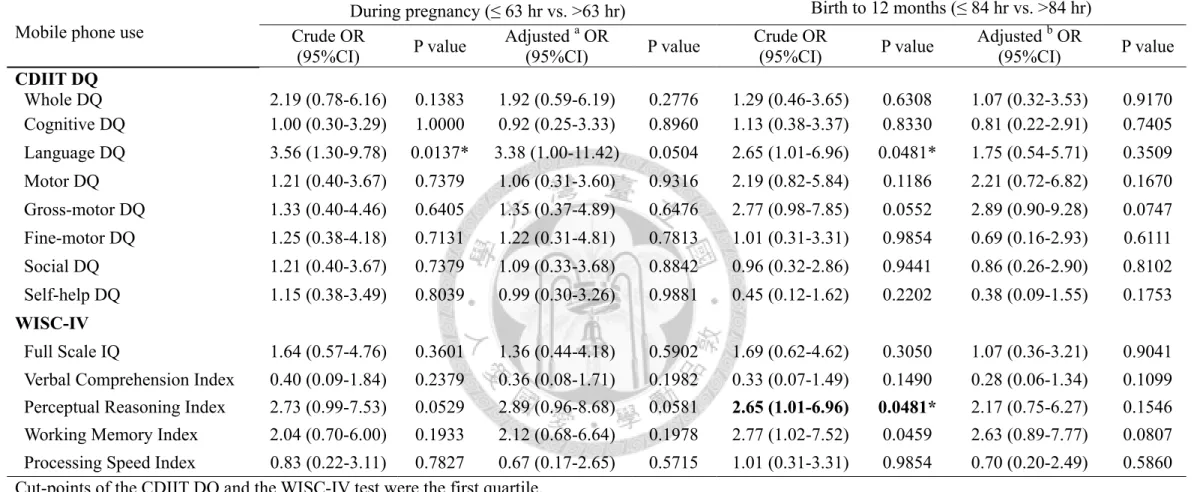
Either prenatal or postnatal mobile phone use, most of the mothers took less than 3 phone calls per days, and the call duration was less than 3 minutes per call. Furthermore, more than 85% of the mothers didn’t use the hands-free equipment, and 94% mothers put their phone in the bags during pregnancy (Table 2, p17). However, there were no significant findings between maternal mobile phone use and child neurodevelopment and intelligence (Table 3 and Table 4, p18 and 19).
Discussion
Our study didn’t find an evidence to support that maternal mobile phone use from pregnancy to 12 months after birth were adversely affected the children’s neurocognitive development. There were two studies published the similar results reporting no association between prenatal mobile phone use and early-childhood neurodevelopment at 6, 14 and 18 months (Divan et al., 2011; Vrijheid et al., 2010).
However, these results were expected, and various potential confounders for neurodevelopment were analyzed and adjusted.
Most of these mothers took less than 3 phone calls per days during pregnancy, but the proportion of heavier user (≥ 4 phone calls per day) in our study is slightly higher than the population in western countries (Divan et al., 2011; Vrijheid et al., 2010; Divan et al., 2010; Divan et al., 2008). And the mothers used the mobile phone directly, only few mothers had ever used the hands-free equipment. Even though the cumulative dose was high, these children’s neurodevelopment and intelligence are still within normal limits.
So far, we have no specific biologic mechanism to explain the association between mobile phone use and adverse effect on neurodevelopment. Some studies focused on the molecular and cellular effect of radiofrequency exposure. Electromagnetic frequency
may affect cell cycle and causing cell damage (Panagopoulos et al., 2007; Zmyslony et al., 2004). According to a recent letter to the editor (Hocking, 2009), Brzezinski (1997) suggested that mobile phone is close to the head when talking on, and the radiofrequency fields from a mobile phone may affect signaling in the unmyelinated nerves and in turn influence melatonin secretion. Furthermore, diverse changes in maternal metabolism or the sex hormone environment may affect development of the fetal brain leading to behavioral problems (Brzezinski, 1997). Only one animal study found that the mice exposed in-utero to radiofrequency were hyperactive, had decreased memory, and decreased anxiety, because the electromagnetic frequency impaired the nervous transmission (Aldad et al., 2012). But the exposure dose was used in animal study is higher and not identical to the human, the extrapolation of this animal model to humans is limited.
During pregnancy, more than 90% of the mothers put their mobile phone in the bag when not in use, so the mobile phone didn’t close to the fetus. To our knowledge, the intensity of radiofrequency field would reduce with distance. In addition, the call duration per phone calls of most mothers was less than 3 minutes. Accordingly, the prenatal exposure to mobile phone use may not high enough to affect the children’s
take more attention and better care to their children, so the children have better neurocognitive development in the postnatal age.
An important limitation of this study is recall bias, because we used the retrospective questionnaires to estimate the past maternal mobile phone use. We considered that mobile phone use might be a habit, and the users didn’t change a lot in different periods. A previous study has shown good accuracy for recalled self-reporting mobile phone use, although the individuals tend to underestimate the number of calls and overestimate the call duration (Vrijheid et al., 2006). Another limitation is that we didn’t ask the children’s use of mobile phone. A study found that the high risk for adverse health effects were for children who both prenatal exposure and use mobile phone directly in postnatal age (Divan et al., 2008). But in this study, mobile phone should be rarely used by these young children, so their postnatal dose of mobile phone exposure might be lower.
Conclusion
Based upon the results of this study, although the dose of maternal mobile phone use is slightly higher than other studies and using the objective tools to estimate the children’s neurocognitive development. However, among these young children, we
didn’t find an evidence to support an association between maternal mobile phone use and children’s neurocognitive development. Our study focused on the 2 and 6-7 years old children and we recruited small sample size, we hope others will be able to explore this question on different age of children or in the large sample size study in the future.
References
Aldad TS, Gan G, Gao XB, Taylor HS 2012. Fetal radiofrequency radiation exposure from 800-1900 mhz-rated cellular telephones affects neurodevelopment and behavior in mice. Scientific reports. 2, 312.
Caldwell BM, Robert HB 2003. Home inventory administration manual,
comprehensive edition: Little Rock, AR: University of Arkansas for Medical Sciences and University of Arkansas at Little Rock.
Brezinski A 1997. Melatonin in humans. The New England Journal of Medicine. 16, 186-195.
Danish National Birth Cohort 2010. Danish National Birth Cohort: 7-year follow up.
Available at:
http://www.ssi.dk/English/RandD/Epidemiology/DNBC/For%20researchers/D ata%20available/7-year%20follow%20up.aspx. Accessed March 25, 2012.
David W 2007. Wechsler intelligence scale for children-IV (Chideses version). Taipei, Taiwan: Chinese Behavioral Science Corporation.
Directorate-General of Budget, Accounting and Statistics, Executive Yuan, R.O.C.
(Taiwan). Report on the survey of family income and expenditure, 2011.
