行政院國家科學委員會專題研究計畫 成果報告
利用腺病毒轉殖增加環氧酵素一型及前列環素合成素的基
因表現,對心臟缺血再灌流後心臟損傷之保護。
計畫類別: 個別型計畫 計畫編號: NSC93-2314-B-002-203- 執行期間: 93 年 08 月 01 日至 94 年 07 月 31 日 執行單位: 國立臺灣大學醫學院內科 計畫主持人: 陳錦澤 共同主持人: 鄭敬楓 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 94 年 10 月 31 日
Abstract
Prsotacyclin (PGI2) has been shown
to prevent ischemic cardiac damage and stable PGI2 analogs are shown to exert
cardioprotective actions by increasing blood flow, reducing platelet aggregation and direct cardiac protection In this study, we delivered the adenovirus containing COX-1/PGIS which is the key productive enzyme of PGI2 , using a catheter-based
technique to investigate the signaling mechanism mediated by PGI2 in
protection against cardiomyocyte apoptosis induced by acute ischemia/reperfusion. After adenovirus-mediated gene delivery, highly efficient and specific expression of green fluorescent protein, or recombinant human COX-1 and PGIS were identified in the left ventricle. Delivery of the PGI2
gene 5 days before ischemia/reperfusion attenuated myocardial apoptosis identified by in situ dUTP nick-end labeling and DNA laddering, and the effect was blocked by the cAMP antagonist which induced signal by PGI2 .
Adv-COX-1/PGIS gene transfer increased phosphorylation of Akt but reduced and caspase-3 activities in the heart. The effects Adv-COX-1/PGIS gene transfer on phosphorylation of Akt and caspase-3 activities were blocked by LY294002(PI3 kinase inhibitor and by CAY(cAMP inhibitor).Furthermore, Adv-COX-1/PGIS gene transfer in cultured cardiomyocytes, also attenuated apoptosis induced by hypoxia/reoxygenation, which was accompanied by increased phospho-AKT and eNOS but reduced caspase-9 and caspase-3 activities. Phospho-AKT and caspase-3 activities were both blocked by Ad.DN-Akt andCAY and LY294002. These results indicate that Adv-COX-1/PGIS gene transfer protects against cardiomyocyte apoptosis induced
by ischemia/reperfusion injury through the PI3 kinase-Akt-eNOS -caspase signaling pathway.
Introduction:
Reperfusion of ischemic myocardium results in an abrupt aggravation of cardiomyocyte injury, demonstrated experimentally by the re-introduction of oxygen into hypoxic myocardium. This injury is due in part to the generation of reactive oxygen species (Bolli et al., 1988), and the injury is limited by antioxidants and free radical scavengers. It has also been suggested that maintenance of endogenous source of PGI2 is important in the limitation of
coronary artery disease and infarct size(Luscher et al., 1993)
Prostacyclin receptor, peroxisome proliferator-activated receptors delta PPARδ), exerts antiapoptotic effects via the AKT1 pathway. Prostacyclin (PGI2)
is produced by archadonic acid metabolism and prostacyclin production key enzymes are cyclooxygenase (COX) and Prostacyclin synthase (PGIS). Prostacyclin (PGI2) are vasodilatory
prostagaandins release by endothelial cells. PGI2exerted antiapoptotic effects through
IP receptors. Hatae et al. (2001) using embryonic kidney 293 cells demonstrated that while extracellular PGI2 exerted antiapoptotic effects through IP receptors, Cutler et al. (2003) demonstrated that stromal production of PGI2 promotes survival of colonocytes through PGI2 receptor, PPAR δ, activation. PGI2
analogue, iloprost, also shown can protect from doxorubicin induced cardiomyocyte injury and attenuates radiocontrast media-induced kidney cells apoptosis Recent findings indicate that the serine-threonine kinase Akt is a powerful survival signal leading to anti-apoptosis and cell survival, and it lies at the
crossroads of activated multiple apoptotic stimuli during myocardial injury. . Akt may thus contribute to antiapoptotic effects of phosphotidal inositol-3 kinase (PI- 3K)/Akt signaling. This is evidenced by the finding that overexpression of a dominant-negative mutant of Akt prevents apoptosis after inhibition of PI-3K. In addition several studies indicate that activation of PI-3K/Akt suppresse caspase-3 activation and DNA fragmentation in a variety of cell lines. However, the role of the interaction and signaling pathway among PGI2, Akt, and
caspase-3 in myocardial apoptosis following ischemia/reperfusion has not been explored.
