行政院國家科學委員會專題研究計畫 成果報告
急性骨髓性白血病多重腫瘤抑制基因 p15INK4B 過度甲基化
的研究及其應用於微量殘存疾病的偵測(2/2)
計畫類別: 個別型計畫 計畫編號: NSC91-2314-B-002-133- 執行期間: 91 年 08 月 01 日至 92 年 07 月 31 日 執行單位: 國立臺灣大學醫學院內科 計畫主持人: 田蕙芬 報告類型: 完整報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 2 月 10 日
SOCS1 Methylation in Patients with Newly Diagnosed
Acute Myeloid Leukemia
Chien-Yuan Chen,1 Jih-Luh Tang,1 Hwei-Ling Shen,1 Shu-Wha Lin,2 Sheng-Yi Huang,1 Ming Yao,1 Woei Tsay,1 Yao-Chang Chen,1,2 Ming-Ching Shen,1,2 Chiu-Hwa Wang,1,2 and Hwei-Fang Tien1*
1
Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
2
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
*Correspondence to: Dr. Hwei-Fang Tien, M.D. Ph.D., Department of Internal Medicine, National Taiwan University Hospital, No. 7, Chung-Shan South Road, Taipei 100, Taiwan.
Tel: 886-2-23123456, Fax: 886-2-23959583, E-mail: hftd@ha.mc.ntu.edu.tw
Supported by: The National Science Council of the Republic of China, NSC 90-2314-B002-267 and 91-2314-B002-133
The proliferation and differentiation of hematopoietic precursor cells depend on various cytokines. The suppressor of cytokine signaling-1 (SOCS1) downregulates Janus kinases / signal transducers and activators of
transcription (JAK / STAT) pathway activity and inhibits the biological effects of cytokines. SOCS1 has been shown to have tumor suppressor activity, and methylation of this gene, resulting in transcriptional silencing, has been found in 65% of hepatocellular carcinoma and has been suggested to play an
important role in the development of the cancer. The methylation status of the
SOCS1 gene in acute myeloid leukemia (AML) has not been reported before.
In this study, we analyzed SOCS1 methylation in 89 patients with newly diagnosed AML and correlated the result with immunophenotypes,
cytogenetics, clinical features, and treatment outcome. SOCS1 methylation was found in the leukemic cells from 53 patients (60%). Thirteen (76%) of the 17 patients with t(15;17) had SOCS1 methylation while this gene was
methylated in only one (11%) of the nine patients with t(8;21). The frequencies of SOCS1 methylation among various cytogenetic subgroups differed
significantly (P=0.014). Other clinical and laboratory parameters and the disease free survival and overall survival were similar between the patients with and without SOCS1 methylation. In conclusion, SOCS1 methylation
occurs in more than half of AML cases, correlates with cytogenetic
abnormalities, and may play an important role in the development of subsets of AML.
INTRODUCTION
The proliferation and differentiation of hematopoietic precursor cells are regulated by various cytokines (Lotem and Sachs, 2002). These cytokines act in part through activation of the Janus kinase / signal transducers and
activators of transcription (JAK/STAT) pathway (Coffer et al., 2000; Ravandi et al., 2002). Inappropriate activation of STAT signaling pathway may play an important role in the pathogenesis of leukemias (Coffer et al., 2000; Lin et al., 2000; Spiekermann et al., 2001, 2002). Constitutive activation of STAT
transcription factors in acute myeloid leukemia (AML) is associated with short disease free survival (Benekli et al., 2002). The suppressor of cytokine
signaling (SOCS) family of proteins negatively regulates the cytokine signaling (Krebs and Hilton, 2001). The members of the SOCS family (SOCS1 to
SOCS7 and CIS) are composed of a poorly conserved amino-terminal region,
a central SH2 domain, and a SOCS box (Hilton et al., 1998). SOCS1 is a negative regulator of the JAK / STAT pathway (Yoshikawa et al., 2001). It inhibits the biological effects of various cytokines, including IL-2, IL-3, IL-4, IL-6, interferon (INF)-γ, and INF-α/β (Endo et al., 1997; Krebs and Hilton, 2001; O’shea et al., 2002). SOCS1 deficient mice die within the first three weeks of life from a myeloproliferative disorder, which is driven by excessive
interferon signaling (Naka et al., 1997; Starr et al., 1998). SOCS1 expression results in suppression of IL-6 and leukemia inhibitory factor (LIF) dependent
STAT3 activation in M1 leukemia cells (Suzuki et al., 1998). Cytokines such as
IL-4, IL-13, INF-γ, LIF and GM-CSF as well as IL-6 induce SOCS1 gene expression in hematological cells (Naka et al., 1997; Starr et al., 1998). In vitro, the interactions between SOCS1 and various cytokines in hematopoietic cells are complex. In vivo, the role for SOCS1 in leukemia has not been
investigated.