Available at: http://win.dgbas.gov.tw/fies/a11.asp?year=99. Accessed March 25, 2012. [In Chinese]
Divan HA, Kheifets L, Obel C, Olsen J 2008. Prenatal and postnatal exposure to cell phone use and behavioral problems in children. Epidemiology. 19, 523-529.
Divan HA, Kheifets L, Obel C, Olsen J 2010. Cell phone use and behavioral problems in young children. Journal of Epidemiology & Community Health.
Divan HA, Kheifets L, Olsen J 2011. Prenatal cell phone use and developmental milestone delays among infants. Scandinavian Journal of Work, Environment
& Health. 37, 341-348.
Hocking B 2009. Maternal Cell Phone Use and Behavioral Problems in Children.
Epidemiology, 20, 312.
Hsieh CJ, Hsieh WS, Su YN, Liao HF, Jeng SF, Tsao FM, Hwang YH, Wu KY, Chen CY, Guo YL, Chen PC 2011. The Taiwan Birth Panel Srudy: a prospective cohort study for environmentally-related child health. BMC research notes. 4, 291.
Raven JC, Styles I, Raven M, 2006. Standard Progressive Matrices (Chinese version).
Taipei, Taiwan: Chinese Behavioral Science Corporation.
Kheifets L, Repacholi M, Sauders R, van Deventer E 2005. The seneitivity of children
to electromagnetic fields. Pediatrics. 116, e303-313.
Liao HF, Pan YL 2005. Test-retest and inter-rater reliability for the Comprehensive Developmental Inventory for Infants and Toddlers diagnostic and screening test. Early human developmeny. 81, 927-937.
Liao HF, Wang TM, Yao G, Lee WT 2005. Concurrent validity of the Comprehensive Developmental Inventory for Infants and Toddlers with the Bayley Scales of Infant Development-II in preterm infants. Journal of the Formosan Medical Association. 104, 731-737.
World Health Organization 2007. Children's EMF research Agenda. Available at:
http://www.who.int/peh-emf/research/children/en/index4.html. Accessed March 25, 2012.
Panagopoulos DJ, Chavdoula ED, Nezis IP, Margaritis LH 2007. Cell death induced by GSM 900-MHz and DCS 1800-MHz mobile telephony radiation. Mutation research. 626, 69-78.
International Telecommunication Union 2010. ITU sees 5 billion mpbile subscriptions globally in 2010: Strong global mobile cellular growth predicted across all regions and all major markets. Available at:
http://www.itu.int/newsroom/press_releases/2010/06.html. Accessed March 25, 2012.
Vrijheid M, Cardis E, Armstrong BK, Auvinen A, Berg G, Blaasaas KG, Brown J, Carroll M, Chetrit A, Christensen HC, Deltour I, Feychting M, Giles G G, Hepworth SJ, Hours M, Iavaronde I, Johansen C, Klaeboe L, Kurttio P, Lagorio S, Lonn S, Mckinney PA, Montestrucq L, Parslow RC, Richardson L, Sadetzki S, Salminen T, Schuz J, Tynes T, Woodward A 2006. Validation of short term recall of mobile phone use for the Interphone study. Occupational and environmental medicine. 63, 237-243.
Vrijheid M, Martinez D, Forns J, Guxens M, Julvez J, Ferrer M, Sunyer J 2010.
Prenatal exposure to cell phone use and neurodevelopment at 14 months.
Epidemiology. 21, 259-262.
Zmyslony M, Politanski P, Rajkowska E, Szymczak W, Jajte J 2004. Acute exposure to 930 MHz CW electromagnetic radiation in vitro affects reactive oxygen species level in rat lymphocytes treated by iron ions. Bioelectromagnetics. 25, 324-328.
Table 1. Characteristics of the study population
Characteristic All population (n = 133) mean ± standard deviation Maternal characteristics
Age (years) 33.0 ± 3.9
Maternal education (%)
High school and below 36.1
University and above 63.9
Prenatal ETS a exposure (%) 24.1
SPM+ b total score 39.6 ± 4.5
Family characteristics Annual family income
≥ NT$ 1,000,000 34.6
< NT$ 1,000,000 65.4
HOME score c (point) 41.2 ± 2.3
Mother accompany with child per day (hr) 7.4 ± 5.2 Infant characteristics
Gender (%)
Male 52.6
Female 47.4
Birth weight (g) 3279.8 ± 396.1
Gestational age (week) 39.0 ± 1.1
Lead in cord blood (μg/L) 1.26 ± 0.66
Postnatal ETS exposure (%) 31.6
CDIIT d (DQ e)
Whole Test 100.0 ± 12.1
Cognitive 99.7 ± 21.0
Language 101.8 ± 12.8
Motor 88.5 ± 11.8
Gross-motor 86.0 ± 13.7
Fine-motor 95.6 ± 10.9
Social 108.8 ± 14.8
Self-help 100.6 ± 14.1
WISC-IV f
FSIQ (Full Scale IQ) 109.4 ± 12.0
VCI (Verbal Comprehension Index) 107.8 ± 13.4
PRI (Perceptual Reasoning Index) 110.0 ± 15.0
WMI (Working Memory Index) 110.0 ± 14.6
PSI (Processing Speed Index) 100.5 ± 12.2
a ETS, environmental tobacco smoke b Standard Progressive Matrices c HOME, Home Observation for Measurement of the Environment d CDIIT, Comprehensive Developmental Inventory for Infants and Toddlers e DQ, developmental quotients f WISC-IV, Wechsler Intelligence Scale for Children, 4th edition
Table 2. Distribution of maternal mobile phone use from pregnancy to 12 months (N = 133) During pregnancy
No. (%)
Birth to 12 months No. (%) Daily phone calls
< 3 73 (54.9) 74 (55.6)
4-10 49 (36.8) 49 (36.8)
> 10 11 (8.3) 10 (7.5)
Time spoken per phone calls (min)
< 1 39 (29.3) 39 (29.3)
1-3 80 (60.2) 75 (56.4)
4-10 13 (9.8) 18 (13.5)
> 10 1 (0.7) 1 (0.8)
Prenatal cumulative dose of mobile phone use (hr)
≤ 63 114 (85.7) --
> 63 19 (14.3) --
Postnatal cumulative dose of mobile phone use (hr)
≤ 84 -- 111 (83.5)
> 84 -- 22 (16.5)
Use of hands-free equipment
Yes 19 (14.3)
No 114 (85.7)
Location of mobile phone
Clothing pocket 8 (6.0)
In the bag 125 (94.0)
Values are numbers and percentages.
