行政院國家科學委員會專題研究計畫 成果報告
界面活性劑增強 luminol 之化學發光及其應用之研究
研究成果報告(精簡版)
計 畫 類 別 : 個別型 計 畫 編 號 : NSC 95-2113-M-002-033- 執 行 期 間 : 95 年 08 月 01 日至 96 年 07 月 31 日 執 行 單 位 : 國立臺灣大學化學系暨研究所 計 畫 主 持 人 : 林萬寅 計畫參與人員: 博士班研究生-兼任助理:陳育錚、陳異仁 碩士班研究生-兼任助理:林郁珊、方巍儒、郭家銘、柯廷 璋、郭奕生 處 理 方 式 : 本計畫可公開查詢中 華 民 國 96 年 11 月 01 日
行政院國家科學委員會補助專題研究計畫
5
成 果 報 告
□期中進度報告
界面活性劑增強 luminol 之化學發光及其應用之研究
計畫類別:5 個別型計畫 □ 整合型計畫
計畫編號:NSC 94-M-2113-002 -033-
執行期間: 95 年 8 月 1 日至 96 年 7 月 31 日
計畫主持人:林萬寅
共同主持人:
計畫參與人員:
成果報告類型(依經費核定清單規定繳交):5精簡報告 □完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
□出席國際學術會議心得報告及發表之論文各一份
□國際合作研究計畫國外研究報告書一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、
列管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:國立台灣大學
中 華 民 國 96 年 10 月 30 日
中文摘要:
陽離子型界面活性劑 CTAC 可以使 luminol、pyrogallol 及 lophine 之氧化產生化 學發光。界面活性劑將發光試劑、氧化劑、 催化劑及增強劑聚集在微胞內部或表面,並 讓反應中間體或放光物質遠離自由基或螢 光消滅劑,而顯著增強化學發光。催化劑、 氧化劑、增強劑濃度對化學發光都有很大的 影響。這些系統可用於檢驗參與或影響化學 發光反應之物質,包括化學發光試劑、增強 劑、抑制劑(如抗氧化劑、尿酸、多巴胺等)。 關鍵詞:化學發光、界面活性劑、luminol AbstractThe presence of cationic surfactant (e.g.,
CTAC) induced the chemiluminescence (CL) from the oxidation of luminol, pyrogallol and
lophine. CL enhancement was achieved by gathering the CL reagent, oxidant, catalyst
and enhancer in the interior or interface of the micelle and shielding the CL
intermediate and emitting species from external scavengers or quenchers. The
concentrations of CL reagent, oxidant, and catalyst dramatically affect the CL emission.
The enhanced CL systems were employed to determine the substances that participate or
influence the CL reactions, such as CL
reagents, enhancers, and inhibitors (e.g., antioxidants, uric acids, dopamine etc.).
Keywords: Chemiluminescence, surfactant,
luminol
計劃緣由與目的:
Methods based on Chemiluminescence
(CL) have the advantages of being simple, fast, sensitive, cheap, and versatile. In the
past few years, we have been interested in the enhancement of CL emission from the
oxidation of luminol. Intense CL emission was observed for the oxidation of luminol
with H2O2 or m-chloroperoxybenzoic acid catalyzed by Fe- or Mn-microperoxidase.1
Moreover some enhancers, such as Na2CO3, Tris, guanidine hydrochloride, are capable
of increasing the CL emission by 1-2 orders of magnitude, depending on the catalysts
and oxidants used.2-5
In this report, we have found that the use of a cationic surfactant (e.g., CTAC) caused a dramatic enhancement of the CL
emission from the oxidation of luminol, pyrogallol, and lophine. We also
investigated the factors that affect the CL emission and the potential applications of
luminol CL) in chemical analysis.
結果與討論:
Effect of CTAC on CL emission
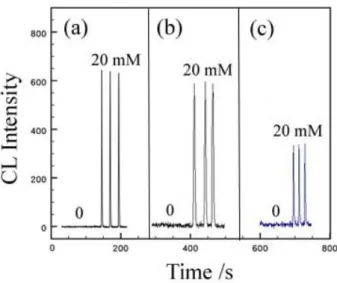
Fig. 1 demonstrated the effect of CTAC
(cetyltrimethylammonium chloride) on the CL emission from the oxidation of luminol with
chloramine T/iodide, pyrogallol with KIO4 and lophine with H2O2 or NaOCl measured by
the technique of flow injection analysis (FIA). The presence of 20 mM CTAC resulted in an
intense CL emission for all three systems, whereas essentially no CL emission was
observed without CTAC.