The expression of inducible SOCS1 is associated with tumor suppressor activity (Rottapel et al., 2002). Aberrant methylation of the SOCS1 gene, which results in transcriptional silencing was recently demonstrated in 17 of 26 human hepatocellular carcinomas (Yoshikawa et al., 2001). The restoration of SOCS1 suppressed growth of tumor cells in which SOCS1 was
methylation-silenced (Yoshikawa et al., 2001). Aberrant DNA methylation in promoter regions of suppressor genes including HIC1 (Issa et al., 1997b),
WT1 (Plass et al., 1999), CDKN2B (Wong et al. 2000; Tien et al., 2001) and CDKN2A (Faderl et al., 2000) can be detected in AML and is usually
associated with a poor prognosis and increased relapse rates. The incidence and the clinical and biological implications of SOCS1 methylation in human
AML are unknown. In this study, we analyzed the methylation status of the
SOCS1 gene in leukemic cells and correlated the result with the clinical and
laboratory characteristics of 89 patients with newly diagnosed AML.
MATERIALS AND METHODS
Patients The methylation status of the SOCS1 CpG island was studied in
bone marrow cells from 89 patients (54 men, 35 women) with newly diagnosed AML in National Taiwan University from 1995 to 2000.
Pretreatment characteristics are shown in Table 1. Eighty-two patients were adults and seven were children, and the median age was 48 years (range 1-85 years). The French-American-British (FAB) subtypes of AML included M1 (23 patients), M2 (30), M3 (17), M4 (14), M5 (4), and M7 (1).
In the patients with AML other than M3 subtype, most received conventional induction chemotherapy with cytarabine (AraC) for 7 days and one
anthracycline (doxorubicin, idarubicin, or mitoxantrone) for 3 days. Some patients with old age and/or poor performance status received no treatment or only low dose AraC 10mg/m2 for 14 to 21 days. The acute promyelocytic leukemia (APL) patients received all trans retinoic acid with or without concurrent induction chemotherapy. After complete remission (CR) was
dose of AraC and one anthracycline or with high dose AraC 2 to 3 g/m2 twice a day for 3~4 days.
Immunophenotype A panel of monoclonal antibodies to myeloid associated
antigens including CD13, CD33, CD11b, CD15, CD14, and CD41a, as well as lymphoid associated antigens including CD2, CD5, CD7, CD19, CD10, and CD20, and lineage nonspecific antigens HLA-DR, CD34, and CD56 was used to characterize the phenotypes of the leukemic cells. Expression of surface antigens on the leukemic cells was shown by an indirect immunoalkaline phosphatase method as described before (Tien et al., 1993).
Cytogenetics Chromosome analyses were carried out as described
previously (Tien et al., 1995). Bone marrow (BM) cells were harvested directly or after 1-3 days of non-stimulated culture. Metaphase chromosomes were banded by trypsin-Giemsa and karyotyped according to ISCN (1995).
Methylation-specific Polymerase Chain Reaction (PCR)
The methylation status of the promoter region of the SOCS1 gene was analyzed by methylation-specific PCR as described (Herman et al., 1996; Tien et al., 2001). Mononuclear cells were isolated from bone marrow aspirates by
Ficoll-Hypaque gradient centrifugation. High-molecular-weight DNA was extracted. DNA (4μg) in a volume of 40μl was denatured by addition of 10 μl of 1 mol/l NaOH (final concentration 0.2 mol/l) for 10 min at 37℃.
