行政院國家科學委員會補助專題研究計畫
■ 成 果 報 告
□期中進度報告
自發性失神癲癇大鼠中 zona incerta 腦合對全面性癲癇與鬍鬚顫抖影響之研究
Role of zona incerta in generalized seizure and whisker tremor in the rat with
spontaneous spike-wave discharges
計畫類別:■ 個別型計畫 □ 整合型計畫
計畫編號:NSC
95-2314-B006-122-MY2
執行期間: 95 年 8 月 1 日至 97 年 7 月 31 日
計畫主持人:蕭富仁
共同主持人:
計畫參與人員:葉耿宏
成果報告類型(依經費核定清單規定繳交):□精簡報告 ■完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、列
管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年■二年後可公開查詢
執行單位:國立成功大學認知科學所
中 華 民 國 97 年 8 月 28 日
自發性失神癲癇大鼠中 zona incerta 腦合對全面性癲癇與鬍鬚顫抖影響之研究
Role of zona incerta in generalized seizure and whisker tremor in the rat with spontaneous
spike-wave discharges
計畫編號:NSC95-2314-B006-122-MY2
執行期限:95 年 8 月 1 日至 97 年 7 月 31 日
主持人:蕭富仁
國立成功大學認知科學所
專任學士級研究助理:葉耿宏
一、中文摘要
視 丘 之 Zona incerta (ZI) 與 視 丘 網 狀 核 (reticular nucleus, TRN))在發育過程中屬於相同的 發生源,這兩個腦核的神經細胞主要是抑制性的 GABAergic 神經細胞。相對於視丘網狀核在清醒-睡眠以及癲癇發作等狀態的精細研究而言,對於 ZI 腦核的了解卻非常少。在大鼠自發性產生與失 神 癲 癇 相 關 之 高 電 位 節 律 性 棘 波 (high-voltage rhythmic spike)時經常會出現在 Long Evans 大鼠, 為了進一步了解 ZI 在高電位節律性棘波與睡眠紡 錘波產生時所扮演的角色,本實驗將兩束電極分別 埋置於桶狀大腦皮層(barrel cortex)以及 ZI 背側 (ZId)位置以記錄其場電位與神經動作電位。相較於 桶狀大腦皮層神經細胞放電而言,ZId 神經細胞具 有顯著高的放電頻率以及較寬廣的感覺接受區域 範圍,同時 ZId 神經細胞的動作電位的時間寬度也 較桶狀皮值神經細胞的時間寬度還要長,這間接支 持 ZId 神經細胞可能是 GABAergic 抑制性神經細 胞。ZId 神經細胞在淺睡時沒有出現與睡眠紡錘波 相關的節率;但在高電位節律性棘波產生時 ZId 會 出現與高電位節律性棘波同步之節律性活性以及 開/關的放電特性,且 ZId 神經細胞活性永遠顯著 落後桶狀神經細胞活性,而 ZId 神經細胞出現節律 性活性時都會觀察到高電位節律性棘波的棘波振 幅顯著變大且震盪頻率變高的現象。另外,ZId 神 經細胞在睡眠紡錘波與高電位節率性棘波發生時 很少出限 burst 放電,這個結果顯著不同於網狀核 神經細胞的反應。所以兩種平行的兩個抑制性 GABAergic 相關腦核(ZI & TRN)在不同的行為狀 態下會對於視丘與大腦有不同的作用。
關鍵詞:失神癲癇、睡眠紡錘波。
Abstract
The zona incerta (ZI) has the same developmental origin as the thalamic reticular nucleus (TRN), and most neurons in both nuclei are GABAergic. In contrast to extensive studies of the TRN in alteration of vigilance and the generation of epileptic activities, only a few reports have
investigated the function of the ZI. Spontaneous high-voltage rhythmic spikes (HVRSs), which may be associated with absence seizure, are often recorded in Long Evans rats. To clarify the role of the ZI in spindle oscillations and HVRS discharges, two sets of microwires were chronically implanted in the dorsal
division of the ZI (ZId) and barrel cortex (BC). Compared to the BC, ZId neurons displayed a higher firing rate and a wider receptive field. They also revealed thin action potentials, which might be related to GABAergic neurons. No spindle-related activity was found in the ZId during light sleep. In contrast, clear intermittent rhythmic activities coincident with paroxysmal HVRS discharges having an on-off pattern were shown in the ZId. ZId neurons always lagged behind those of the BC in the onset of HVRS discharges and within HVRS activities. Moreover, rhythmic ZId activities often occurred during high-amplitude HVRS activities. Interestingly, ZId neurons displayed rare bursts during spindle oscillations and HVRS discharges, which differed markedly from those of TRN neurons. Accordingly, these two parallel GABAergic controls of the ZId and TRN may exert distinct inhibitory actions on cortical and thalamic neurons under different behavioral states.