Using a systemic gene transfer approach, we previously reported that human Adv-COX-1/PGIS gene delivery
protected against brain
ischemia-reperfusion damage in MCA model rats. In this study, we established a catheter-based technique for local Adv-COX-1/PGIS gene transfer to investigate the effects of PGI2 on
apoptosis and cell survival and its signaling mechanism in protection against ischemia/reperfusion (I/R)-induced myocardial apoptosis in vivo and in vitro. Our findings show that the PI3kinase/Akt/caspase-3–dependent pathway plays an important role in mediating the protection of PGI2 against
myocardial apoptosis.
Methods
Replication-Deficient Adenoviral
Vectors
Adenoviral vectors harboring human
COX-1 and PGIS
(Adv.-`HPGKcox-1/PGIS), green fluorescent protein (Ad.CMV-GFP), cDNA under the control of the HPGK promoter or without a reporter gene
(Ad.Null) were prepared as described.21
Catheter-Based Gene Delivery
Wistar mice (male; weight, 15 to 20 g; Harlan) were used in this study. The study complied with the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (National Academy of sinica). Five days before coronary occlusion, gene delivery was performed with the use of a catheter-based strategy as described.22,23 Briefly,mices were anesthetized, intubated, and mechanically ventilated before surgery. The chest was entered through a left intercostal approach. A
24-gauge catheter (BD) containing 300uL of virus solution (2X1010 pfu/mL in PBS) was advanced from the apex of the left ventricle to the aortic root. The aorta and pulmonary trunk were clamped distal to the site of the catheter, and the solution was injected. The clamp was maintained for 15 seconds. After injection, the exposed heart was monitored for 5 minutes for resumption of normal sinus rhythm. Mean arterial pressure (MAP) and electrocardiography were recorded throughout the experimental period.
Myocardial I/R Animal Model
Acute myocardial I/R models were established as previously described. 24 Briefly, a 6-0 polypropylene suture (Ethicon) was passed loosely around the left anterior descending (LAD) coronary artery near its origin. Once hemodynamics were stabilized, LAD occlusion was performed by tightening the suture loop for 30 minutes followed by 120 minutes of reperfusion. At the end of reperfusion, the ischemic regions were then removed for further analysis.24 Mices were randomly divided into 6 groups. In the sham group, the chest was opened and injected with saline (control, n=8). The I/R control group (n=7) was also injected
with saline. The third group received AdV-HPGJ .Null (n=7) and the fourth group received Ad-HPGK –COX-1/PGIS (n=8). The fifth group received AdV-.HPGK –COX-1/PGIS together with administration of antagonist.
In Situ Nuclear DNA Fragmentation and DNA Laddering
DNA fragmentation was determined by means of a terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) detection kit (Roche).24 The ratio of TUNEL-positive cardiomyocytes to the total number of cardiomyocytes was calculated. DNA laddering was analyzed as previously described.24 DNA fragments were separated by agarose gel electrophoresis and visualized under ultraviolet light.
In Vitro Cell Culture and
Hypoxia/Reoxygenation
Cardiomyocytes were isolated from the hearts of 2- to 3-day-old Wistar rats (Harlan) with an enzymatic technique, then cultured in DMEM/F-12 medium with 10% FBS. Cardiomyocyte origin was confirmed immunohistochemically using antibody to α-actin (Sarcomeric, Sigma). Cultured cells were growth-arrested in serum-free medium for 18 hours at 37°C before the experiments. Cells were transduced with Ad.CMV-COX-1/PGIS, Ad.Null, at MOI 50 for 24hours followed by 24-hour hypoxia (95% N2 and 5% CO2) and 24-hour reoxygenation (95%
O2 and 5% CO2). Before
hypoxia/reoxygenation (H/R), myocytes were treated with 20 mmol/L CAY(cAMP _ inhibitor) for 30 minutes or 100 _mol/L LY294002(PI3 kinase inhibitor) for 60 minutes. Apoptotic cardiomyocytes were identified by TUNEL staining (Roche), as previously described.26 Cell viability was determined by trypan blue eliminating
assay.