Table 3. Linear regression models of the child neurodevelopment and mobile phone use (N=133)
Mobile phone use During pregnancy (≤ 63 hr vs. >63 hr) Birth to 12 months (≤ 84 hr vs. >84 hr)
Crude β (SE) P value Adjusted a β (SE) P value Crude β (SE) P value Adjusted b β (SE) P value CDIIT DQ
Whole DQ -2.18 (2.99) 0.4669 -0.74 (2.77) 0.7910 1.61 (2.62) 0.5390 1.41 (2.67) 0.5993 Cognitive DQ -3.93 (5.32) 0.4618 -2.05 (5.38) 0.7042 0.30 (5.08) 0.9523 0.50 (5.15) 0.9234 Language DQ -6.03 (3.13) 0.0562 -3.79 (2.97) 0.2158 -0.43 (2.83) 0.8800 -0.28 (2.89) 0.9237 Motor DQ 2.22 (3.00) 0.4615 2.56 (3.01) 0.3969 -0.28 (2.85) 0.9214 -0.10 (2.89) 0.9717 Gross-motor DQ 1.30 (3.42) 0.7046 1.43 (3.49) 0.6825 -1.50 (3.30) 0.6498 -1.26 (3.34) 0.7066 Fine-motor DQ 1.03 (2.78) 0.7126 1.79 (2.68) 0.5068 -0.03 (2.54) 0.9910 0.29 (2.57) 0.9097 Social DQ -0.85 (3.67) 0.8171 0.07 (3.55) 0.9850 0.48 (3.36) 0.8857 -0.74 (3.35) 0.8262 Self-help DQ -0.39 (3.52) 0.9128 0.62 (3.37) 0.8549 4.34 (3.17) 0.1729 4.41 (3.23) 0.1756 WISC-IV
Full Scale IQ -2.37 (2.99) 0.4294 -2.32 (2.94) 0.4317 -3.93 (2.82) 0.1662 -2.80 (2.81) 0.3222 Verbal Comprehension Index 3.05 (3.38) 0.3680 3.83 (3.25) 0.2416 1.32 (3.20) 0.6817 2.46 (3.19) 0.4407 Perceptual Reasoning Index -4.91 (3.67) 0.1836 -5.46 (3.69) 0.1416 -4.99 (3.47) 0.1535 -3.92 (3.54) 0.2705 Working Memory Index -3.99 (3.65) 0.2767 -4.30 (3.65) 0.2411 -6.47 (3.43) 0.0616 -6.20 (3.48) 0.0768 Processing Speed Index -0.74 (3.12) 0.8119 -0.80 (3.06) 0.7950 -1.84 (2.94) 0.5342 -1.19 (2.98) 0.6917
a Models adjusted for maternal education, maternal age, family income, infant sex, lead levels in the cord blood, HOME score, prenatal ETS exposure as reported on a questionnaire, and mothers intelligence.
b Models adjusted for maternal education, maternal age, family income, infant sex, lead levels in the cord blood, HOME score, prenatal ETS exposure and postnatal ETS exposure as reported on a questionnaire, mother accompany with child per day (hr), and mothers intelligence.
Table 4. Logistic regression models of the child neurodevelopment and mobile phone use (N=133) Mobile phone use
During pregnancy (≤ 63 hr vs. >63 hr) Birth to 12 months (≤ 84 hr vs. >84 hr) Crude OR
(95%CI) P value Adjusted a OR
(95%CI) P value Crude OR
(95%CI) P value Adjusted b OR
(95%CI) P value CDIIT DQ
Whole DQ 2.19 (0.78-6.16) 0.1383 1.92 (0.59-6.19) 0.2776 1.29 (0.46-3.65) 0.6308 1.07 (0.32-3.53) 0.9170 Cognitive DQ 1.00 (0.30-3.29) 1.0000 0.92 (0.25-3.33) 0.8960 1.13 (0.38-3.37) 0.8330 0.81 (0.22-2.91) 0.7405 Language DQ 3.56 (1.30-9.78) 0.0137* 3.38 (1.00-11.42) 0.0504 2.65 (1.01-6.96) 0.0481* 1.75 (0.54-5.71) 0.3509 Motor DQ 1.21 (0.40-3.67) 0.7379 1.06 (0.31-3.60) 0.9316 2.19 (0.82-5.84) 0.1186 2.21 (0.72-6.82) 0.1670 Gross-motor DQ 1.33 (0.40-4.46) 0.6405 1.35 (0.37-4.89) 0.6476 2.77 (0.98-7.85) 0.0552 2.89 (0.90-9.28) 0.0747 Fine-motor DQ 1.25 (0.38-4.18) 0.7131 1.22 (0.31-4.81) 0.7813 1.01 (0.31-3.31) 0.9854 0.69 (0.16-2.93) 0.6111 Social DQ 1.21 (0.40-3.67) 0.7379 1.09 (0.33-3.68) 0.8842 0.96 (0.32-2.86) 0.9441 0.86 (0.26-2.90) 0.8102 Self-help DQ 1.15 (0.38-3.49) 0.8039 0.99 (0.30-3.26) 0.9881 0.45 (0.12-1.62) 0.2202 0.38 (0.09-1.55) 0.1753 WISC-IV
Full Scale IQ 1.64 (0.57-4.76) 0.3601 1.36 (0.44-4.18) 0.5902 1.69 (0.62-4.62) 0.3050 1.07 (0.36-3.21) 0.9041 Verbal Comprehension Index 0.40 (0.09-1.84) 0.2379 0.36 (0.08-1.71) 0.1982 0.33 (0.07-1.49) 0.1490 0.28 (0.06-1.34) 0.1099 Perceptual Reasoning Index 2.73 (0.99-7.53) 0.0529 2.89 (0.96-8.68) 0.0581 2.65 (1.01-6.96) 0.0481* 2.17 (0.75-6.27) 0.1546 Working Memory Index 2.04 (0.70-6.00) 0.1933 2.12 (0.68-6.64) 0.1978 2.77 (1.02-7.52) 0.0459 2.63 (0.89-7.77) 0.0807 Processing Speed Index 0.83 (0.22-3.11) 0.7827 0.67 (0.17-2.65) 0.5715 1.01 (0.31-3.31) 0.9854 0.70 (0.20-2.49) 0.5860 Cut-points of the CDIIT DQ and the WISC-IV test were the first quartile.
a Models adjusted for maternal education, maternal age, family income, infant sex, lead levels in the cord blood, HOME score, prenatal ETS exposure as
Appendix 1
Appendix 1. Paper reviews of exposure to prenatal and postnatal cell phone use and child neurodevelopment
Country Population Study year Exposure assessment Findings Reference
Denmark General population March 1996 to November 2002 Retrospective questionnaires No evidence of developmental milestone delays among infants
Divan HA et al., 2011 Denmark General population March 1996 to November 2002 Retrospective questionnaires ↑Risk of behavioural problems Divan HA et al., 2010
(both prenatal and postnatal exposure)
Spain General population July 2004 to July 2006 Prospective questionnaires ↓Psychomotor score at 14 months Vriheid et al., 2010 (Heaviest users)
Denmark General population March 1996 to November 2002 Retrospective questionnaires Emotional and hyperactivity problems at 7 years of age
Divan HA et al., 2008
Appendix 2
Questionnaires of mobile phone use
G.電磁波概況
G1. 請問您(孩子母親) 懷孕前一年到現在是否曾使用手機?□1沒有【跳答至 G12.】 □2 有
手機使用頻率
G2.