Keywords: zona incerta, absence seizure, sleep spindle.
二、緣由與目的
The zona incerta (ZI) and thalamic reticular nucleus (TRN) share developmental origins in the ventral thalamus (Jones, 1985), and most neurons of both nuclei are GABAergic (Ficalora and Mize, 1989; Benson et al., 1992; Mitrofanis, 2005). The TRN has been demonstrated to serve as a pacemaker of spindle activity and to modulate thalamocortical networks resulting in alterations of vigilance states (Steriade et al., 1997). Moreover, it is also linked to the
generation of epileptic discharges (Steriade et al., 1997). Whether the ZI plays a similar role to that of the TRN remains unresolved. Although cortical activities are not altered after lesions to the ZI (Jurkowlaniec et al., 1990), neuronal activities of the ZI under wake-sleep states remain obscure (Steriade et al., 1982; Bartho et al., 2007; Urbain and
Deschenes, 2007).
In the past two decades, anatomical tracing evidence has indicated that incertal neurons are densely connected with various brain regions, including the superior colliculous (Kim et al., 1992; Nicolelis et al., 1992), dorsal thalamus (Power et al., 1999; Bartho et al., 2002; Lavallee et al., 2005),
cerebral cortex (Lin et al., 1990; Nicolelis et al., 1992), and the ZI itself (Mitrofanis, 2005). The superior colliculus strongly innervates the facial motor nucleus for controlling facial motor activity (Kleinfeld et al., 2006; van Luijtelaar and Sitnikova, 2006). A somatotopic map was also found in the ZI (Nicolelis et al., 1992), particularly for an extensive representative area of the head. Electrical or chemical stimulation of the ZI results in changes in behavior or posture (Milner and Mogenson, 1988; Supko et al., 1991; Murer and Pazo, 1993; Dybdal and Gale, 2000; Périer et al., 2002). Recently, the advantage of ZI stimulation in parkinsonian tremors and disabilities was also reported (Voges et al., 2002; Benazzouz et al., 2004; Plaha et al., 2006; Guehl et al., 2008). Moreover, the ZI displays susceptibility to generalized seizures (Brudzynski et al., 1995; Deransart et al., 1996; Hamani et al., 2002). In the first year of this project, we provide evidence of microstimulation and lesion to support the incertal control of HVRS discharges and perioral tremor. Based on prior knowledge, the ZI is involved in the generation or development of generalized absence seizures and related myoclonus.
To the development of absence epilepsy, a “cortical focus theory” is recently proposed (Meeren et al., 2005) based on numerous electrophysiological and pharmacological results in rats with spontaneous absence epileptic discharges (Meeren et al., 2002; Manning et al., 2004; Gurbanova et al., 2006). The perioral representative areas of the cerebral cortex, particularly of the barrel cortex (BC), can initiate spontaneous absence epileptic discharges (Meeren et al., 2002; Manning et al., 2004). Spontaneous high-voltage rhythmic spike (HVRS) discharges, which may be associated with absence seizure, are observed in Long Evans rats (Kaplan, 1985; Buzsáki et al., 1990; Shaw, 2004, 2007). Consequently, correlates of neuronal activities between the ZI and BC during HVRS discharges may be of benefit to understand the mechanism of HVRS generation.