Western Blot Analysis
Heart tissues and cultured cardiomyocytes were homogenized in the protein extraction buffer containing 10 mmol/L Tris, pH 7.4, 100 mmol/L NaCl, 0.1% SDS, 1% Triton X-100, 5 mmol/L EDTA, 2 mmol/L Na3VO4, 1:100 protease inhibitor cocktail (Sigma). Aliquots were resolved on SDS-PAGE. Proteins were transferred to a nitrocellulose membrane and then incubated with the primary antibodies that recognize Akt, phospho-Akt, phospho-GSK-3_, GSK-3_ and cleaved caspase-3 (cell signaling) at 4°C overnight. Bound antibodies were detected by a secondary antibody conjugated to horseradish peroxidase and visualized by ECL chemiluminescence (NEN Life Science Products).
Immunohistochemistry
Expression and localization of human COX-1 pr PGIS in rat ventricles after gene delivery were identified immunohistochemically by antibody to human AM (Phoenix Pharmaceuticals).
Statistical Analysis
Data are expressed as mean_SEM and were compared between experimental groups with the use of 1-way ANOVA followed by Fisher protected least squares difference. Probability values of P_0.05 were considered statistically significant.
Result
Catheter-Based Adenoviral Gene Transfer for
Global Expression in Rat Heart
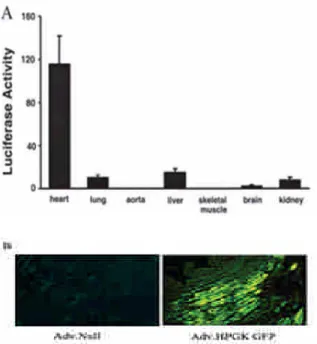
Using a catheter-based gene transfer technique, the highest levels of luciferase activity were detected in the myocardium, but very low levels were found in the liver, lung, brain, and kidney (Figure 1A). Left
ventricles transfected with Ad.CMV-GFP showed homogenous expression of green fluorescence, whereas the Ad.Null-transfected myocardium showed little background fluorescence (Figure 1B). The expression and localization of human recombinant AM in the left ventricle was confirmed and identified immunohistochemically at 4 to 6 days after local gene transfer. No specific staining was found in the left ventricle of the Ad.Nullinjected group (Figure 2).
Adv-COX-1/PGIS Gene Delivery
Attenuates Apoptosis in the Acute I/R Rat Model
Figure 3A shows representative apoptotic cardiomyocytes identified by TUNEL staining in the I/R-injured region. The ratio of TUNEL-positive cardiomyocytes to total number of cardiomyocytes in the Adv-COX-1/PGIS. group was significantly reduced as compared with the Ad.Null group (22.7 ±4.8% versus 43.4±6.1%, n=8 and=7, P<0.001). This beneficial effect was abolished by Results of TUNEL detection were in accordance with DNA fragmentation assay (Figure 3C). DNA laddering was not visualized in the sham-operated heart tissue, whereas I/R markedly increased DNA fragmentation. Adv-COX-1/PGIS gene transfer abrogated I/R-induced DNA fragmentation. These results indicate that expression of Adv-COX-1/PGIS gene transfer in the heart protects against I/R-induced cardiomyocyte apoptosis. Effects ofAdv-COX-1/PGISon Akt, , and Caspase-3 in I/R Injury
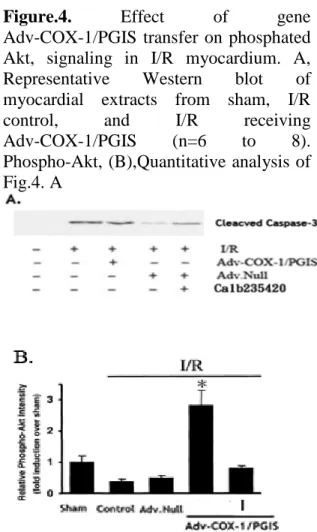
Western blot analysis showed that Adv-COX-1/PGIS delivery resulted in increased Akt phosphorylation in the heart after I/R as compared with the controls, with or without delivery of Ad.Null. The effects of Adv-COX-1/PGIS on Akt were abolished by the COX non-specific
antagonist anpirin (Figures 4A ,B). Similarly, cleaved caspase-3, which is a downstream proapoptotic signal, was markedly reduced after Adv-COX-1/PGIS gene delivery, whereas the inhibitory effect of on caspase-3 activation was
abrogated by caspase-3
inhibitor(Calbiochem 235420) (Figures 5A and 5B).