是否有使 用手機
G3.
每天約幾通
G4.
每通平均幾分鐘
G5.
使用哪 一耳接 聽電話
否 是
3 通 以下
4‐10 通
10 通 以上
1 分 鐘內
1‐3 分鐘
3‐10 分鐘
10 分 鐘以
上 右 耳
左 耳 a.懷孕前一年以上 □1 □2 □1 □2 □3 □1 □2 □3 □4 □1 □2 b.懷孕前半年 □1 □2 □1 □2 □3 □1 □2 □3 □4 □1 □2 c.懷孕第 1~3 個月 □1 □2 □1 □2 □3 □1 □2 □3 □4 □1 □2
d.懷孕第 4~6 個月 □1 □2 □1 □2 □3 □1 □2 □3 □4 □1 □2
e.懷孕第 7 個月~分娩 □1 □2 □1 □2 □3 □1 □2 □3 □4 □1 □2
f.兒童 0~1 歲 □1 □2 □1 □2 □3 □1 □2 □3 □4 □1 □2 g.兒童 1~2 歲 □1 □2 □1 □2 □3 □1 □2 □3 □4 □1 □2 h.兒童 3~5 歲 □1 □2 □1 □2 □3 □1 □2 □3 □4 □1 □2
G6. 請問您(孩子母親)除了直接接聽手機外,使用手機免持聽筒接聽的頻率?
使用頻率 使用手機免持聽筒接聽的頻率
一直都是 經常 有時 很少 從不
a.懷孕前一年以上 □1 □2 □3 □4 □5
b.懷孕前半年 □1 □2 □3 □4 □5
c.懷孕第 1~3 個月 □1 □2 □3 □4 □5
d.懷孕第 4~6 個月 □1 □2 □3 □4 □5
e.懷孕第 7 個月~分娩 □1 □2 □3 □4 □5
f.兒童 0~1 歲 □1 □2 □3 □4 □5
g.兒童 1~2 歲 □1 □2 □3 □4 □5
h.兒童 3~5 歲 □1 □2 □3 □4 □5
G7. 請問您(孩子母親)除了直接接聽手機外,使用耳機接聽的頻率?
使用頻率 使用耳機接聽的頻率
一直都是 經常 有時 很少 從不
a.懷孕前一年以上 □1 □2 □3 □4 □5
b.懷孕前半年 □1 □2 □3 □4 □5
c.懷孕第 1~3 個月 □1 □2 □3 □4 □5
d.懷孕第 4~6 個月 □1 □2 □3 □4 □5
e.懷孕第 7 個月~分娩 □1 □2 □3 □4 □5
f.兒童 0~1 歲 □1 □2 □3 □4 □5
g.兒童 1~2 歲 □1 □2 □3 □4 □5
h.兒童 3~5 歲 □1 □2 □3 □4 □5
G8. 請問您(孩子母親)除了直接接聽手機外,使用藍芽接聽的頻率?
使用頻率 使用藍芽接聽的頻率
一直都是 經常 有時 很少 從不
a.懷孕前一年以上 □1 □2 □3 □4 □5
b.懷孕前半年 □1 □2 □3 □4 □5
c.懷孕第 1~3 個月 □1 □2 □3 □4 □5
d.懷孕第 4~6 個月 □1 □2 □3 □4 □5
e.懷孕第 7 個月~分娩 □1 □2 □3 □4 □5
f.兒童 0~1 歲 □1 □2 □3 □4 □5
g.兒童 1~2 歲 □1 □2 □3 □4 □5
h.兒童 3~5 歲 □1 □2 □3 □4 □5
G9. 在您(孩子母親)懷孕時,您的手機通常置於何處?
使用頻率 上衣口袋 洋裝或
裙子口袋
包包裡
c.懷孕第 1~3 個月 □1 □2 □3
d.懷孕第 4~6 個月 □1 □2 □3
e.懷孕第 7 個月~分娩 □1 □2 □3
G10. 您(孩子母親)是否隨時帶著您的行動電話
□1 是的,隨時
□2 是的百分之五十以上的時機間都帶著
□3 是的大約百分之五十的時間帶著
□4是的但低於百分之五十的時間
□5幾乎都不帶在身上
G15a 何時開始使用?
□1懷孕前一年以上 □2懷孕前半年 □3懷孕第 1~3 個月 □4懷孕第 4~6 個月
□5懷孕第 7 個月~分娩 □6兒童 0~1 歲 □7兒童 1~2 歲 □8兒童 3~5 歲
G16a 何時開始使用?
□1懷孕前一年以上 □2懷孕前半年 □3懷孕第 1~3 個月 □4懷孕第 4~6 個月
□5懷孕第 7 個月~分娩 □6兒童 0~1 歲 □7兒童 1~2 歲 □8兒童 3~5 歲 G11. 請問您的寶貝除了直接接聽手機外,使用手機免持聽筒接聽的頻率?
使用頻率 使用手機免持聽筒
一直都是 經常 有時 很少 從不
a.兒童 0~1 歲 □1 □2 □3 □4 □5
b.兒童 1~2 歲 □1 □2 □3 □4 □5
c.兒童 3~5 歲 □1 □2 □3 □4 □5
G12. 請問您的寶貝除了直接接聽手機外,使用耳機接聽的頻率?
使用頻率 使用耳機接聽的頻率
一直都是 經常 有時 很少 從不
a.兒童 0~1 歲 □1 □2 □3 □4 □5
b.兒童 1~2 歲 □1 □2 □3 □4 □5
c.兒童 3~5 歲 □1 □2 □3 □4 □5
G13. 請問您的寶貝除了直接接聽手機外,使用藍芽接聽的頻率?
使用頻率 使用藍芽接聽的頻率
一直都是 經常 有時 很少 從不
a.兒童 0~1 歲 □1 □2 □3 □4 □5
b.兒童 1~2 歲 □1 □2 □3 □4 □5
c.兒童 3~5 歲 □1 □2 □3 □4 □5
G14. 請問您(孩子母親)懷孕前一年到現在,家中是否使用無線電話?
□1否 【跳答至 G16.】 □2是
G15. 您家中是否使用無線上網?
□1否 【跳答至 G17.】 □2是
G18a 何時開始使用?
□1懷孕前一年以上 □2懷孕前半年 □3懷孕第 1~3 個月 □4懷孕第 4~6 個月
□5懷孕第 7 個月~分娩 □6兒童 0~1 歲 □7兒童 1~2 歲 □8兒童 3~5 歲 G18a 何時開始有行動電話基地台?