In second-year part of this project, we tried to correlate the neuronal activities between the BC and ZI during natural sleep spindle rhythm and
spontaneous HVRS discharges. Neural bursts of the BC and ZI neurons in the two conditions were analyzed.
三、結果與討論
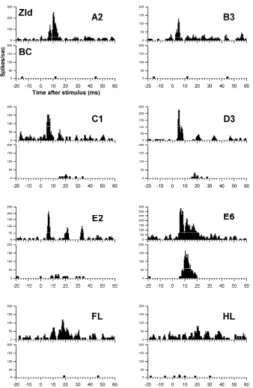
Figure 1 depicts the activities of dorsal ZI (ZId) neurons and BC neurons in response to peripheral stimulation. ZId neurons showed a strong response to the stimulation of almost entire body, and BC neurons only responded to a single whisker. The receptive field and firing frequency of ZId and BC neurons were summarized in Table 1. The
distribution showed a significant difference between the ZId and BC neurons (P < 0.005 by χ test).
Figure 2 depicts the firing pattern of ZId and BC neurons during natural sleep. Burst firing of BC neurons was clearing seen in BC neurons, but not shown in ZId neurons.
Figure 3 depicts an example of ZId and BC neurons during a spontaneous HVRS discharge. ZId neurons showed a part of rhythmic activity during HVRS discharges and a transient increase of firing frequency at the beginning and end of a HVRS episode. In addition, BC neurons led to ZId neurons in all HVRS bouts.
Figure 4 depicts burst firing analysis of ZId and BC neurons. BC neurons showed a clear burst firing in a Poincare plot, but not obvious in ZId neurons. Additionally, clear rhythmic activities were seen in the BC and ZId neurons.
Figure 5 depicts burst firing proportion of BC and ZId neurons. BC neurons showed significant higher proportion in burst firing compared to ZId neurons under both sleep spindle and HVRS discharges.
Figure 6 depicts ensemble BC and ZId neuronal activities in a rat. In HVRS discharges, BC showed a 7-12 Hz rhythmic activity, but ZId neurons also displayed a comparable rhythm in a part of HVRS bout added on transient increase activity at the beginning and end of the HVRS bout. By contrast, BC neurons showed a spindle rhythm, but no spindle rhythm was observed in ZId.
In summary, we found the ZI involving in the modulation of HVRS discharges but being not related to spindle activity. Specifically, ZI seems to be able to terminate spontaneous HVRS discharge.
To date, we have published a paper in the
British Journal of Anaesthesia and 3 conference
papers. One manuscript has been submitted into the journal Epilepsia, and the other one is under preparation.
四、參考文獻
Bartho, P., Freund, T.F. & Acsady, L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur. J. Neurosci. 16, 999-1014 (2002). Bartho, P., Slezia, A., Varga, V., Bokor, H., Pinault,
D., Buzsaki, G. & Acsady, L. Cortical control of zona incerta. J. Neurosci. 27, 1670-1681 (2007). Benazzouz, A., Tai, C.H., Meissner, W., Bioulac, B.,
Bezard, E. & Gross, C. High-frequency stimulation of both zona incerta and subthalamic nucleus induces a similar normalization of basal ganglia
metabolic activity in experimental parkinsonism.
FASEB J. 18, 528-530 (2004).
Benson, D.L., Isackson, P.J., Gall, C.M. & Jones, E.G. Contrasting patterns in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience 46, 825-849 (1992). Brudzynski, S.M., Cruickshank, J.W. & McLachlan,
R.S. Cholinergic mechanisms in generalized seizures: important of the zona incerta. Can. J.
Neurol. Sci. 22, 116-120 (1995).
Buzsáki G, Smith A, Berger S, Fisher LJ & Gage FH. Petit mal epilepsy and parkinsonian tremor: hypothesis of a common pacemaker. Neuroscience 36, 1-14 (1990).
