INTRODUCTION
There are 4 species of Anguilla eels in Taiwan. A. japonica is the most abundant, followed by A. marmorata. The other 2 tropical species, A. bicolor pacifica and A. celebesensis, are rare and only occa-sionally found in the estuaries of Taiwan (Tzeng 1982, 1983, Tzeng & Tabeta 1983, Tzeng et al. 1994, Han et al. 2001). A. japonica is a temperate species, occurring from Taiwan through to Japan in NE Asia (Ege 1939, Tesch 1977). The eels spawn west of the Mariana Islands (Tsukamoto 1992). Their leaf-like leptoce-phalus larvae take ca. 4 to 5 mo to migrate with the North Equatorial and Kuroshio Currents, and meta-morphose into transparent glass eels on the edge of
the NE Asian continental shelf (Tzeng 1990, 2003, Tzeng & Tsai 1994, Cheng & Tzeng 1996). The glass eels become pigmented elvers in the estuaries and live in the river for approximately 5 to 8 yr, during which they develop from elvers to the yellow and sil-ver eel stages. The sexually maturing silsil-ver eels migrate to the spawning ground, where they die after spawning.
Anguilla marmorata is widely distributed in NE Asia and the Indo-Pacific Ocean. It is an endangered spe-cies in Taiwan. Budimawan (1997) and Arai et al. (2002a,b) have reported its age at recruitment and sea-sonal occurrence in the estuaries of Japan, Taiwan and other Pacific countries. They may spawn west of the Mariana Islands, like A. japonica (Miller & Tsukamoto
© Inter-Research 2003 · www.int-res.com *Corresponding author. Email: wnt@ccms.ntu.edu.tw
Disparities in habitat use and migratory behavior
between tropical eel
Anguilla marmorata and
temperate eel
A. japonica in four Taiwanese rivers
J. C. Shiao
1, Y. Iizuka
2, C. W. Chang
3, W. N. Tzeng
3, 4,*
1Institute of Zoology, and 2Institute of Earth Sciences, Academia Sinica, 128, Section 2, Academia Road, Nankang, Taipei,
Taiwan 115, ROC
3Department of Zoology, National Taiwan University, 1, Section 4, Roosevelt Road, Taipei, Taiwan 106, ROC
4Present address: Institute of Fisheries Sciences, National Taiwan University, Taipei, Taiwan 106, ROC
ABSTRACT: Strontium (Sr):calcium (Ca) ratios in otoliths of the eels Anguilla japonica and A. mar-morata caught in 4 Taiwanese rivers were examined to reconstruct their migratory environmental history. In all sampling locations, each eel species preferred a different environment and all were dif-ferently distributed in the river. A. japonica was more abundant than A. marmorata in the lower reach, accounting for 76 to 86% of the eel population. In contrast, A. marmorata was more abundant than A. japonica in the upper reach, accounting for 76 to 100% of the eel population. A. japonica con-sisted of diversified migratory contingents, including freshwater, brackish-water and seawater eels,
but A. marmorata tended to reside in freshwater and seemed to avoid seawater during the yellow eel
stage. This disparity in migratory behaviors and habitat use between species may reflect interspecific competition and adaptive radiation. The flexible migratory behavior and adaptation to different salinities of A. japonica may be an advantageous evolutional fitness when facing competition, heavy fishing pressure and environmental stress. The freshwater-restricted A. marmorata is more easily threatened by both fishing pressure and continuous habitat degradation than A. japonica.
KEY WORDS: Anguilla japonica · Anguilla marmorata · Otolith microchemistry · Environmental history · Migratory behaviors · Adaptive radiation
2001). The species is relatively long-lived; a 17 yr old female A. marmorata was found in the Pearl River of south China (Williamson 1993). Knowledge of this eel’s life history is very limited.
Anguilla japonica and A. marmorata coexist in Tai-wanese rivers, and may share the same niches, use the same demersal habitats and forage for the same prey (Tzeng et al. 1995). Thus, interspecific competition might play an important role in regulating their habitat use and population size. Mechanisms may exist to avoid interspecific competition and attain maximum benefit for each eel species in the river. Habitat segre-gation and behavior differentiation might be the effec-tive means. Fishermen’s experience has indicated that A. japonica is dominant in the lower reach of the river and estuary, while A. marmorata dominates in the middle to upper reaches of the river. However, in many small brooks of Taiwan, ecological niches are seriously compressed, so that there is no apparent boundary to distinguish lower reach from middle reach, or middle reach from upper reach. Consequently, the presumed segregative distribution of the 2 eel species in the rivers needs evaluation. Tzeng et al. (2002) found that A. japonica tended to stay in brackish waters in the rivers of Taiwan. The other temperate eels, A. anguilla in Europe, and A. rostrata in North America, also have a flexible migratory life cycle, i.e. a portion of the eel population might skip freshwater residence and live in estuarine and coastal waters until maturation (Tzeng et al. 1997, 2000a, Tsukamoto et al. 1998, Tsukamoto & Arai 2001, Jessop et al. 2002). Whether the tropical eel A. marmorata has a similarly flexible migratory behav-ior or merely resides in freshwater, is still unclear and intriguing.
Fish otoliths are metabolically inert and grow by sea-sonal accretion throughout the life of the fish (Pannella 1971). Strontium (Sr) can substitute for calcium (Ca) in the process of otolith deposition, because it has a simi-lar ionic charge and radius to Ca (Payan et al. 1999). The concentration of Sr is approximately 100-fold
greater in seawater (8.7 × 10– 5 M) than freshwater (9 ×
10– 7 M) (Campana 1999) and the Sr:Ca ratios in fish
otoliths are higher in marine and brackish waters than in freshwater (Tzeng 1996a). Thus, otolith Sr:Ca ratios are extensively used to study the migratory environ-mental history of diadromous fishes (Radtke et al. 1988, 1990), including freshwater eels (Otake et al. 1994, Tzeng & Tsai 1994, Arai et al. 1997, 1999, Tzeng et al. 1997, 2000a, Kawakami et al. 1998, Jessop et al. 2002).