Hydroquinone (30μl of 10 mmol/l) (Sigma, St Louis, MO, USA) and 520μl of 1.5 mol/l sodium bisulfite (Sigma) at PH 5 were added and mixed, and
samples were incubated under mineral oil at 50℃ for 16h. Modified DNA was purified using the Wizard DNA purification resin and Vacuum Mainfold,
according to the manufacturer’s instruction (Promega, Madison, WI, USA), and then eluted into 100 μl of water. Final desulphonation was achieved by treatment with 50 μl of 1 mol/l NaOH (final concentration 0.3 mol/l) at room temperature for 5 min, followed by ethanol precipitation. DNA was
resuspended in 45μl of water and used immediately or stored at -20℃ before use.
The bisulfite-modified DNA was amplified by PCR using either a methylation-specific or unmethylation specific primer set, designed by Yoshikawa et al. (2001). The methylation-specific primer sequences were 5’-TTCGCGTGTATTTTTAGGTCGGTC-3’ (sense) and
5’-CGACACAACTCCTACAACGACCG-3’ (antisense). The unmethylation-specific primer sequences were
5’-TTATGAGTATTTGTGTGTATTTTTAGGTTGGTT-3’ (sense) and 5’-CACTAACAACACAACTCCTACAACAACCA-3’ (Antisense). Negative controls (normal DNA and distilled water) were used in each experiment. The hepatoma cell lines Hep3B and SNU423 were used as positive controls; the former had an amplified band in PCR using methylation-specific primers, but not in PCR using unmethylation-specific primers, and the latter had positive bands in both conditions. To avoid contamination, each DNA sample was aliquoted and analyzed using at least two different PCR.
Statistics Comparisons were made with the t-test. Survival curves were
plotted using Kaplan-Meier method; differences between curves were analyzed by the log-rank test. All statistical analyses were performed using the SPSS 8.0 for Window (SPSS, Chicago, IL, USA). P values less than 0.05 were considered significant.
RESULTS
SOCS1 Methylation in AML and Its Correlation With Clinical Features
Methylation of the promoter region of the SOCS1 gene was detected in 53 (60%) of the 89 patients with newly diagnosed AML (Fig. 1). Blood cells from eight normal donors of hematopoietic stem cells showed no methylation. The
FAB M1 and M3 subtypes had higher incidences of SOCS1 methylation (74% and 77%, respectively) than the M2, M4, and M5 subtypes (47%, 50%, and 25% respectively, Table 1). However, the difference did not reach statistical significance (M1&M3 vs M2, M4&M5: 75% vs 46%, P=0.626). Other clinical and laboratory features, including age, sex, WBC count, hemoglobin, platelets, and lactate dehydrogenase (LDH), were similar between the patients with and without SOCS1 methylation (Table 1). In addition to the amplified bands shown by PCR using methylation-specific primers, samples from 49 of the 53 patients with SOCS1 methylation could also be amplified with
unmethylation-specific primers (Fig 1). This may be explained by
contamination of normal cells or presence of unmethylated alleles in the AML cells (Cameron et al., 1999; Tien et al., 2001).
Correlation of SOCS1 Methylation With Cytogenetics and Immunophenotypes
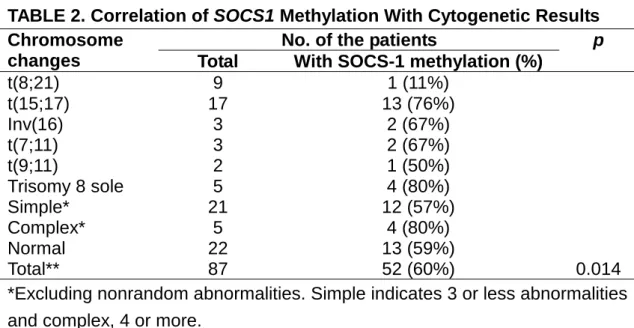
The cytogenetic studies were performed before treatment. Two patients showed no metaphase cells for analysis. The cytogenetic result of the remaining 87 patients is shown in Table 2. The incidence of SOCS1 methylation was low (11%) in AML with t(8;21), but high (76%) in APL with
t(15;17); the difference among various cytogenetic subgroups was statistically significant (P=0.014, Table 2).