Deransart, C., Marescaux, C. & Depaulis, A. Involvement of nigra glutamatergic inputs in the control of seizures in a genetic model of absence epilepsy in the rat. Neuroscience 71, 721-728 (1996).
Dybdal, D. & Gale, K. Postural and anticonvulsant effects of inhibition of the rat subthalamic nucleus.
J. Neurosci. 20, 6728-6733 (2000).
Ficalora, A.S. & Mize, R.R. The neurons of the substantia nigra and zona incerta which project to the cat superior colliculus are GABA
immunoreactive: a double-label study using GABA immunocytochemitry and lectin retrograde
transporter. Neuroscience 29, 567-581 (1989). Guehl, D., Vital, A., Cuny, E., Spampinato, U.,
Rougier, A., Bioulac, B. & Burbaud, P. Postmortem proof of effectiveness of zona incerta stimulation in Parkinson disease. Neurology 70, 1489-1490 (2008).
Gurbanova, A.A., Aker, R., Berkman, K., Onat, F.Y., van Rijn, C.M. & van Luijtelaar, G. Effect of systemic and intracortical administration of phenytoin in two genetic models of absence epilepsy. Br. J. Pharmacol. 148, 1076-1082 (2006). Hamani, C., Sakabe, S., Bortolotto, Z.A., Cavalheiro, E.A. & Mello, L.E.A.M. Inhibitory role of the zona incerta in the pilocarpine model of epilepsy.
Epilepsy Res. 49, 73-80 (2002).
Jones, E.G. The Thalamus. Plenum, New York, (1985).
Jurkowlaniec, E., Trojniar, W. & Tokarski, J. The EEG activity after lesions of the diencephalic part of the zona incerta in rats. Acta Physiol. Polonica 41, 85-97 (1990).
Kaplan, B.J. The epileptic nature of rodent
electrocortical polyspiking is still unproven. Exp.
Neurol. 88, 425-436 (1985).
Kim, U., Gregory, E. & Hall, W.C. Pathway from zona incerta to the superior colliculus. J. Comp.
Neurol. 321, 555-575 (1992).
Kleinfeld, D., Ahissar, E. & Diamond, M.E. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr. Opin. Neurobiol. 16, 435-444 (2006).
Lavallee, P., Urbain, N., Dufresne, C., Bokor, H., Acsady, L. & Deschenes, M. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J. Neurosci. 25, 7489-7498 (2005).
Lin, C.S., Nicolelis, M.A.L., Schneider, J.S. & Chapin, J.K. A major direct GABAergic pathway from zona incerta to neocortex. Science 248, 1553-1556 (1990).
Manning, J.P., Richards, D.A., Leresche, N., Crunelli, V. & Bowery, N.G. Cortical-area specific block of genetically determined absence seizures by ethosuximide. Neuroscience 123, 5-9 (2004). Meeren, H., van Luijtelaar, G., Lopes da Silva, F., &
Coenen, A. Evolving concepts on the
pathophysiology of absence seizures. The cortical focus theory. Arch. Neurol. 62, 371-376 (2005). Meeren, H.K.M., Pijn, J.P.M., van Luijtelaar,
E.L.J.M., Coenen, A.M.L. & Lopes da Silva, F.H. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J. Neurosci. 22, 1480-1495 (2002).
Milner, K. & Mogenson, G.J. Electrical and chemical activation of the mesencephalic and subthalamic locomotor regions in freely moving rats. Brain Res. 452, 273-285 (1988).
Mitrofanis, J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience 130, 1-15 (2005).
Murer, M.G. & Pazo, J.H. Circling behaviour induced by activation of GABAA receptors in the
subthalamic nucleus. Neuroreport 4, 1219-1222 (1993).
Nicolelis, M.A.L., Chapin, J.K. & Lin, R.C.S. Somatotopic maps within the zona incerta relay parallel GABAergic somatosensory pathways to the neocortex, superior colliculus, and brain stem.