The aim of this study is to (1) reconstruct the migratory environmental history of Anguilla marmorata and A. japonica in Taiwanese rivers by examining otolith Sr:Ca ratios using an electron probe microanalyzer (EPMA) and to determine their age by examining otolith annuli, (2) examine the adaptive distribution of A. japonica and A. marmorata in the rivers, and (3) compare the distrib-ution and migratory environment of these 2 species, to improve understanding of the species-specific habitat use and migratory behavior of anguillid eels.
MATERIALS AND METHODS
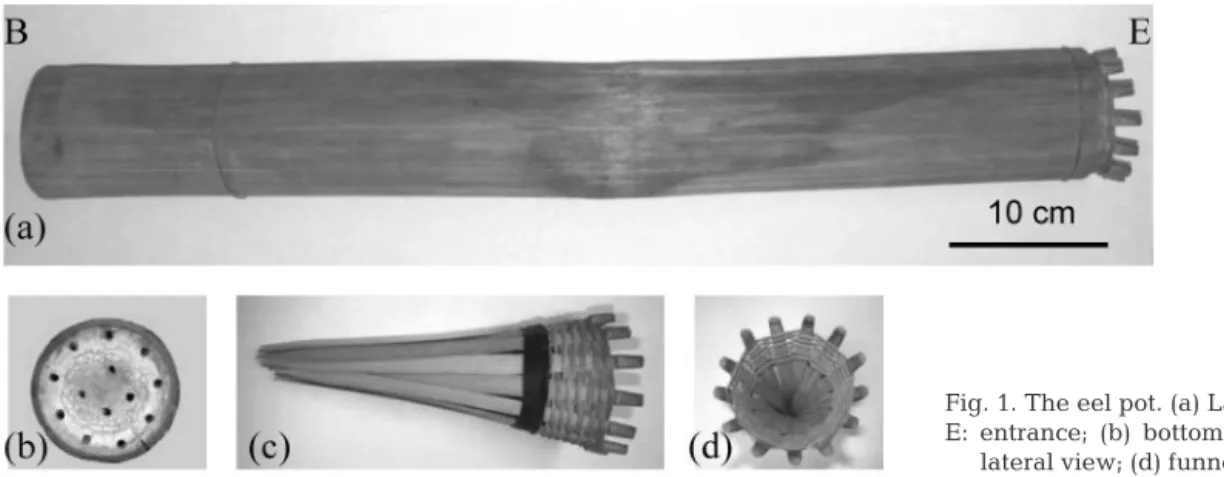
Fish collection and sampling area.Anguilla japon-ica and A. marmorata of yellow and silver eel stages were collected with a bamboo eel pot (Fig. 1). The pot was approx. 100 cm long and 10 to 15 cm in diameter, and an earthworm was placed inside as bait. The eel pot was set on the river bottom, with the entrance fac-ing downstream, and was retrieved the followfac-ing morning to check the catch. Each eel collected was measured to the nearest 5 mm in total length and dis-sected to determine its sex by gonadal histology (Han et al. 2001). The developmental stage of each eel was determined by its morphological characteristics, e.g. size, color, diameter of eyes and degree of sexual mat-uration. The specimens used for otolith Sr:Ca ratio analysis and age determination from each location and species are listed in Table 1.
Fig. 1. The eel pot. (a) Lateral view, B: bottom, E: entrance; (b) bottom view; (c) funnel cap,
Eels were collected between 1998 and 2001 from the Touchien River, northern Taiwan, the Kaoping and Linpien Rivers in southern Taiwan, and the Hsiukuluan River in eastern Taiwan. A single survey, duration approx. 3 to 5 d, was conducted in each of the Touch-ien, LinpTouch-ien, and Hsiukuluan Rivers. In the Kaoping River, 6 surveys were carried out. The eels in the Touchien River were sampled at the head of tidal influence, just down-stream of a small dam, and updown-stream in the middle reach of the river (Fig. 2).
The Kaoping River is the largest river in Taiwan, approx. 171 km long with a drainage area of 3256 km2. The lower reach (Lin-yuan)
of the river is an estuary containing environ-ments from freshwater to brackish water and a salinity range from 0 to 32 ppt. The middle reach (Chi-san) of the river is a freshwater environment about 40 km from the coast.
Fig. 2. Anguilla japonica and A. marmorata.
Spe-cies composition of eels collected in the lower and middle reaches of the 4 rivers. d: Sampling site;
s: no eel was collected
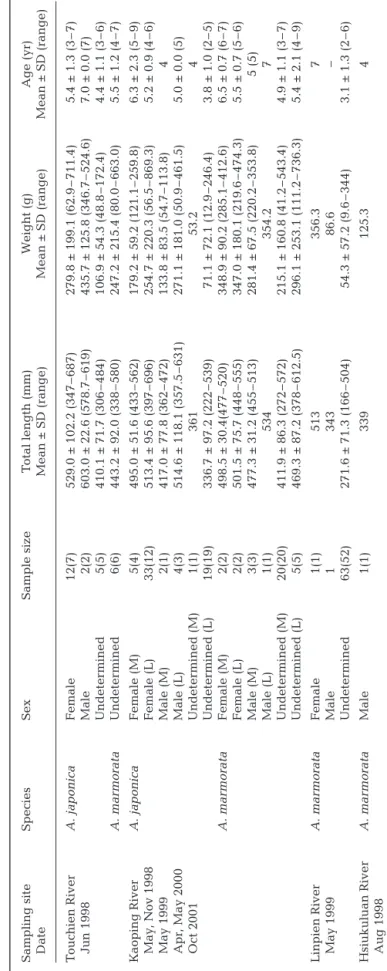
T able 1. Anguilla japonica and A. mar morata. Mean (±
SD) total length and body weight, by developmental stages and sex, fr
om the middle (M) and lower r
eaches (L) of
4 rivers in T
aiwan. Numerals in par
entheses under ‘Sample size’ ar
e the numbers of specimens aged
Sampling site Species Sex Sample size T otal length (mm) W e ight (g) Age (yr) Date Mean ± SD (range) Mean ± SD (range) Mean ± SD (range) T ouchien River A . japonica Female 12(7) 529.0 ± 102.2 (347 – 687) 279.8 ± 199.1 (62.9 – 711.4) 5.4 ± 1.3 (3 – 7 ) Jun 1998 Male 2(2) 603.0 ± 22.6 (578.7 – 619) 435.7 ± 125.8 (346.7 – 524.6) 7.0 ± 0.0 (7) Undeter mined 5(5) 410.1 ± 71.7 (306 – 484) 106.9 ± 54.3 (48.8 – 172.4) 4.4 ± 1.1 (3 – 6 ) A . mar morata Undeter mined 6(6) 443.2 ± 92.0 (338 – 580) 247.2 ± 215.4 (80.0 – 663.0) 5.5 ± 1.2 (4 – 7 ) Kaoping River A . japonica Female (M) 5(4) 495.0 ± 51.6 (433 – 562) 179.2 ± 59.2 (121.1 – 259.8) 6.3 ± 2.3 (5 – 9 ) May , Nov 1998 Female (L) 33(12) 513.4 ± 95.6 (397 – 696) 254.7 ± 220.3 (56.5 – 869.3) 5.2 ± 0.9 (4 – 6 ) May 1999 Male (M) 2(1) 417.0 ± 77.8 (362 – 472) 133.8 ± 83.5 (54.7 – 113.8) 4 Apr , May 2000 Male (L) 4(3) 514.6 ± 118.1 (357.5 – 631) 271.1 ± 181.0 (50.9 – 461.5) 5.0 ± 0.0 (5) Oct 2001 Undeter mined (M) 1(1) 361 53.2 4 Undeter