□1懷孕前一年以上 □2懷孕前半年 □3懷孕第 1~3 個月 □4懷孕第 4~6 個月
□5懷孕第 7 個月~分娩 □6兒童 0~1 歲 □7兒童 1~2 歲 □8兒童 3~5 歲
G19a 何時開始使用?
□1懷孕前一年以上 □2懷孕前半年 □3懷孕第 1~3 個月 □4懷孕第 4~6 個月
□5懷孕第 7 個月~分娩 □6兒童 0~1 歲 □7兒童 1~2 歲 □8兒童 3~5 歲
G20a 何時開始有行動電話基地台?
□1懷孕前一年以上 □2懷孕前半年 □3懷孕第 1~3 個月 □4懷孕第 4~6 個月
□5懷孕第 7 個月~分娩 □6兒童 0~1 歲 □7兒童 1~2 歲 □8兒童 3~5 歲 G16. 您住家附近是否有行動電話基地台?
□1否 【跳答至 G18.】 □2是 □3不清楚
□4其他電磁波產生器,如高壓電塔,廣播電台發射塔,變電所等,請說明______
G17. 您工作場所是否使用無線電話?
□1否 【跳答至 G19.】 □2是
G18. 您工作場所是否使用無線上網?
□1否 【跳答至 G20.】 □2是
G19. 您工作場所附近是否有行動電話基地台?
□1否 【跳答至下一頁】 □2是 □3不清楚
□4其他電磁波產生器,如高壓電塔,廣播電台發射塔,變電所等,請說明_____
Part II
二、臍帶血中酚類化合物與胎兒生長發育之相關性 Association between phenolic compounds in umbilical cord
blood and child growth
中文摘要
研究背景與目的:雙酚A(BPA)、壬基酚(NP)與辛基酚(OP)為工業上常用之化學物
質,也時常存在日常生活的用品中。塑膠奶瓶、玩具、水瓶、罐頭食品、清潔劑…
等,皆會釋放出微量的酚類化合物。一般來說,這些酚類物質大多經由飲食而進 入人體,飲食攝入也是日常生活中最主要的暴露途徑。這些酚類物質也被歸類為 環境賀爾蒙之汙染物,研究指出此種物質可能會影響生殖系統之發育或胎兒之生 長與發育。然而,目前對於產前暴露酚類物質與其胎兒之出生結果與往後生長發
育之相關性仍不清楚。因此,本研究目的為分析臍帶血中雙酚A、壬基酚與辛基
酚濃度,並探討其與胎兒出生結果與往後生長發育之相關性。
材料與方法:本研究之401 位產後婦女與其單胞胎之胎兒,皆選自於台北出生世
代追蹤研究(Taiwan Birth Panel Study)。產後利用結構式問卷收集產婦產前之生活 習慣調查,並於生產時收集胎兒臍帶血。以極致液相層析─串聯質譜儀
(UPLC-MS/MS)分析臍帶血中酚類化合物之濃度。並進一步使用迴歸模式分析臍 帶血中酚類化合物與其胎兒之出生結果與往後生長發育之相關性。
結果:臍帶血中雙酚A、壬基酚與辛基酚之中位數濃度分別為 1.50、60.97 以及
2.30 ng/mL。在迴歸模式中,調整相關之潛在干擾因子後,發現壬基酚和出生時 的頭圍(per ln unit: β = -0.13, 95% CI: -0.23, -0.03)呈顯著之負相關;關於往後生長 發育的部分,發現壬基酚和頭圍(per ln unit: β = -0.10, 95% CI: -0.19, -0.01 for 0-18 months)與身高(per ln uint: β = -0.05, 95% CI: -0.10, -0.002 for 0-6 years)呈負相關。
結論:本研究結果顯示,臍帶血中壬基酚濃度和胎兒之出生頭圍與其往後的頭圍 和身高之發展呈負相關。但仍需要更多完整且長期的研究來進一步探討產前酚類
Abstract
Background and objective: Bisphenol A, nonylphenol, and octylphenol are widely
used in our life, and they all classified as endocrine-disrupting compounds (EDCs) which could lead the adverse effect on children’s growth. Previous studies investigated the association between prenatal phenols exposure and birth outcomes, and the findings were inconsistent. In addition, most of these studies measured the levels of phenols in maternal blood or urine during pregnancy to regard as the prenatal exposure.
Few studies measured the phenols levels in cord blood to represent the prenatal exposure of fetus. The objective of this study was to measure the phenols levels in cord blood and explore the effects of prenatal phenols exposure on birth size and growth.
Methods: The study was a part of the Taiwan Birth Panel Study. A total of 401 pairs
of parents and their infants were recruited in this study. We used the ultra-performance liquid chromatography – tandem mass spectrometry (UPLC/MS/MS) to measure the free form BPA, NP and OP in cord blood. Birth outcomes were obtained after delivery, and we followed up these children to obtain the growth data up to 6 years old. The association between phenols levels in cord blood and child growth was assessed using linear regression and mixed models.
Results: The median of BPA, NP, and OP concentration in cord blood were 3.10, 74.4,
and 2.30 ng/mL, respectively. For birth outcomes, after adjusting the confounders, head circumference decreased by 0.13 cm (95% CI: -0.23, -0.03) in association with a 1-unit increase in ln-transformed NP concentration. Regarding the child growth, a 1-unit increase of ln-NP was significantly associated with a decrease in head circumference (β = -0.11, 95% CI: -0.21, -0.02 for 0-18 months), and height z-score (β
= -0.06, 95% CI: -0.11, -0.01 for 0-6 years).