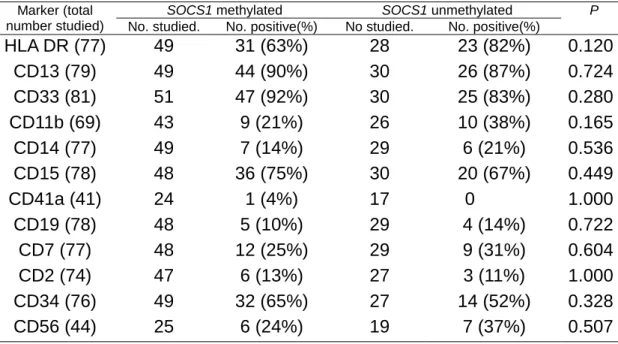
The patients with SOCS1 methylation had a somewhat lower incidence of HLA DR and CD11b expression on the leukemia cells than those without methylation (63% vs 82%, P=0.12 and 21% vs 38%, P=0.165, respectively, Table 3). There was no difference in the expression of other antigens between the two groups of patients.
Correlation of SOCS1 Methylation With Treatment Outcome
Among the 68 patients who received standard induction chemotherapy, 34 (83%) of the 41 patients with SOCS1 methylation and 24 (89%) of the 27 without methylation obtained a complete remission. The median disease free survival was 15 months in the former group and 10 months in the latter group (P=0.97). Also the overall survival was not different between the two groups (median, 30 months vs 58 months, P=0.524).
DISCUSSION
This is the first report concerning SOCS1 methylation in AML. Sixty percent of newly diagnosed AML were identified to have aberrant methylation in the
SOCS1 CpG island. SOCS1 is a negative regulator of the JAK / STAT
signaling pathway, and inappropriate activation of JAK and STAT proteins has been associated with the oncogenic process (Bowman et al., 2000; Coffer et al., 2000). Direct implication of the JAK/STAT signaling pathway in human hematological malignancies has been demonstrated by the identification of translocations involving JAK and STAT encoding genes. For example, the
STAT5B gene is fused to the retinoic acid receptor alpha (RARA) gene in an
acute promyelocytic-like leukemia (Arnould et al., 1999) and a translocation involving the JAK2 and ETV6 genes, which results in constitutive activation of the JAK2 protein tyrosine kinase, has been described in leukemia (Lacronique et al., 1997). SOCS1 interferes with the ETV6/JAK2-induced phosphorylation and activates proteasome-dependent degradation (Frantsve et al., 2001). Moreover, constitutive expression of SOCS1 blocks the proliferation of cells transformed with ETV6/JAK2, BCR/ABL or v-ABL (Rottapel et al., 2002). Dysregulation of the SOCS1 gene may hence play a role in leukemogenesis.
The CpG islands of genes are essentially unmethylated in normal tissues (Bird, 1986), but become hypermethylated in some tumor suppressor genes in malignancies, including leukemias (Issa et al., 1997a; John et al., 1999). Aberrant methylation of CpG islands is associated with gene inactivation
(Singal and Ginder, 1999) and may contribute to the pathogenesis of neoplasia. SOCS1 has been shown to have tumor suppressor activity (Rottapel et al., 2002). Yoshikawa et al. (2001) demonstrated that aberrant methylation in the CpG island of the SOCS1 gene resulted in its
transcriptional silencing in hepatocellular carcinoma and that restoration of
SOCS1 suppressed growth of tumor cells in which SOCS1 was
methylation-silenced and JAK2 was constitutively activated. Because SOCS1 methylation was demonstrated in most newly diagnosed AML, we suggest that SOCS1 may collaborate with other genetic abnormalities to facilitate the development of leukemia.
It is noteworthy that the incidence of SOCS1 methylation is different among the various cytogenetic subgroups, being higher in APL with t(15;17), and lower in AML with t(8;21). Because the signaling cascades in AML with
different cytogenetic abnormalities are not fully understood, it is not clear why
SOCS1 methylation occurs frequently in some AML but not in others.