mined (L) 19(19) 336.7 ± 97.2 (222 – 539) 71.1 ± 72.1 (12.9 – 246.4) 3.8 ± 1.0 (2 – 5 ) A. mar m orata Female (M) 2(2) 498.5 ± 30.4(477 – 520) 348.9 ± 90.2 (285.1 – 412.6) 6.5 ± 0.7 (6 – 7 ) Female (L) 2(2) 501.5 ± 75.7 (448 – 555) 347.0 ± 180.1 (219.6 – 474.3) 5.5 ± 0.7 (5 – 6 ) Male (M) 3(3) 477.3 ± 31.2 (455 – 513) 281.4 ± 67.5 (220.2 – 353.8) 5 (5) Male (L) 1(1) 534 354.2 7 Undeter mined (M) 20(20) 411.9 ± 86.3 (272 – 572) 215.1 ± 160.8 (41.2 – 543.4) 4.9 ± 1.1 (3 – 7 ) Undeter mined (L) 5(5) 469.3 ± 87.2 (378 – 612.5) 296.1 ± 253.1 (111.2 – 736.3) 5.4 ± 2.1 (4 – 9 ) Linpien River A . mar morata Female 1(1) 513 356.3 7 May 1999 Male 1 343 86.6 – Undeter mined 63(52) 271.6 ± 71.3 (166 – 504) 54.3 ± 57.2 (9.6 – 3 44) 3.1 ± 1.3 (2 – 6 ) Hsiukuluan River A . mar morata Male 1(1) 339 125.3 4 Aug 1998
Two small agricultural irrigation dams occur in the middle and upper reaches of the river. The annual river discharge of the river was approx. 8455 million m3, with 761 million m3flowing during the dry season
(October to March) and 7694 m3 during the rainy
sea-son (April to September).
The Linpien River, 42 km long, has a drainage area of 344 km2. No water reservoirs or dams occur in this small
river except in the upper reach. The sampling site was in freshwater about 6 km from the coast. The water level at the sampling site was about 50 cm deep but became al-most dry about 5 km further upstream. The sampling site, although not far from the coast, should be regarded as a middle-reach environment in this short river.
The sampling sites in the middle and lower reach of the Hsiukuluan River are also freshwater environment. The river, 81 km long, is the largest river in eastern Taiwan and has a drainage area of 1790 km2 that
drains into the Pacific Ocean.
Otolith preparation and Sr:Ca analysis. Otoliths were embedded in epofix resin, ground and polished until their primordium was exposed. For electron probe microanalysis, the polished otolith was carbon-coated under a high vacuum evaporator. Sr and Ca concentrations from the primordium to the otolith edge were quantified using an EPMA. A JEOL JXA-8900R system, equipped with wavelength dispersive X-ray spectrometers, was used. Quantitative analyses were conducted using beam conditions of 15 kV for the acceleration voltage, 3 nA for the current, and a 5 × 4 µm rectangular scanning beam. The quantitative data were corrected by the ZAF method to calculate oxide compositions (Goldstein et al. 1984), using the standard calibration of calcite (CaCO3, NMNH 136321)
and strontiantite (SrCO3, NMNH R10065). Sr
concen-tration was measured for 80 s at Sr Lα peak positions and 20 s at both the lower and upper sides of the
base-line. Ca was measured for 20 s at the Ca Kα peak and for 10 s at both sides of the baseline. After Sr:Ca ratio analysis, the otolith was polished again to remove the carbon layer and etched for 1 to 2 min with 5% EDTA to reveal annular marks for age determination. Then, the time spent by the eel in different habitats was determined by examining the temporal trend in the Sr:Ca ratio, relative to their observed value ranges in freshwater, estuarine and marine conditions.
RESULTS
Species composition of the eels
The species composition of the eels differed among sampling sites (Fig. 2). In the lower reach of the Touch-ien River, 76% (n = 25) of eels were Anguilla japonica and 24% were A. marmorata, while no eels were found in the middle reach of the river. In the lower reach of the Kaoping River, 86% (n = 59) of eels were A. japon-ica and 14% were A. marmorata. In the middle reach of the Kaoping River, 24% (n = 33) of eels were A. japonica and 76% were A. marmorata. All eels in the lower reach of the Linpien River (n = 65) and the mid-dle reach of the Hsiukuluan River (n = 1) were A. mar-morata (100%). No eels were caught in the lower reach of the Hsiukuluan River.