Brain Res. 577, 134-141 (1992).
Périer, C., Tremblay, L., Féger, J. & Hirsch, E.C. Behavioral consequences of bicuculline injection in the subthalamic nucleus and the zona incerta in rat.
J. Neurosci. 22, 8711-8719 (2002).
Plaha, P., Ben-Shlomo, Y., Patel, N.K. & Gill, S.S. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in
improving contralateral parkinsonism. Brain 129, 1732-1747 (2006).
Power, B.D., Kolmac, C.I. & Mitrofanis, J. Evidence for a large projection from the zona incerta to the
dorsal thalamus. J. Comp. Neurol. 404, 554-565 (1999).
Shaw, F.-Z. Is spontaneous high-voltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J. Neurophysiol. 91, 63-77 (2004). Shaw, F.-Z. 7-12 Hz high-voltage rhythmic spike
discharges in rats evaluated by antiepileptic drugs and flicker stimulation. J. Neurophysiol. 97, 238-247 (2007).
Steriade, M., Parent, A., Ropert, N. & Kitsikis, A. Zona incerta and lateral hypothalamic afferents to the midbrain reticular core of cat – an HRP and eletrophysiological study. Brain Res. 238, 13-28 (1982).
Steriade, M., Jones, E.G. & McCormick, D.A. Thalamus. Vol. I Organization and Function. Elsevier, New York, (1997).
Supko, D.E., Uretsky, N.J. & Wallace, L.J. Activation of AMPA/kanic acid glutamate receptors in the zona incerta stimulates locomotor activity. Brain
Res. 564, 159-163 (1991).
Urbain, N. & Deschenes, M. Motor cortex gates vibrissal responses in a thalamocortical projection pathway. Neuron 56, 714-725 (2007).
van Luijtelaar, G. & Sitnikova, E. Global and focal aspects of absence epilepsy: the contribution of genetic models. Neurosci. Biobehav. Rev. 30, 983-1003 (2006).
Voges, J. et al. Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson disease: correlation of therapeutic effect with anatonomical electrode position. J. Neurosurg. 96, 269-279 (2002).
Fig. 1. Peri-stimulus histograms of single ZId and BC neurons to innocuous stimulation in various body parts. A prominent short-latency evoked peak (4-10 ms) was observed in the ZId. The ZId neuron responded to the stimulation in a large body part, including many whiskers and the forelimb. BC neurons only responded to the whisker E6 and displayed a prominent peak in the range of 10-15 ms. A1, B3, C1, D3, E2, and E6 are whisker number. FL, forelimb; HL, hind limb.
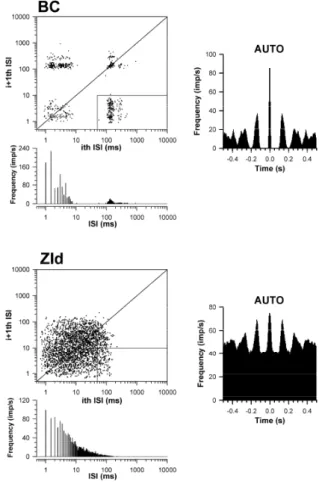
Fig. 2. Representative example of ZId and BC activities during sleep. (A) Field potentials (FPs) and multiunit activities (MUAs) of the ZId and BC during sleep. Several spindle oscillations, which oscillated at
7-14 Hz and recurred at 1-5 s, were observed in BC FPs. Hyperpolarization (arrow) was followed by spindle oscillation. However, no coincident spindle activity was observed in ZId traces. Portraits of action potentials (APs) of ZId and BC neurons are shown in the rightmost panel. Durations of APs of ZId neurons were obviously shorter than those of BC neurons (dashed lines). Slopes of ZId APs (dV/dt in the rightmost panel) were also steeper than those of BC neurons. Maximal slopes of ZId APs (1.82 ± 0.08 V/s) were higher than those of BC neurons (1.14 ± 0.05 V/s). (B) Firing characteristics of BC and ZId single units during spindle oscillations. Two clusters of rhythmic activities under spindle oscillations were found in interspike interval (ISI) histograms of the BC. A clear 12.5-Hz spindle oscillation is shown in the autocorrelogram (AUTO). A high portion of burst activities (19.97%), characterized in the right bottom box in the Poincaré plot, was also observed in BC neurons. By contrast, no spindle-compatible oscillation was observed in either AUTO or ISI histograms of the ZId. In addition, rare burst firing (3.95%) was observed in the ZId. Binwidth, 5 ms.