The SOCS1 methylation had no impact on disease free survival or overall survival in this study. Methylation of the CpG islands in HIC1, WT1, CDKN2B, and CDKN2A is associated with poor outcome and high relapse rate in
Wong et al., 2000; Tien et al., 2001). In contrast, methylation of the estrogen receptor gene has been associated with improved prognosis in AML (Li et al., 1995). Thus, the correlation of CpG island methylation and prognosis is still controversial, and the clinical implications of DNA methylation possibly depend on the function of the involved genes.
REFERENCES
Arnould C, Philippe C, Bourdon V, Gr goire MJ, Berger R, Jonveaux P. 1999. The signal transducer and activator of transcription STAT5b gene is a new partner of retinoid receptor alpha in acute promyelocytic-like leukemia. Hum Mol Genet 8:1741-1749.
Benekli M, Xia Z, Donohue KA, Ford LA, Pixley LA, Baer MR, Baumann H, Wetzler M. 2002. Constitutive activity of signal transducer and activator of transcription 3 protein in acute myeloid leukemia blasts is associated with short disease free survival. Blood 99:252-257.
Bird AP. 1986. CpG-rich islands and the function of DNA methylation. Nature 321:209-213.
Bowman T, Garcia R, Turkson J, Jove R. 2000. STATs in oncogenesis. Oncogene 19:2474-2488.
Cameron EE, Baylin SB, Herman JG. 1999. p15INK4B CpG Island Methylation in Primary Acute Leukemia Is Heterogeneous and Suggests Density as a Critical Factor for Transcriptional silencing. Blood 94: 2445-2451
Coffer PJ, Koenderman L, de Groot RP. 2000. The role of STATs in myeloid differentiation and leukemia. Oncogene 19:2511-2522.
Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. 1997. A new protein containing an SH2 domain that inhibits JAK kinase. Nature 387:921-924.
Faderl S, Kantarjian HM, Estey E, Manshouri T, Chan CY, Rahman Elsaied A, Kornblau SM, Cortes J, Thomas DA, Pierce S, Keating MJ, Estrov Z, Albitar M. 2000. The prognostic significance of p16(INK4a)/p14(ARF) locus
deletion and MDM-2 protein expression in adult acute myelogenous leukemia. Cancer. 89:1976-82.
SOCS-1 inhibits TEL-JAK2 mediated transformation of hematopoietic cells through inhibition of JAK2 kinase activity and induction of
proteasome –mediated degradation. Mol Cell biol 21:3547-3557 Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. 1996.
Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93:9821-9826.
Hilton DJ, Richardson RT, Alexander WS, Viney EM, Wilson TA, Spring NS, Starr R, Nicholson SE, Metcalf D, Nicola NA. 1998. Twenty proteins containing a C-terminal SOCS box from five structural classes. Proc Natl Acad Sci USA 95:114-119.
Huang SY, Tang JL, Liang YJ, Wang CH, Chen YC, Tien HF. 1997. Clinical, hematological and molecular studies in patients with chromosome
translocation t(7;11): a study of four Chinese patients in Taiwan. Br J Haematol 96:682-687.
ISCN: An International System for Human Cytogenetic Nomanclature. Mitelman F ed. 1995. Basel Switzerland: Karger.
Issa JP, Baylin SB, Herman JG. 1997a. DNA methylation changes in hematologic malignancies: biological and clinical implications. Leukemia 11:s7-s11
Issa JP, Zehnbauer BA, Kaufmann SH, Biel MA, Baylin SB. 1997b. HIC1 methylation is a late event in hematopoietic neoplasms. Cancer Res 57:1678-1681.
John RM, Paul CV, Susan JC. 1999. Concurrent DNA methylation of multiple genes in acute myeloid leukemia. Cancer Res 59:3730-3740.
Krebs DL, Hilton DJ. 2001. SOCS proteins: Negative regulator of cytokine signaling. Stem Cells 19:378-387.
Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, Bernard OA. 1997. A
Science 278:1309-1312.
Lin TS, Mahajan S, Frank DA. 2000. STAT signaling in the pathogenesis and treatment of leukemias. Oncogene 19:2496-2504.
Li Q, Kopecky KJ, Mohan A, Willman CL, Appelbaum FR, Weick JK, Issa JP. 1995. Estrogen receptor methylation is associated with improved survival in adult myeloid leukemia. Clin Cancer Res 5:1077-1084.