Size and relative growth
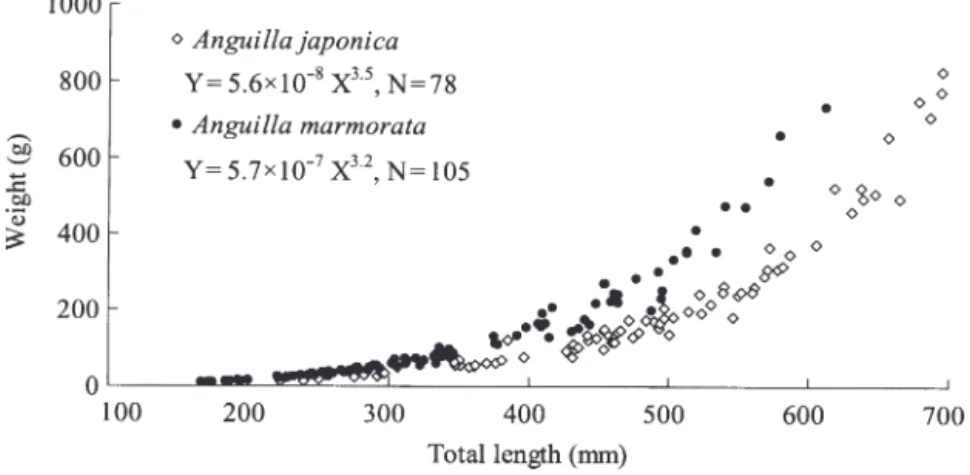
Mean (± SD) total lengths of the eels from the 4 Tai-wanese rivers ranged from 271.6 to 501.5 mm, and weights ranged from 54.3 to 348.9 g for Anguilla mar-morata, and from 336.7 to 603.0 mm and 71.1 to 435.7 g
for A. japonica (Table 1). The size ranges overlapped
between species and rivers. The sexes of 2 to 4 yr old A. marmorata from the middle reach of the Kao-ping River and lower reach of the Linpien River were mostly undeter-mined because of gonadal immatu-rity. The length (X)–weight (Y) relationship was Y = 5.6 × 10– 8X3.5
for A. japonica and Y = 5.7 × 10– 7
X3.2for A. marmorata (Fig. 3). The
relative growth of length and weight was significantly different between these 2 eel species (analy-sis of covariance, F = 12.2, p < 0.001, t = 3.5, p < 0.001 for slope, t = 12.5, p < 0.001 for intercept). A. marmorata was heavier than A. japonica at similar lengths.
Growth check and corresponding Sr:Ca ratios in otolith before elver stage
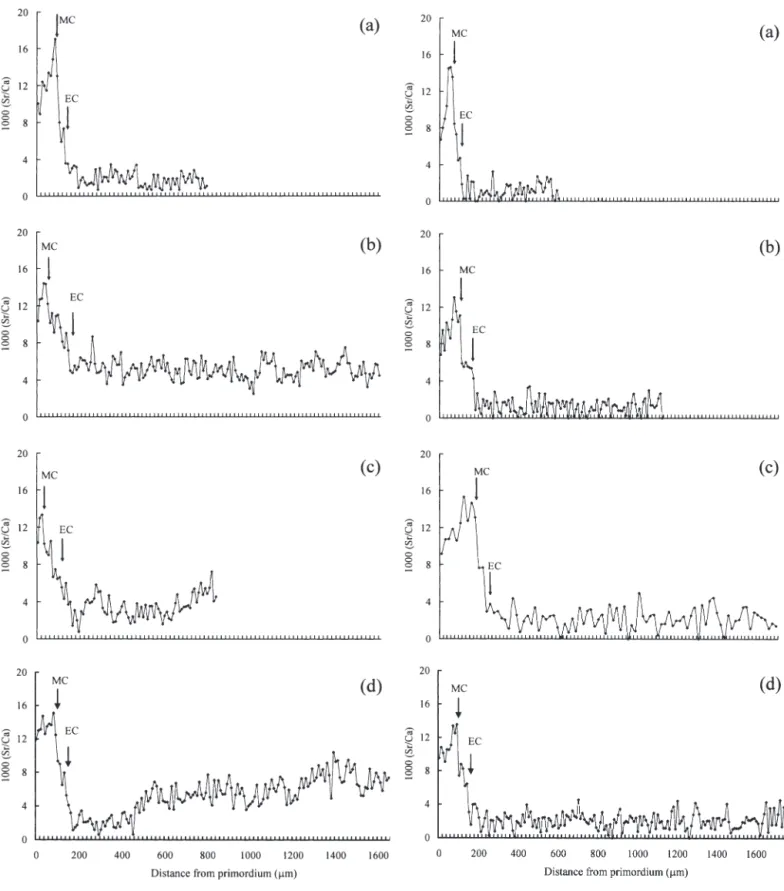
The electron probe created an approx. 1 µm deep hole in the sectioned surface of the otolith during the measurement of Sr:Ca ratios. The position of the hole in combination with the growth check could be used as a time marker to understand the chronological change of the Sr:Ca ratios in otoliths, such as the check of metamorphosis from leptocephalus to glass eel, the check for the elver at estuarine arrival, and the annu-lus of the adult eel (Fig. 4a,b,d).
The Sr:Ca ratios in the otolith of a selected Anguilla
japonica increased from approx. 10 × 10– 3in the
pri-mordium to a peak of approx. 16 × 10– 3at a distance of
110 µm from the primordium, which corresponded to the timing between metamorphosis from the lepto-cephalus to the glass eel stage. The Sr:Ca ratios decreased to approx. 7 × 10– 3 when the glass eel
became an elver at estuarine arrival (Fig. 4c). Similar changes in both otolith growth check and Sr:Ca ratios were also found in A. marmorata (Fig. 4d,e). This indi-cates that the environmental history of these 2 eels was similar during the marine leptocephalus stage. How-ever, the patterns of the Sr:Ca ratios beyond the elver
stage were greatly different between these 2 species (Figs. 5 & 6).