The use of anionic (e.g., SDS) or neutral (e.g., triton-X) surfactants or cyclodextrins did
not have any enhancing effect on the CL
emission. These results suggest that the cationic micellear intersurface may play a
significant role in CL enhancement. It is proposed that the attraction of negatively
charged reagents (I−, IO4−, OCl−, luminol anion, pyrogallol anion) onto the micellear
interface and the hydrophobic reagents (chloramine T, lophine, CL intermediates) to
the micellar interior will bring all the reactants to close vicinity for an efficient reaction to
occur. Meanwhile, the incorporation of the CL intermediates and the emitting species in the
micelle will protect them from potential radical scavengers or fluorescence quenchers
in the solution, thereby increasing the CL efficiency and the fluorescence quantum yield.
All these factors will contribute to the CL enhancement.
CL from the oxidation of luminol by chloramine T and iodide was found to be
strongly dependent on the concentrations of chloramine T, iodide, CTAC and pH. Fig. 2
showed the effect of the concentrations of chloramine T on the CL emission. No CL
emission was observed in the absence of the oxidant chloramine T. The CL intensity increased rapidly when 0.5-10 μM of chloramine T was added, indicating that
chloramine T was essential for CL emission.
Fig. 1. FIA CL emission for the oxidation of (a) luminol (b) pyrogallo, and (c) lophine with and without the presence of 20 mM CTAC.
The effect of the concentration of iodide on the CL emission was illustrated in Fig. 2.
No CL emission was observed in the absence of iodide. The CL intensity increased rapidly when 0.3-10 μM of iodide was added, indicating that iodide was also essential for
CL emission.
In the chloramine T/I−/luminol system, the actual oxidant for the CL reaction is iodine.
Oxidation of iodide by chloramine T produced iodine, which is more soluble in the micelle
than in the solution. The iodine in the micelle reacts with luminol anion in the interface,
initiating the follow-up CL reactions.
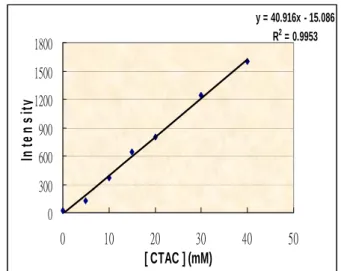
The effect of the concentration of CTAC
on the CL emission was demonstrated in Fig. 4. No CL emission was observed in the
absence of CTAC. The CL intensity increased linearly when 0-40 mM of CTAC was added,
indicating that CTAC was essential for CL emission.
Detection of iodide and doapmine
The enhanced CL systems can be used to
determine a variety of substances. For
example, the chloramine T/I−/luminol/CTAC system can be used to determine chloramine T
(0-10 μM) and iodide (0-10 μM). The detection limit (3σ) is 0.1 μM. This CL system is selective for iodide. The interference
Fig. 2. FIA-CL emission for the oxidation of luminol (50 μM) with chloramine T (0-10 μM) at pH 10.10 in the presence of I− (5 μM) and CTAC (20 mM).
0 0.5 1 1.5 2 2.5 3 4 5 6 8 10 Time /s CL 0 0.3 0.6 0.8 1 2.5 3 5 7 9 10 Time /s CL 1.5 2 y = 40.916x - 15.086 R2 = 0.9953 0 300 600 900 1200 1500 1800 0 10 20 30 40 50 [ CTAC ] (mM) In te n s it y
Fig. 3. FIA-CL emission for the oxidation of luminol (50 μM) with chloramine T (5 μM) at pH 10.10 in the presence of I− (0-10 μM) and CTAC (20 mM).
Fig. 4. FIA-CL emission for the oxidation of luminol (50 μM) with chloramine T (5 μM) at pH 10.10 in the presence of I− (5 μM) and CTAC (0-40 mM).
studies showed that anions (F−, Cl−, Br−, SO42−,
PO43−, NO3−) and cations (Mg2+, Ca2+, Sr2+, Ba2+, alkali metal ions) at 100 folds will not interfere with the CL emission. The CL
method was successfully employed to determine the iodide content in iodized salt
and in seawater (data not shown).