Lotem J, Sachs L. 2002. Cytokine control of developmental programs in normal hematopoiesis and leukemia. Oncogene 21:3284-3294.
Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. 1997. Structure and function of a new STAT induced STAT inhibitor. Nature 387:924-929. O’shea JJ, Gadina M, Schreiber RD. 2002. Cytokine signaling in 2002: New
surprises in the Jat / Stat pathway. Cell 109:S121-131.
Plass C, Yu F, Yu L, Strout MP, El-Rifai W, Elonen E, Knuutila S, Marcucci G, Young DC, Held WA, Bloomfield CD, Caligiuri MA. 1999. Restriction
landmark genome scanning for aberrant mehylation in primary refractory and relapsed acute myeloid leukemia: involvement of the WIT-1 gene. Oncogene 18:3159-3165.
Ravandi F, Talpaz M, Kantarjian H, Estrov Z. 2002. Cellular signaling pathways: New targets in leukemia therapy. Br J Haematol 116:57-77. Rottapel R, Ilangumaran S, Neale C, La Rose J, Ho JM, Nguyen MH, Barber
D, Dubrenil P, deSepulveda P. 2002. The tumor suppressor activity of SOCS-1. Oncogene 21:4351-4362.
Singal R, Ginder GD. 1999. DNA methylation. Blood 93:4059-4070. Spiekermann K, Biethahn S, Wilde S, Hiddemann W, Alves F. 2001.
Constitutive activation of STAT transcription factors in acute myelogenous leukemia. Eur J Hematol 67:63-71
Spiekermann K, Pau M, Schwab R, Schmieja K, Franzrahe S, Hiddemann W. 2002. Constitutive activation of STAT3 and STAT5 is induced by leukemic fusion proteins with protein tyrosine kinase activity and is sufficient for transformation of hematopoietic precursor cells. Exp Hematol 30:262-271. Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ ,
Alexander WS, Metcalf D, Nicola NA, Hilton DJ. 1997. A family of cytokine-inducible inhibitors of signaling. Nature 387:917-921.
Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, Hilton DJ, Alexander WS. 1998. Liver degeneration and lymphoid deficiencies in mice lacking suppressor cytokine signaling-1. Proc Natl Acad Sci USA
95:14395-14399.
Suzuki R, Sakamoto H, Yasukawa H, Masuhara M, Wakioka T, Sasaki A, Yuge K, Komiya S, Inoue A, Yoshimura A. 1998. CIS3 and JAB have different regulatory roles in interleukin-6 mediated differentiation and STAT 3 activation in M1 leukemia cells. Oncogene 17:2271-2278.
Tien HF, Wang CH, Chen YC, Shen MC, Lin DT, Lin KH. 1993.
Characterization of acute myeloid leukemia (AML) coexpressing lymphoid markers: different biologic features between T-cell antigen positive and B-cell antigen positive AML. Leukemia. 7:688-95.
Tien HF, Wang CH, Lin MT, Lee FY, Liu MC, Chuang SM, Chen YC, Shen MC, Lin KH, Lin DT. 1995. Correlation of cytogenetic results with
immunophenotype, genotype, clinical features and ras mutation in acute myeloid leukemia. A study of 235 Chinese patients in Taiwan. Cancer Genet Cytogenet 84: 60-68.
Tien HF, Tang JL, Tsay W, Liu MC, Lee FY, Wang CH, Chen YC, Shen MC. 2001. Methylation of the p15 (INK4B) gene in myelodysplastic syndrome: it can be detected early at diagnosis or during disease progression and is highly associated with leukemic transformation. Br J Haematol.
112:148-154.
Wong IH, Ng MH, Huang DP, Lee JC. 2000. Aberrant p15 promotor
subtypes: potential prognostic implications. Blood 95:1942-1949.
Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE, Harris CC, Herman JG. 2001. SOCS-1, a negative regulator of the
JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 28:29-35.