Effect of PGI2 analogue, beraprost (BPS), on H/R-Induced Apoptosis and Viability in Cultured Cardiomyocytes
Based on findings in vivo, we examined the effects of PGI2 on signal events in
relation to cardiomyocyte apoptosis and cell survival subjected to H/R. Treated with BPS significantly reduced cardiomyocyte apoptosis factor (caspase-3, cytochrome C) and increased viability factor(BCL-XL and phosphated AKT) compared with cells subjected to H/R as westerm blot including caspase-3, cytochrome C, BCL-XL(Fig.6A). Consistent with western blotting result, cell necrosis level, LDH, also indicated that BPS attenuated H/R induced cell damage in cardiomyocyte(Fig.6B)
BPS mediateds the eNOS expression
To further investigate the signaling mechanisms mediating BPS protection in myocardial apoptosis, we examined eNOS production effect of BPS in neonatal primary cultured cardiomyocytes. Treatment of cardiomyocytes with BPS resulted in a significant gene expression of eNOS compared to control in time or dose dependent manner (Fig.7).
BPS protect cardiomyocyte from H/R induced apoptosis via PI3 kinase signal pathway induce eNOS
The classical signaling pathway of PGI2
uses a G protein-coupled cell surface receptor termed IP. Activation of IP by an agonist leads to increased production of
intracellular cAMP via stimulation of adenylyl cyclase. Crdiomyocytes induced eNOS procution was significantly inhibit
gene expression by cAMP
inhibitor,CAY10441, as RT-PCR(Fig.8A) and Western blot analysis also showed that the treatment of beraprost in cardiomyocytes resulted in reduced cleaved caspase-3 formation but cleaved caspase-3 activity is increased when cardiomyocytes adopted with cAMP inhibitor ,CAY1401, or PI3 kinase inhibitor,LY(Fig.8B). The results indicate that beraprost increases cAMP production and thus activation of PI3 kinase activity and eNOS, leading to inhibition of caspase-3 activation.
Discussion
This is the first study to demonstrate that Adv-COX-1/PGIS gene transfer protects against myocardial apoptosis through activation of the cAMP-PI3 kinase-Akt- mediated signaling mechanism. Using the Catheter-Based Gene Delivery model, we showed that the leuciferase activity of recombinant GFP in cardiac cells is about 120 more than in lung or liver or kidney is about 15 to 20. Furthermore, Using the I/R mice model, we showed that the expression of recombinant Adv-COX-1/PGIS in cardiac cells activates Akt, leading to inhibition of caspase-3 activation and thus attenuation of cell death. In cultured cardiomyocytes, PGI2 analogue, BPS, also protects against
H/R-induced apoptosis through the cAMP-PI3kinase-Akt-caspase–dependent pathway and this anti-apoptotic effect is induced by eNOS production.
Evidence from recent studies indicates that PGI2 acts upon vascular
tissues and platelets as a potent vasodilator and anticoagulator and these characteristics are mimicked by
various PGI2agonists. Exogenous PGI2 or
cAMP-elevating agents showed an anti-apoptotic response this result is consist with our data. Except for exogenous PGI2 influence cell fate,
endogenously produced PGI2 apparently
uses PPARδ to modulate apoptotic process as well. In previously report, the authors show that endogenous production of PGI2 activates endogenously expressed
PPARδin a kidney cell line, leading to an apoptotic response. It is interesting to note that addition endogenously PGI2 of
opposing effects following I/R. These results are suggestive of possible crosstalk between PPARδand certain cytoplasmic signaling pathways.