Fig. 3. Representative example of ZId and BC activities during high-voltage rhythmic spike (HVRS) discharges. (A) FPs and MUAs of the ZId and BC during an HVRS discharge. Coincident activity was found between the ZId and BC during HVRS discharges with different configurations. Hyperpolarization (arrow) was occasionally followed by an HVRS discharge in the BC, but none was observed in the ZId. A persistent spike discharge was found in the BC, but several episodes of rhythmic wax-and-wane activities were intermittently observed in the ZId. In a comparison of the onset of the HVRS discharge, the BC was 6 ms ahead of the ZId. Transient elevation of neuronal activities was seen in the spike count histogram of the ZId at the beginning of HVRS discharges. Binwidth, 10 ms. (B) Relation between FPs and MUAs of the BC and ZId during HVRS discharges by means of spike-triggered averages. BC neuronal activities preceded the FP spike by 2 ms, while ZId neuronal activities lagged behind the FP spike by 1 ms. FPs of the BC and ZId are presented as the mean (solid lines) ± S.D. (dashed lines). Binwidth, 1 ms. (C) Phase plots of FPs between the BC and ZId at different time courses of an HVRS discharge. Numbers shown in boxes were correlated with the number indicated under the FP trace of the ZId in part A. FPs of the BC preceded those of the ZId in many cycles, particularly during the middle phase (middle box). An
alternative structure between the ZId and BC in the middle box was caused by the notch of the ZId FP (arrow in part B).
Fig. 4. Firing characteristics of BC and ZId single units under HVRS discharges. Two clusters of rhythmic activities were found in ISI histograms of the BC under HVRS discharges. A 9-Hz oscillation was observed in AUTOs. A high propensity for burst firing (31.08%) was shown in Poincaré plot of the BC, but low (4.89%) in the ZId. A compatible 9-Hz oscillation, which stood on a platform, was illustrated in the AUTO of the ZId. High-frequency firing with irregular tonic discharges was observed in the Poincaré plot, thereafter resulting in the platform of AUTO. Binwidth, 5 ms.
Fig. 5. Comparison of the proportion of bursts in BC and ZId neurons under sleep spindles and HVRS discharges. The proportion of burst firings in the BC under both oscillations was significantly higher than that of the ZId. * p < 0.0001 by Mann-Whitney rank sum test.
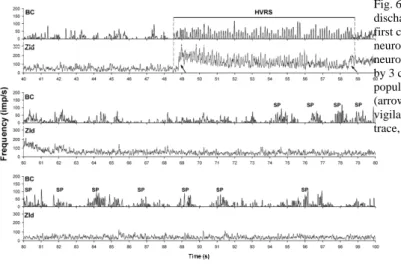
Fig. 6. Ensemble activity of 23 BC and 18 ZId neurons during HVRS discharges and sleep spindle (SP). Ensemble activity is shown by the first component of the principal component analysis. The cortical neurons showed a synchronous oscillation (~9 Hz) during HVRS. ZId neurons showed an identical oscillation, but lagged behind BC activity by 3 cycles. Transient increases were also observed in ZId neuronal population activities at both the beginning and end of HVRS discharges (arrows). An HVRS discharge was recorded before the transition of vigilance states. Spindle oscillatory activities can be seen in the BC trace, but not in the ZId trace. Binwidth, 10 ms.