Conclusion: In this study, we observed an evidence of prenatal NP exposure may have
an adverse effect on head circumference at birth and child growth in the early childhood. However, among these young children, we didn’t find an effect of prenatal BPA and OP exposure on child growth. Therefore, further studies are needed to explore the association between prenatal phenols exposure and child growth
Key words: prenatal exposure, phenol, fetal and child growth, young children
Contents
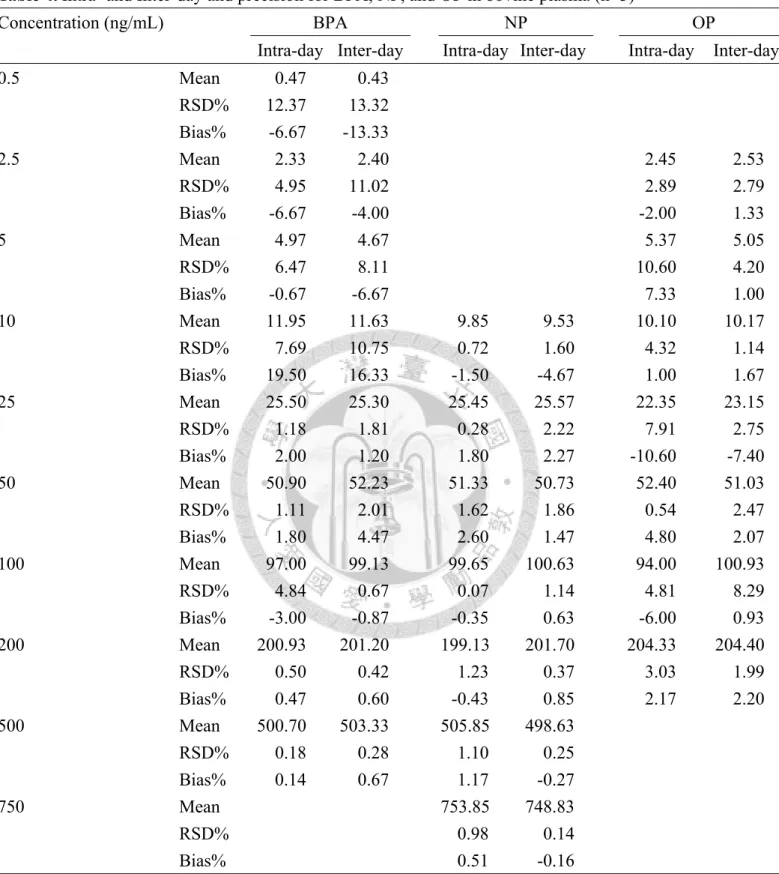
中文摘要 ... i Abstract ... ii Figure of contents ... v Table of contents ... vi Introduction ... 26 Materials and Methods ... 30 Study population ... 30 Measurement of BPA, NP, OP in cord blood ... 31 Chemicals and reagents ... 31 Sample preparation and calibration experiments ... 31 Instrumental analysis ... 33 Evaluation of matrix effect and extraction efficiency of sample pretreatment 34 Method validation and quantification ... 35 Infant’s birth outcomes and child growth ... 37 Statistical analysis ... 37 Results ... 39 Method performance, matrix effect, recovery and method validation ... 39 Phenols levels in cord blood ... 40 Prenatal phenols exposure and child growth ... 41 Discussion ... 43 References ... 48 Appendix 1 ... 70 Appendix 2 ... 72 Appendix 3 ... 73
Figure of contents
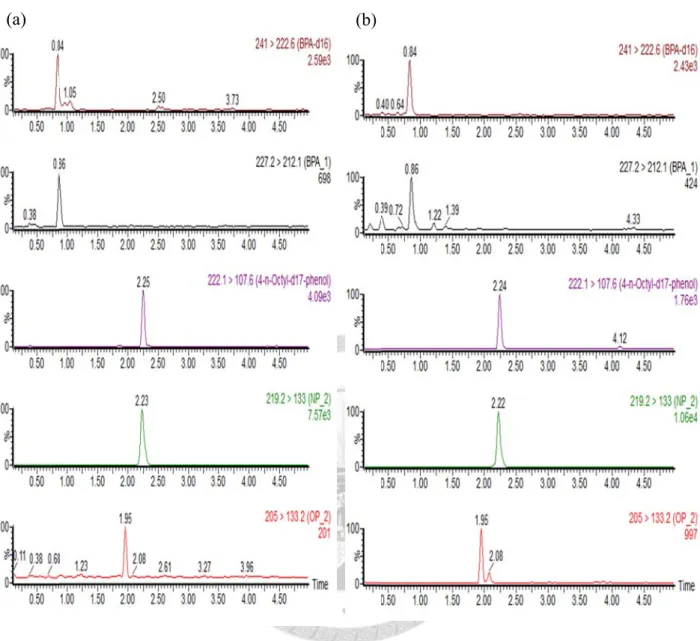
Figure 1. The flow chart of study population ... 51 Figure 2. The flow chart of sample preparation ... 52 Figure 3. UPLC/MS/MS chromatogram of solvent blank and matrix blank ... 53 Figure 4. UPLC/MS/MS chromatogram of bovine plasma and human cord blood ... 54
Table of contents
Table 1. Selected reaction monitoring (SRM) transitions, individual collision energy and cone voltage of the analytes ... 55 Table 2. Instrumental parameters of the mass spectrometer ... 56 Table 3. Matrix effect factor (%), extraction efficiency and recovery percentages of analytes with different concentration in bovine plasma ... 57 Table 4. Intra- and Inter-day and precision for BPA, NP, and OP in bovine plasma .... 58 Table 5. Quantification of the spiked samples in human cord blood plasma ... 59 Table 6. The detection rate and concentration of BPA, NP, OP in cord blood plasma . 60 Table 7. Characteristics of 401 mother-infants pairs included in this study ... 61 Table 8. Phenols levels distribution in maternal and infant’s characteristics ... 62 Table 9. Results from univariable linear/mixed model analysis ... 63 Table 10. Linear regression models of phenols levels in cord blood and birth outcomes ... 64 Table 11. Mixed models of phenols levels in cord blood and child growth from birth to 12 months ... 65 Table 12. Mixed models of phenols levels in cord blood and child growth from birth to 2 years ... 66 Table 13. Mixed models of phenols levels in cord blood and child growth from birth to 5 years ... 67 Table 14. Mixed models of phenols levels in cord blood and child growth from birth to 6 years ... 68 Table 15. Dose-response of prenatal NP exposure and child growth ... 69
Introduction
Bisphenol A (BPA) used in the manufacture of polycarbonate plastics (PC) and epoxy resins. BPA had been found to release from the plastic products, including toys, baby bottles, dental sealant, food and water containers, also the lining of beverages and food cans (Cao et al., 2009; Maragou et al., 2008). Nonylphenol (NP) and octylphenol (OP) are widely used and the most common surfactants in domestic detergents, pesticide formulations, industrial products, and release from the wax used for coating fruits and vegetables, plastic food packaging (Ying et al., 2002). BPA, NP, and OP are widely spread in the environment, they all classified as endocrine-disrupting compounds (EDCs). Endocrine disruptors are the chemicals that can interfere with hormone system in animals and humans, and then affect the natural hormones in the body responsible for the maintenance of homeostasis and the regulation of developmental processes (Kavlock, 1999).
Exposure sources and routes are ubiquitous make human exposure nearly universal, especially in dietary ingestion. Specific products or activities had been examined for potential exposure of human, including canned food, microwave containers
dietary ingestion for general population, especially for preschool children. Dietary ingestion of BPA accounted for > 95% of the children excreted amounts of urinary BPA (Morgan et al., 2011). The average daily NP intake in Taiwan is at least 4-fold higher than daily intake in western countries due to the dietary habit of Taiwanese (Lu et al., 2007). However, phenols exposure of pregnant mothers is an important issue and should be concerned about.
Phenols levels had been measured in maternal blood, placental tissue and cord blood of pregnant mothers in previous studies (Chen et al., 2008; Schönfelder et al., 2002). These findings supported that phenols can accumulate in the maternal-fetal-placental unit, and have the maternal-fetal transfer when the barrier of placenta does not exist. Placental transfer can lead to the prenatal exposure to fetus, and the potential effect of prenatal phenols exposure on fetal and children growth is a concern.