Caspase-3 is thought to be activated during the final step of the proapoptotic signaling pathway in many cell lines, and it represents a potential therapeutic target for suppression of cardiomyocyte apoptosis. In human cardiomyopathy, apoptosis of cardiac muscle cells is associated with release of cytochrome c and activation of caspase-3.38 I/R induces apoptosis in the myocardium, whereas inhibition of caspase activity has been shown to attenuate both I/R injury and apoptosis in cardiomyocytes. In the present study, we confirmed that Akt-mediated signaling effects in regulating cardiac myocyte death is executed by caspase-3. Cardiomyocyte apoptosis was inhibited when BPS was administered. These findings provide new insights into the role and signaling mechanisms mediated by PGI2 in
protection against I/R-induced cardiac injury and apoptosis and may have significance in the development of molecular targets for future therapeutic application.
Fig.1 Expression and tissue distribution of adenovirusmediated gene delivery 2 days after catheter-based technique. A, Tissue distribution of luciferase activities in the heart, lung, aorta, liver, skeletal muscle, brain, and kidney. B, High efficiency of GFP expression in heart compared with control after injection of Ad.Null and Ad.CMV-GFP (n=5 and 5). Original magnification x400.
Figure 2. Expression and localization of
human COX-1 and PGIS in mice myocardium after catheter-based Adv-COX-1/PGIS gene delivery. Human COX-1 and PGIS was identified immunohistochemically at 5 days after Adv-COX-1/PGIS injection. Ad.Null-injected myocardium was used as control. Original magnification X400.
Figure 3. Effect of Adv-COX-1/PGIS gene transfer on cardiomyocyte apoptosis after acute I/R injury. A, Representative photomicrographs of DNA fragments detection by TUNEL assay. B, Quantitative analysis of apoptotic cardiomyocytes expressed as percentage of TUNEL-positive nuclei in cardiomyocytes from I/R-injured ventricular sections of no treated mice as control, Ad.Null or Adv-COX-1/PGIS treated mice hearts (mean=SEM, n=6 to 8). *P_0.01 vs other groups. C, Representative DNA laddering analysis. Nucleosomal DNA ragmentation of myocardial extracts was analyzed from 3 separate experiments.
Figure.4. Effect of gene
Adv-COX-1/PGIS transfer on phosphated Akt, signaling in I/R myocardium. A, Representative Western blot of myocardial extracts from sham, I/R control, and I/R receiving Adv-COX-1/PGIS (n=6 to 8). Phospho-Akt, (B),Quantitative analysis of Fig.4. A
Fig.5. Effect of gene Adv-COX-1/PGIS
transfer on cleaved caspase-3 signaling in I/R myocardium. A, Representative Western blot of myocardial extracts from sham, I/R control, and I/R receiving Adv-COX-1/PGIS (n=6 to 8). Phospho-Akt, (B),Quantitative analysis of Fig.5. A
Figure6. Effect of BPS mitochondria-dependent signaling in cultured cardiomyocyte following H/R. A,Western blot for cleaved-caspase-3 cytochromeC, BCL-XL and phosphated AKT, GPDH was loading control. Representative data are from 4 independent experiments. B, Quantitative cell cytotoxicity base in LDH release level on pretreating cardiomyocyte with indicated beraprost following H/R.
Fig.7. BPS increases iNOS gene
transcription. Dose and time dependence of iNOS mRNA levels and eNOS protein levels in response to BPS.
Fig.8. Effect of BPS treatment on eNOS and PI3 kinase signaling in cultured cardiomyocyte in the presence of cAMP inhibitor,CAY10441 or PI3 kinase inhibitor, A. representative of photograph of RT-PCR cardiomyocyte subjected to BPS or CAY10441 following H/R.. B. Western blot of cleaved-caspase-3 .