Sr:Ca ratios in Anguilla japonica otolith
For the 6 eels collected from the middle reach of the Kaoping River, the otolith Sr:Ca ratios beyond the elver check were all lower than 4 × 10– 3, with a highest value
of 3.7 × 10– 3 (Fig. 5a, Table 2). The sampling site of
these eels was a purely freshwater environment about 40 km from the coast and completely free from tidal influence. Thus, eels with otolith Sr:Ca ratios lower than 4 × 10– 3can be considered to be freshwater
resi-dents and eels with Sr:Ca ratios larger than 4 × 10– 3can
be regarded as estuarine or marine residents. Based on these criteria, the environmental history of Anguilla japonica beyond the elver stage was classified into 3 different migratory contingents:
Type 1 (freshwater contingent): all Sr:Ca ratios from elver mark to the edge of otolith were less than 4 × 10– 3. All eels from the middle reach of the river were
Type 1, as were 14% of the eels from the lower reach of the Kaoping River (Table 2). The mean (± SD) Sr:Ca ratios in otoliths of Type 1 eels in the middle reach Fig. 4. Anguilla japonica and A. marmorata. (a,b,d) Growth checks and (c,e) corresponding changes of Sr:Ca ratios in otoliths of A. japonica (a–c) and A. marmorata (d,e). Panel (b) was magnified from (a). Thick arrows in (a), (b) and (d): spots of Sr:Ca
Fig. 5. Anguilla japonica. Temporal changes in the Sr:Ca
ratios of otoliths of different migratory contingents, collected from the middle and lower reaches of the Kaoping River. (a) Type 1 (freshwater), (b) Type 2 (seawater), (c,d) Types 3a and 3b (estuarine). EC: elver check, MC: metamorphosis check. Panel (a): 41.8 cm yellow eel, (b): 63.5 cm silver eel,
(c): 45.1 cm silver eel, (d): 59.3 cm silver eel
Fig. 6. Anguilla marmorata. Temporal changes in the Sr:Ca
ratios of otoliths of different migratory contingents collected from the lower reach of the Linpien River. (a,b) Type 1 (fresh-water), (c,d) Type 3a (estuarine). Panel (a): 22.1 cm yellow eel; (b): 32.5 cm yellow eel; (c): 43.1 cm yellow eel;
(1.8 ± 0.2 × 10– 3, range: 0.0 to 3.7 × 10– 3) were very
close to those in the lower reach of the Kaoping River (2.2 ± 0.8 × 10– 3, range: 0.0 to 4.5 × 10– 3) (Table 2).
Three Anguilla japonica (25%) from the Touchien River were also Type 1, with a mean Sr:Ca ratio of 1.98 ± 0.9 × 10– 3, which was also very close to that of
the Type 1 eels from the Kaoping River (Table 2). Type 2 (seawater contingent): of the 58 eels collected in the lower reach of the Kaoping River, 3 (5%) were Type 2. The mean Sr:Ca ratio in the otoliths of Type 2 eels beyond the elver stage was 5.5 ± 1.1 × 10– 3(range
2.5 to 8.8 × 10– 3) (Table 2). The higher and varied Sr:Ca
ratios in the otoliths of Type 2 eels indicated that they seasonally migrated to high-salinity seawater during their stay in the lower reach (Fig. 5b).
Type 3 (estuarine contingent): of the 58 eels collected from the lower reach of the Kaoping River, 47 (81%) were Type 3 (Table 2). After the elver stage, the mean Sr:Ca ratios in the otoliths of Type 3 eels varied consid-erably among individuals. Type 3 eels did not have consistently low or high Sr:Ca ratios as did those of Types 1 and 2. Type 3 eels illustrated a variety of tem-poral patterns in the Sr:Ca ratios of their otoliths (Fig. 5c,d). Type 3 eels were further divided into 2 subtypes, according to the duration of their estuarine residence, which varied from several months to years. Type 3a (freshwater-favoring contingent) had Sr:Ca ratios less than 4 × 10– 3 over more than half of their life span,
while Type 3b (seawater-favoring contingent) had Sr:Ca ratios greater than 4 × 10– 3over more than half of
their life span. Type 3a eels were approx. 3 times more abundant than Type 3b, indicating that this species preferred freshwater when they migrated into the estuary (Table 2). In the Touchien River, 75% of Anguilla japonica (9 eels) were Type 3a, with a mean Sr:Ca ratio of 2.2 ± 1.1 × 10– 3.
Sr:Ca ratios in Anguilla marmorata otolith
The mean Sr:Ca ratios in Type 1 Anguilla marmorata otoliths were similar to those of Type 1 A. japonica (Table 2). No Type 2 or 3b eels were found for A. morata. The pattern of Sr:Ca ratios in otoliths of A. mar-morata of Type 3a (Fig. 6c,d) was more similar to that for Type 1 (Fig. 6a,b) than for Type 3a of A. japonica (Fig. 5c).
The A. marmorata of Type 3a had few spots with Sr:Ca
ratios higher than 4 × 10– 3, indicating that A. marmorata
did not frequently inhabit a highly saline environment. In the Linpien River, 46 Anguilla marmorata were Type 1 (88%) and 6 were Type 3a (12%). The mean otolith Sr:Ca ratios of Type 1 (1.6 ± 0.9 × 10– 3) and Type
3a (1.7 ± 1.0 × 10– 3) eels were similar (Table 2). In the
middle reach of the Kaoping River, of 20 eels, 8 (40%) were Type 1 and 12 (60%) were Type 3a, with mean
T able 2. Anguilla japonica and A. mar morata . Mean (±
SD) Sr:Ca ratios in otoliths, classified by their life histor
y patter ns. T ype 1: fr eshwater , T ype 2: seawater , T ype 3a : estuarine with fr eshwater pr edominant, wher e mor
e than half of the otolith Sr:Ca ratio measur
ements wer e less than 4 × 10 –3 , T
ype 3b: estuarine with seawater pr
edomi-nant, wher
e mor
e than half of the otolith Sr:Ca ratio measur
ements wer e gr eater than 4 × 10 –3
. Mean Sr:Ca ratios wer
e calculated fr
om elver check to otolith edge.