Previous studies which investigated the association between prenatal phenols exposure and birth outcomes were limited and inconsistent. In the general population, few studies found the adverse effect of prenatal BPA exposure on birth outcomes such as gestational age and birth weight (Chou et al., 2011; Cantonwine et al., 2010).
Increased BPA exposure was associated with slightly increase in head circumference (Philippat et al., 2012). Another study didn’t find the association between prenatal BPA
exposure and birth outcomes (Wolff et al., 2008). In addition, the effect of prenatal NP exposure on the birth size of offspring was only shown in the animal studies (Jie et al., 2010). Regarding the body growth at postnatal age, the effect of prenatal BPA exposure on body weight was only shown in animal studies (Honma et al., 2002; Rubin et al., 2001). Overall, the association between prenatal phenols exposure and child growth is not explicit. Additionally, most of these previous studies measured the levels of phenols in maternal blood or urine during pregnancy to regard as the prenatal exposure of fetus. Few studies used the phenols levels in cord blood to represent the prenatal exposure.
According to the metabolism of bisphenol A in human, BPA were rapidly absorbed from the gastrointestinal tract in human. Conjugation with glucuronic acid in the liver, and rapidly cleared from blood by elimination with urine (Völkel et al., 2002).
The half-life of BPA and NP from the blood was 5.3 and 2-3 hours, respectively (Völkel et al., 2002; Müller et al., 1998). Although the half-life of phenols is short, the exposure sources and routes are ubiquitous in our life. BPA glucuronide does not exert hormonal activity (Matthews, 2001; Snyder et al., 2000), therefore only unconjugated BPA is possibly expected the hormonal effects and cause the health effect. Also, there
potential estrogenic effects. Furthermore, the deconjugation of BPA glucuronide in
utero by β-glucuronidase, an enzyme that is present in high concentrations in placenta
and various other tissues (Ginsberg and Rice, 2009). This creates the potential for local activation of the conjugated from back to free BPA in numerous tissues and may be important to resultant fetal exposure. The risk of phenols exposure does not be negligible despite the rapid metabolism in human.
In this study, we measured the free form BPA, NP and OP levels in cord blood.
Moreover, we explored the effect of prenatal phenols exposure on birth size and child growth among the young children.
Materials and Methods
Study population
This study was based on the Taiwan Birth Panel Study (TBPS), a prospective cohort study, which was conducted between April 2004 and January 2005 (Hsieh et al., 2011). Informed consents were obtained from study participants before delivery to collect umbilical cord blood at birth and stored at -80℃ until laboratory analysis.
After delivery, all subjects were interviewed by trained interviewers to obtain the information during prenatal life style. The protocols used in this study were approved by the Ethical Committee of National Taiwan University Hospital.
In this study, five mothers who reported smoking over 5 months during pregnancy, four mothers who reported drinking more than 100 mL during pregnancy, four infants who were very preterm (gestational age < 32 weeks), and six twin babies were excluded from the analysis. Combined with the appropriate cord blood for phenols measurement, finally, a total of 401 mother-infant pairs were remained in our study.
Measurement of BPA, NP, OP in cord blood
Chemicals and reagents
Bisphenol A was supplied by AccuStandard (New Haven, Connecticut, USA).
Bisphenol A-D16, 4-tert-octylphenol and the technical mixture of nonylphenol were obtained from Sigma/Aldrich (Saint Louis, MO, USA). 4-n-Octyl-d17-phenol was supplied by C/D/N Isotopes (Pointe-Claire, Quebec, Canada; purity > 98%). Stock solutions of each compound were prepared at a concentration of 500 μg/mL in methanol and stored at -20℃. Milli-Q water was obtained from a Millipore water purification system (Milford, MA,USA). N-methylmorpholine (purity > 99.5%) were provided by J.T. Baker (Phillipsburg, NJ, USA). Solvents including methanol and acetonitrile were all LC/MS grade (J.T. Baker). Bovine plasma were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Sample preparation and calibration experiments
All glassware was rinsed with methanol before being used for experiments. The concentration of BPA, NP, and OP in the cord blood were quantified using modified analytical methods previously described (Anari et al., 2002). We conducted a small
experiment to compare two methods of derivatized with Dansyl chloride and underivatized analysis. We chose the better method by comparing the linear range of calibration and the feasibility between derivatization and non derivatization (the results showed in Appendix1). Finally, the underivatization method was used in this study.
A 50 μL aliquot plasma was diluted with 0.5 mL of water and added in 50 μL of internal standard (Bisphenol A-D16 and 4-n-Octyl-d17-phenol; 200 ng/mL in methanol).
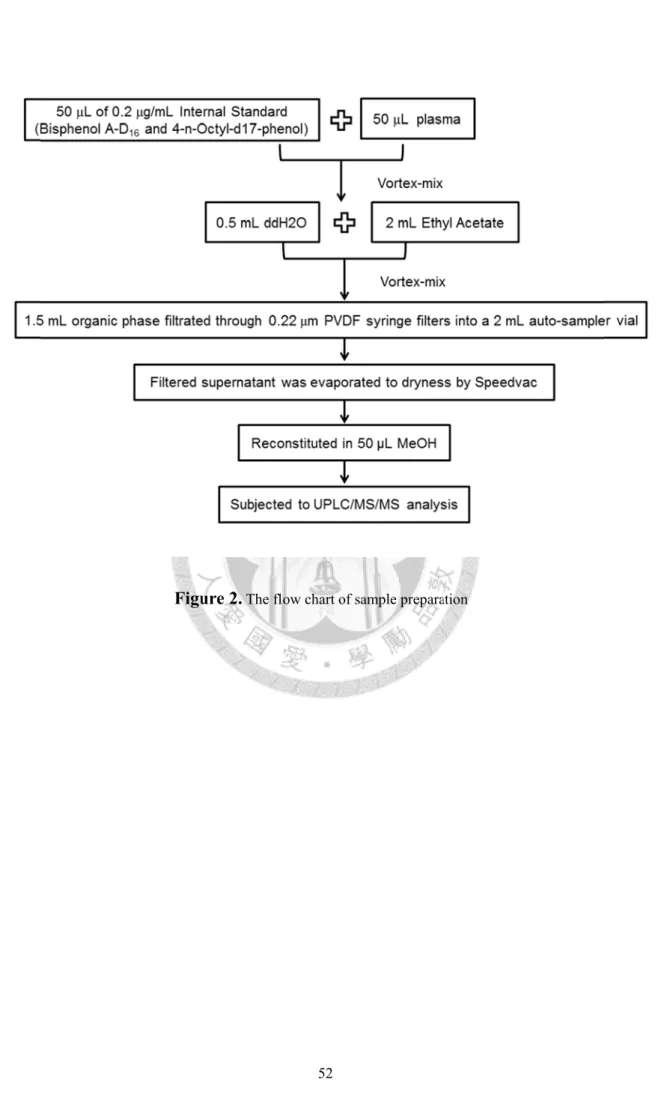
After gentle mixing, samples were added in 2 mL of ethyl acetate to each test tube. The samples were vortex-mixed and the organic phase (1.5 mL) of the samples was transferred to another tube, then filtrated through 0.22 μm PVDF syringe filters into a 2 mL auto-sampler vial, and evaporated to dryness by SpeedVac concentrator (Thermo Savant SPD 1010, Holbrook, NY, USA). The residues were reconstituted with 50 μL of methanol and transferred to 150-μL insert for UPLC/MS/MS analysis.