TABLE 1. Clinical Characteristics of the 89 AML Patients Before Treatment. Total (n=89) SOCS-1 Methylated (n=53) SOCS-1 Unmethylated (n=36) p Age 0.416 ≧60 24 13 11 <60 65 40 25 Sex (Male/Female) 54/35 32/21 22/14 0.111 WBC (1000/㎜3)* 21.2±68.9 14.3±65.2 0.818 Hemoglobin (g/dl)* 8.1±2.2 7.4±2.8 0.529 Platelets (1000/㎜3)* 34.0±37.0 29.0±54.0 0.857 Lactate dehydrogenase (IU)* 1043±1373 927±1350 0.730
FAB subtype 0.278 M1 23 17 (74%) 6 (26%) M2 30 14 (47%) 16 (53%) M3 17 13 (77%) 4 (23%) M4 14 7 (50%) 7 (50%) M5 4 1 (25%) 3 (75%) M7 1 1 (100%) 0 Cytogenetics** 0.122 Favorable 29 16 13 Intermediate 50 30 20 Unfavorable 8 6 2
* median ± standard deviation
**Chromosomal study in two patients showed no metaphase cells for analysis. Favorable: including t(8;21), t(15;17), and inv(16). Unfavorable: t(7;11)
(Huang et al., 1997) and complex chromosomal changes. Intermediate: Normal karyotype and other chromosomal abnormalities.
TABLE 2. Correlation of SOCS1 Methylation With Cytogenetic Results No. of the patients
Chromosome
changes Total With SOCS-1 methylation (%)
p t(8;21) 9 1 (11%) t(15;17) 17 13 (76%) Inv(16) 3 2 (67%) t(7;11) 3 2 (67%) t(9;11) 2 1 (50%) Trisomy 8 sole 5 4 (80%) Simple* 21 12 (57%) Complex* 5 4 (80%) Normal 22 13 (59%) Total** 87 52 (60%) 0.014
*Excluding nonrandom abnormalities. Simple indicates 3 or less abnormalities and complex, 4 or more.
TABLE 3. Surface Antigen Expression in Patients With and Without SOCS1 Methylation
SOCS1 methylated SOCS1 unmethylated
Marker (total
number studied) No. studied. No. positive(%) No studied. No. positive(%)
P HLA DR (77) 49 31 (63%) 28 23 (82%) 0.120 CD13 (79) 49 44 (90%) 30 26 (87%) 0.724 CD33 (81) 51 47 (92%) 30 25 (83%) 0.280 CD11b (69) 43 9 (21%) 26 10 (38%) 0.165 CD14 (77) 49 7 (14%) 29 6 (21%) 0.536 CD15 (78) 48 36 (75%) 30 20 (67%) 0.449 CD41a (41) 24 1 (4%) 17 0 1.000 CD19 (78) 48 5 (10%) 29 4 (14%) 0.722 CD7 (77) 48 12 (25%) 29 9 (31%) 0.604 CD2 (74) 47 6 (13%) 27 3 (11%) 1.000 CD34 (76) 49 32 (65%) 27 14 (52%) 0.328 CD56 (44) 25 6 (24%) 19 7 (37%) 0.507
TABLE 4. SOCS1 Methylation and Treatment Outcome SOCS1 methylated (n=41) SOCS1 unmethylated (n=27) P Complete remission (%) 34 / 41 (83%) 24 / 27 (89%) 0.242 Consolidation chemotherapy 0.579 Conventional regimen 29 / 41 13 / 27
High dose regimen 12 / 41 14 / 27
Stem cell transplantation 9 5 0.117
Disease free survival
(median, months) 15 10 0.245
Overall survival
Figure legends
Figure 1 Methylation-specific polymerase chain reaction analysis of the
SOCS1 promoter region in 12 patients with newly diagnosed
AML (lanes 1 to 5 in experiment I, left panel, and lanes 1 to 7 in experiment II, right panel). Five patients show SOCS1
methylation (upper row, lanes 2 and 4 in experiment I and lanes 3, 5, and 6 in experiment II). Amplified bands of unmethylated DNA can also be seen in three of them (lower row, lanes 2 and 4 in experiment I and lane 6 in experiment II), but not in the other two. In experiment I, lane 6 was normal control, lanes 7 and 8 were the hepatoma cell lines SNU-423 and Hep3B, respectively, and lane 9 was water.