n: sample size, LP: Linpien River
, Mid-KP: middle r
each of Kaoping River
, Low-KP: lower r
each of Kaoping River
, TC: T ouchien Ri ver , HKL: Hsiukuluan River Life histor y
Mean Sr:Ca ratio
× 10 –3 patter n ————————————————— A. mar morata ————————————————— —————————— A. japonica —————————— nL Pn Mid-KP n Low-KP n T C n HKL T otal n M id-KP n Low-KP n T C T otal T ype 1 4 6 1.6 ± 0.9 0 8 2.0 ± 0.9 1 2.1 ± 0.2 4 2.0 ± 1.0 1 1.8 ± 0.9 60 6 1.8 ± 0.2 0 8 2.2 ± 0.8 0 3 2.0 ± 0.9 17 T ype 2 0 0 0 3 5.5 ± 1.1 0 3 T ype 3a 0 6 1.7 ± 1.0 12 2.0 ± 1.0 6 2.0 ± 1.0 2 2.2 ± 0.9 26 35 3.1 ± 1.5 0 9 2.2 ± 1.1 44 T ype 3b 12 4.6 ± 1.5 12 T otal 52 20 7 6 1 8 6 6 58 12 76
Sr:Ca ratios of 2.0 ± 0.9 × 10– 3and 2.0 ± 1.0 × 10– 3,
respectively (Table 2). In the lower reach of the Kao-ping River, of 7 eels, 1 was Type 1 (14%) and 6 (86%) were Type 3a, with mean Sr:Ca ratios of 2.1 ± 0.2 × 10– 3
and 2.0 ± 1.0 × 10– 3, respectively (Table 2). The mean
otolith Sr:Ca ratios were not significantly different between Types 1 and 3a eels, whether they were from the middle or lower reach of the Kaoping River (2-way ANOVA, p= 0.83 for Types 1 and 3a, p = 0.38 for mid-dle and lower reaches, and p= 0.19 for interaction). In the Touchien River, 4 eels were Type 1 (67%) and 2 eels (33%) were Type 3a, with mean Sr:Ca ratios of 2.0 ± 1.0 × 10– 3 and 2.2 ± 0.9 × 10– 3, respectively
(Table 2). The only A. marmorata caught in the Hsiukuluan River was also Type 1, with a mean otolith Sr:Ca ratio of 1.8 ± 0.9 × 10– 3. Of the total 86 eels, 60
individuals (70%) were of the completely freshwater contingent (Type 1) and 26 individuals (30%) resided in brackish water for a short period (Type 3a, Table 2). This indicates that A. marmorata is a freshwater-ori-ented species in the growth phase (yellow eel stage).
DISCUSSION
Habitat segregation
The difference in percentage composition of An-guilla japonica and A. marmorata between middle and lower reaches of the Kaoping River (Fig. 2) indicated that A. marmorata tended to reside in the upper reach while A. japonica tended to reside in the lower reach of the river. In contrast, all of the 65 eels caught in the lower reach of the Linpien River approx. 6 km from the river mouth were A. marmorata, and no A. japonica were found. Although the lower reach of the Linpien River was not far from the coast, it was free from the influence of tidal flux. The sampling site in the Linpien River, which consisted of shallow water and fast cur-rent, was more similar to a middle reach than to a lower reach. Thus, this environment was not attractive
to A. japonica but was attractive to A. marmorata,
which became dominant in the lower reach of the Lin-pien River. Most (88%) of the 65 eels from the LinLin-pien River were of the freshwater type (Table 2). This also supported the hypothesis of a geographic separation in the distribution of these 2 species within the river.
Anguilla marmorata and A. japonica also differed in microhabitat preferences. A. marmorata preferred deep pools in the river, whereas A. japonica preferred sandy and muddy substrates with shelters. Eels of different size also differed in habitat preference. The larger eels tended to reside in deep pools in the upper reach, but small eels chose shallow waters in the lower reach of the river. The deep pool in the middle reach of the
Kaoping River was approximately 2 m deep, which was suitable for adult A. marmorata. The upper reach of the Linpien River was not suitable, however, because it became too shallow during the dry winter season. The drought conditions commonly found in the steep and short rivers of Taiwan may negatively influ-ence the migratory behavior and habitat use of the eel by confining it to isolated, deep pools and interrupting their movement between pools.
Different migratory behavior
Anguilla japonica has a more phenotypic plasticity than A. marmorata, and can be classified into freshwater, seawater and estuarine contingents (Table 2, Fig. 5). Approx. 80% of A. japonica in the lower reach of the Kaoping River were estuarine-oriented, as was noted by Tzeng et al. (2002, 2003). In contrast, A. marmorata was mainly freshwater-oriented and apparently avoided res-idence in a brackish water environment, as inferred from the few Sr:Ca ratios higher than 4 × 10– 3in the otoliths of
the Type 3 eels (Table 2, Fig 6c,d). One possible expla-nation for this difference in migratory behavior and tat use between species is that the brackish water habi-tat was overwhelmingly dominated by A. japonica. Con-sequently, no surplus estuarine habitats were available
for A. marmorata. Therefore, A. marmorata was expelled
to the upper reaches, where the habitats were less pro-ductive and unstable. In contrast, if A. marmorata occu-pies fresh water habitats in the middle and upper reaches of rivers, A. japonica would be expelled to the lower reach.
These 2 eel species may have coped with interspecific resource competition by allopatric rather than sympatric distribution (Fig. 2). Interspecific competition possibly plays an important role in the distribution of the 2 eel species in the river. Migrating to the upper river takes greater risk and costs more energy than residing in es-tuary and the lower river. If no interspecific interaction exists, it seems difficult to explain the geographic sepa-ration of the 2 eel species in the river. Environmental fac-tors as well as the interspecific interactions might influ-ence the habitat choice of fish. The specific differinflu-ences in habitat choice should be carefully interpreted because the difference might not be a simple interaction among competitors (Helfman et al. 1997). A species occurs where it does because the fish functions best there, i.e. the choice of habitat reflects the use of physiologically optimal environments rather than being the result of in-teractions with other species over limiting resources. If this is what governs the habitat choice of Anguilla japon-ica and A. marmorata, brackish waters may be the opti-mal environment for A. japonica and freshwater for A. marmorata. Chloride cells in the gill epithelium of
A. japonica markedly increased during their first week after transfer from freshwater to seawater (Shirai & Utida 1970). When the seawater adapted eel was transferred to freshwater, their chloride cells degenerated slowly over the following 10 wk. The ability of chloride cells to de-velop quickly is essential for euryhaline fish such as A. japonica, to maintain a constant osmolality under vari-able salinity environments such as the estuary. No study on the salinity tolerance of A. marmorata has yet been conducted, and we do not know if it can adapt to large changes in salinity during the yellow stage. If it can, it should have evolved a phenotypic behavior similar to that of A. japonica, and may then be expected to com-pete for the same resources. However, A. marmorata does not have as many migratory contingent types as A. japonica.