Matrix-matched standard calibration solution was prepared in bovine plasma and through the same procedure of sample preparation. The linear range were 0.5-500 ng/mL for BPA, 10-750 ng/mL for NP, 2.5-200 ng/mL for OP, and spiked 200 ng/mL of internal standard (BPA-d16 and 4-n-Octyl-d17-phenol ) in each solution.
Instrumental analysis
We measured the concentration of bisphenol A (BPA), nonylphenol (NP), and octylphenol (OP) in cord bloods using ultra-performance liquid chromatography tandem mass spectrometry (UPLC/MS/MS). The UPLC/MS/MS was performed using a Waters Acquity UPLC system (Waters Corporation, Milford, MA, USA) and controlled by MassLynx V4.1 with QuanLynx Application Manager. An ACQUITY UPLC BEH C18 column (2.1 mm 50 mm, 1.7μm) was used, the temperature and the flow rate were maintained at 60℃ and 0.5 mL/min, respectively. The mobile phase was composed of 10 mM N-methylmorpholine (pH 9.5) and acetonitrile. The initial composition of gradient program was 40% acetonitrile for 0.5 min, followed by a linear gradient to 60% acetonitrile in 0.5 min, then 95% acetonitrile in 2 min and held at 95% acetonitrile for 1 min before being returned to the initial condition. At last, the column was re-equilibrated at 40% acetonitrile for 1 min. The total run time of gradient program was 5 minutes and the sample injection volume was 5 μL.
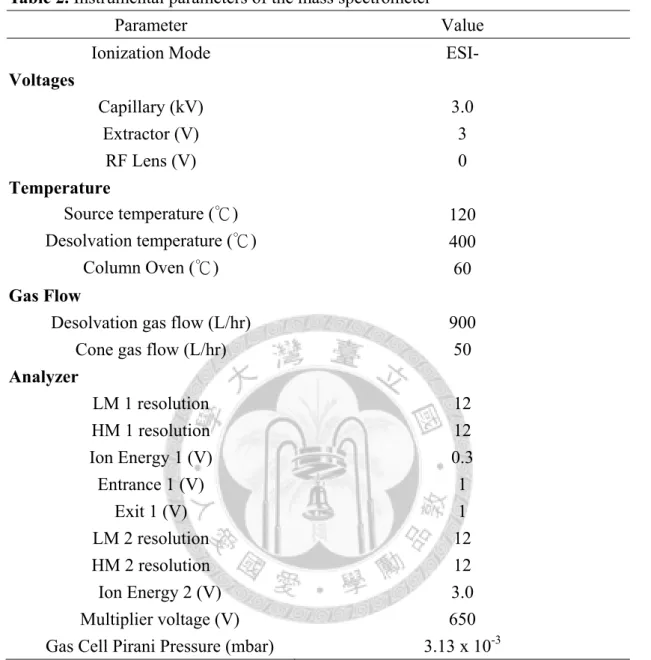
To achieve maximal analyte signal intensities, the instrumental parameters were referenced to previously described (Lien et al., 2009). The mass spectrometer was performed in native electrospray ionization (ESI-) and the capillary voltage was maintained at 3.0 kV. The desolvation gas flow, con gas flow, desolvation temperature,
and source temperature were set at 900 L/hr, 50 L/hr, 400℃ and 120℃, respectively.
Extractor voltage was 3.0 V and RF lens voltage was 0 V. Collision gas was argon at 3.13×10-3 mbar. Ion energy 1 and 2 were set at 0.3 and 3, respectively. Both LM 1 and LM 2 resolution were set at 12. The multiplier voltage was set at 650 V. The dwell time for NP and OP were 0.08 second, then for BPA was 0.1 second. Ions were monitored by selected reaction monitoring (SRM) as shown in Table 1 (p55) as well as the individual collision energy and cone voltage.
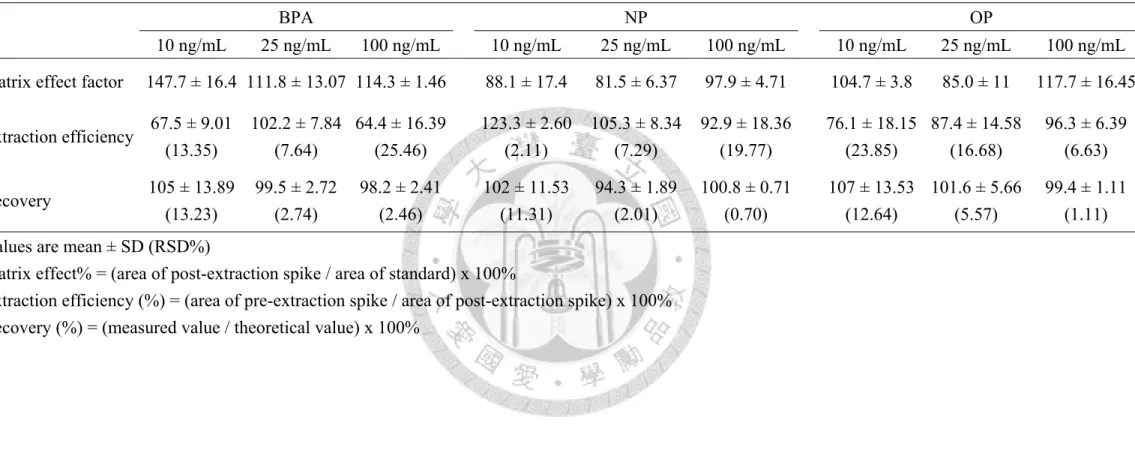
Evaluation of matrix effect and extraction efficiency of sample pretreatment
Three duplicates of bovine plasma which were spiked three different levels of BPA, NP and OP (10, 25 and 100 ng/mL) before or after the procedure of sample preparation. For matrix effect, the same levels of all analytes were spiked into solvent solutions. The percent matrix effect was calculated by the following formula: matrix effect% = (area of post-extraction spike / area of standard) × 100. Then, the extraction efficiency was tested to measure the analytes loss during sample preparation. We used the following formula to calculate the extraction efficiency: absolute recoveries% =