A hypothesis of adaptive radiation
To date, 3 temperate eels, Anguilla anguilla, A. ros-trata and A. japonica, have been found to have flexible migratory patterns, including freshwater, seawater and estuarine contingents (Tzeng et al. 2000b, 2002, 2003, Tsukamoto & Arai 2001, Jessop et al. 2002). Whether the other 2 temperate eelsA. australis and A. dieffenbachii have similar behavior is not clear. A. dieffenhachii and A. australis are usually found in the brackish estuaries of New Zealand (Jellyman et al. 1997) and numerous A. australis reside in a salty in-land lake in South Australia (L. McKinnon pers. comm.). A. australis and A. dieffenbachii yellow eels are evidently able to regulate the plasma osmolality in hyper-saline environments. Thus, they are likely to have different migratory contingents.
Temperate eels are believed to have evolved from tropical eels (Ege 1939, Aoyama et al. 1996, Aoyama et al. 2001, Lin et al. 2001). If the tropical species, such as A. marmorata, conserved the freshwater preference in the growth phase, the temperate species have to adapt to seawater to find vacant niches, and this may have led them to develop diversified migratory contingents. The salinity choice by the congeners of the eel may be re-garded as ‘character displacement’ similar to that for other species for adaptive radiation (Brown & Wilson 1956, Schluter 1994, 2000). Estuarine and coastal wa-ters have higher productivity than freshwater rivers in the temperate zone, while freshwater rivers have higher productivity than seawater in the tropical zone (Gross 1987). Occupation of the lower reach of rivers and coastal waters in the temperate area could have op-timal evolutionary fitness for temperate eels such as A. japonica. The tropical eel A. marmorata is an ancient species of the freshwater eel that seems to have a more conservative, freshwater-oriented migratory life cycle
in which they migrate upstream to expand their habitat. But in subtropical Taiwan, where the temperate and tropical species coexist, the most productive lower reach of the river would be best for both species groups. Since these 2 eel species are apparently geographically segregated in distribution, productivity of the river seems not to be the only or determinant factor in eel distribution, especially when similar species coexist in the same river. Otherwise, A. marmorata would be as abundant as A. japonica in the estuary and lower reach, where the productivity is generally higher than in the upper reach of the river. A disparity in habitat prefer-ences reflects the biological differprefer-ences between the species, such as growth, feeding preferences, and pref-erences for water temperature, depth and bottom type. A. marmorata may simply out-compete A. japonica in the habitat typical of the upper reaches of freshwater streams, and conversely A. japonica may out-compete A. marmorata in the lower reaches and estuary, forcing them upstream. Competition combined with ‘evolution-arily developed preferences’ seems a more plausible explanation for the geographic segregation in A. japon-ica and A. marmorata distribution.
Acknowledgements. This study was conducted with financial
support from the National Science Council (contract no. NSC91-2313-B002-291, awarded to W.N.T.). We thank Mr. G. H. Cheng for otolith preparation, Mr. B. M. Jessop, and the anonymous reviewers for their helpful comments on an early draft of the paper.
LITERATURE CITED
Aoyama J, Kobayashi T, Tsukamoto K (1996) Phylogeny of eels suggested by mitochondrial DNA sequences. Nippon Suisan Gakkaishi 62:370–375
Aoyama J, Mutsumi N, Tsukamoto K (2001) Molecular phy-logeny and evolution of the fresh water eel, genus
Anguilla. Mol Phylogenet Evol 20:450–459
Arai T, Otake T, Tsukamoto K (1997) Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla japonica. Mar Ecol Prog Ser 161:17–22
Arai T, Limbong D, Otake T, Tsukamoto K (1999) Metamor-phosis and inshore migration of tropical eels, Anguilla
spp., in the Indo-Pacific. Mar Ecol Prog Ser 182:283–293 Arai T, Marui M, Miller MJ, Tsukamoto K (2002a) Growth
his-tory and inshore migration of the tropical eel, Anguilla marmorata, in the Pacific. Mar Biol 140(2):309–316
Arai T, Marui M, Otake T, Tsukamoto K (2002b) Inshore migration of a tropical eel, Anguilla marmorata, from
Tai-wanese and Japanese coasts. Fish Sci 68(1):152–157 Brown WL Jr, Wilson EO (1956) Character displacement. Syst
Zool 5:46–64
Budimawan (1997) The early life history of the tropical eel
Anguilla marmorata (Quoy & Gaimard, 1824) from 4
Pacific estuaries, as revealed from otolith microstructural analysis. J Appl Ichthyol 13:57–62
Campana (1999) Chemistry and composition of fish otoliths: pathways, mechanism and applications. Mar Ecol Prog Ser 188:263–297
Cheng PW, Tzeng WN (1996) Timing of metamorphosis and estuarine arrival across the dispersal range of the Japan-ese eel Anguilla japonica. Mar Ecol Prog Ser 131:87–96
Ege V (1939) A revision of the genus Anguilla Shaw: a
sys-tematic, phylogenetic and geographical study. Dana Rep 16:1–256
Goldstein JI, Newbury DE, Echlin P, Joy DC, Fiori C, Lifshin E (1984) Scanning electron microscopy and x-ray micro-analysis — a text for biologists, materials scientists, and geologists. Plenum Press, New York
Gross MR (1987) Evolution of diadromy in fishes. In: Dadswell MJ, Klauda RJ, Moffitt CM, Saunders RL, Rulifson RA, Cooper JE (eds) Common strategies of anadromous and catadromous fishes. Am Fish Soc Symp 1, Bethesda, MD, p 14–25
Han YS, Chang CW, He JT, Tzeng WN (2001) Validation of the occurrence of short-finned eel Anguilla bicolor paci-fica in natural waters of Taiwan. Acta Zool Taiwanica 12:
9–19
Helfman GS, Collette BB, Facey DE (1997) The diversity of fishes. Blackwell Science, Oxford
Jellyman DJ, Glova GJ, Sagar PM, Sykes JR (1997) Spatio-temporal distribution of fish in the Kakanui River estuary, South Island, New Zealand. NZ J Mar Freshw Res 31: 103–118
Jessop BM, Shiao JC, Iizuki Y, Tzeng WN (2002) Migratory behaviour and habitat use by American eels Anguilla ros-trata as revealed by otolith microchemistry. Mar Ecol Prog
Ser 233:217–229
Kawakami Y, Mochioka N, Morishita K, Toh H, Nakazono A (1998) Determination of the fresh water mark in otoliths of Japanese eel elvers using microstructure and Sr:Ca ratios. Environ Biol Fish 53:421–427
Lin YS, Poh YP, Tzeng CS (2001) A phylogeny of fresh water eels inferred from mitochondrial genes. Mol Phylogenet Evol 20:252–261
Miller MJ, Tsukamoto K (2001) Evidence of a spawning area of Anguilla marmorata in the North Equatorial Current of the western North Kaciric. J Taiwan Fish Res 9(1&2): 191–198
Otake T, Ishii T, Nakahara M, Nakamura R (1994) Drastic changes in otolith strontium:calcium ratios in leptocephali and glass eels of Japanese eel Anguilla japonica. Mar Ecol
Prog Ser 112:189–193
Pannella G (1971) Fish otolith: daily growth layers and peri-odical patterns. Science 173:1124–1127
Payan P, Edeyer A, De Pontual H, Borelli G, Boeuf G, Mayer-Gostan N (1999) Chemical composition of saccular endo-lymph and otolith in fish inner ear: lack of spatial unifor-mity. Am J Physiol 277 46:123–131
Radtke RL, Kinize III RA, Folsom SD (1988) Age at recruit-ment of Hawaiian freshwater gobies. Environ Biol Fish 23: 205–213
Radtke RL, Townsend DW, Folsom SD, Morrison MA (1990) Strontium:calcium concentration ratios in otoliths of her-ring larvae as indicators of environmental histories. Envi-ron Biol Fish 27:51–61
Schluter D (1994) Experimental evidence that competition promotes divergence in radiation. Science 266:298–801 Schluter D (2000) Ecological character displacement in
adap-tive radiation. Am Nat 156:4–16
Shirai N, Utida S (1970) Development and degeneration of the chloride cell during seawater andfresh water adaptation of the Japanese eel, Anguilla japonica. Z Zellforsch 103:
247–264
Tesch FW (1977) The eel-biology and management of Anguilla eel. Chapman & Hall, London
Tsukamoto K (1992) Discovery of the spawning area for Japanese eel. Nature 356:789–791
Tsukamoto K, Arai T (2001) Facultative catadromy of the eel
Anguilla japonica between freshwater and seawater
habi-tats. Mar Ecol Prog Ser 220:265–276
Tsukamoto K, Nakai I, Tesch WV (1998) Do all freshwater eels migrate? Nature 396:635–636
Tzeng WN (1982) New record of the elver of Anguilla celebe-sensis Kaup from Taiwan. Bioscience 19:57–66
Tzeng WN (1983) Species identification and commercial catch of the Anguillid elvers from Taiwan. China Fish Mon 366:16–23
Tzeng WN (1990) Relationship between growth rate and age at recruitment of Anguilla japonica elvers in a Taiwan
estuary as inferred from otolith growth increments. Mar Biol 107:75–81
Tzeng WN (1996a) Effects of salinity and ontogenetic move-ments on strontium:calcium ratios in the otoliths of the Japanese eel, Anguilla japonica Temminck & Schlegel.
J Exp Mar Biol Ecol 199:111–122
Tzeng WN (1996b) Short- and long-term fluctuations in catches of elvers of the Japanese eel, Anguilla japonica, in
Taiwan. In: Hancock DA, Smith DC, Grant A, Beumer JP (eds) Developing and sustaining world fisheries resources — the state of science and management world fisheries congress. CSIRO Publishing, Collingwood, p 85–89 Tzeng WN (2003) The processes of onshore migration of
Japanese eel Anguilla japonica as revealed by otolith
microstructure. In: Aida K, Tsukamoto K, Yamauchi K (eds) Advances in eel biology. Springer-Verlag, Tokyo, p 181–190
Tzeng WN, Tabeta O (1983) First record of the short-finned eel Anguilla bicolor pacifica from Taiwan. Bull Jpn Soc Sci
Fish 49:27–32
Tzeng WN, Tsai YC (1994) Changes in otolith microchemistry of the Japanese eel, Anguilla japonica, during its
migra-tion from the ocean to the rivers of Taiwan. J Fish Biol 45: 671–683
Tzeng WN, Cheng PW, Lin FY (1994) Relative abundance, sex ratio and population structure of the Japanese eel
Anguilla japonica in the Taushui River System of northern
Taiwan. J Fish Biol 46:183–201
Tzeng WN, Hsiao JJ, Shen HP, Chern YT, Wang YT, Wu JY (1995) Feeding habit of the Japanese eel Anguilla japon-ica in the streams of northern Taiwan. J Fish Soc Taiwan
22:279–302
Tzeng WN, Severin KP, Wickström H (1997) Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla. Mar Ecol Prog Ser 149:
73–81
Tzeng WN, Wang CH, Wickström H, Reizenstein M (2000a) Occurrence of the semi-catadromous European eel An-guilla anAn-guilla (L.) in Baltic Sea. Mar Biol 137:93–98
Tzeng WN, Lin HR, Wang CH, Xu SN (2000b) Differences in size and growth rates of male and female migrating Japanese eels in Pearl River, China. J Fish Biol 57: 1245–1253
Tzeng WN, Shiao JC, Iizuka Y (2002) The use of otolith Sr:Ca ratios to study the riverine migratory behaviours of Japan-ese eel Anguilla japonica. Mar Ecol Prog Ser 245:213–221
Tzeng WN, Iizuka Y, Shiao JC, Yamada Y, Oka HP (2003) Identification and growth rates comparison of divergent migratory contingents of Japanese eel (Anguilla japonica).
Aquaculture 216:77–86
Williamson GR (1993) The eels Anguilla marmorata and A. japonica in the Pearl River, China, and Hong Kong. Asian
Fish Sci 6:129–138
Editorial responsibility: Otto Kinne (Editor), Oldendorf/Luhe, Germany
Submitted: January 13, 2003; Accepted: June 14, 2003 Proofs received from author(s): October 6, 2003