The chloramine T/I−/luminol/CTAC system can also be used to determine the concentration of dopamine as illustrated in.
Fig. 5. 0 20 40 60 80 100 120 140 0 200 400 600 800 1000 1200 Inte n sity Time (sec)
Injection : 50uM luminol + 20mM CTAC + dopamine ( pH 10.10)
流入 1 : 10uM CT + 10uM KI 流入 2 : 50mM Na2CO3- NaHCO3 buffer ( pH10.09) 0uM 1uM 10uM 100uM
Significant inhibition of the CL emission was observed upon addition of dopamine
(1-10 μM), allowing the determination of dopamine at sub-μM level.
The chloramine T/I−/luminol/CTAC CL system can also be used to determine
polyphenols other than dopamine (e.g.,
catechol, adrenaline, some flavonoids). The CL detection in conjunction with HPLC
separation will provide a sensitive and powerful means to determine mixtures of
polyphenols. We also found some antioxidants (e.g., vitamin C, trolox, 3-hydroxyflavone)
that are capable of scavenging radicals can also be determined by this CL system.
CL emission from pyrogallol
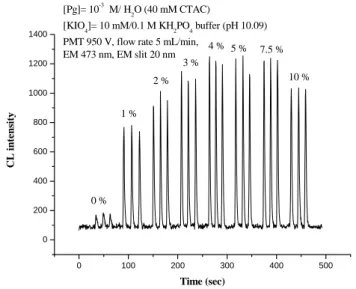
Pyrogallol is another CL reagent that requires CTAC for detecting CL emission (Fig.
1). We found that CL from the oxidation of pyrogallol with periodate was enhanced
significantly by the presence of acetaldehyde as illustrated in Fig. 6. 0 100 200 300 400 500 0 200 400 600 800 1000 1200 1400 10 % 7.5 % 5 % 4 % 3 % 2 % 1 % [Pg]= 10-3 M/ H 2O (40 mM CTAC) [KIO 4]= 10 mM/0.1 M KH2PO4 buffer (pH 10.09)
PMT 950 V, flow rate 5 mL/min, EM 473 nm, EM slit 20 nm C L in tens ity Time (sec) 0 %
The presence of 4% CH3CHO enhanced the CL emission by one order of magnitude.
Fig. 5. FIA-CL emission for the oxidation of luminol (50 μM) with chloramine T (5 μM), I− (5 μM) and
CTAC (20 mM) at pH 10.10 in the presence of. dopamine (0-100 μM)
Fig. 6. FIA-CL emission for the oxidation of pyrogallol (1 mM) with KIO4 (10 mM) and CTAC (40 mM) in the
The reason for the CL enhancement caused by CH3CHO is still not clear and requires further
investigation.
The CL emission from pyrogallol was
found to be dependent on the concentrations of pyrogallol (0-1 mM), KIO4 (0-10 mM), and
CTAC (0-40 mM). The CL intensity of this system is much weaker than that of luminol.
However, it is more easily affected than luminol by other substances, allowing the
detection of those substances.
Detection of uric acid
The pyrogallol system can be used
to determine the concentration of uric acid as shown in Fig. 7. Addition of uric acid (0-80 μM) caused a significant inhibition of the CL emission from pyrogallol, allowing its
quantification. 0 50 100 150 200 250 300 100 200 300 400 500 600 700 800 900 80 μM 60 μM 40 μM 20 μM [Pg]= 10-3 M/ H2O (20 mM CTAC)+uric acid 0.1 M KH2PO4 buffer (pH 10.02; 10 % CH3CHO) [KIO4]= 10 mM/0.1 M KH2PO4 buffer (pH 10.08)
PMT 950 V, flow rate 5 mL/min, EM 473 nm, EM slit 20 nm
C L int e ns it y Time (s) 0 μM Conclusion
We have found that CTAC is capable of
enhancing the CL emission from the oxidation of luminol, pyrogallol, and lophine with
appropriate oxidants. The enhancement in CL caused by surfactant was attributed to the
attraction of the relevant CL reagents to the interior or interface of the micelle for efficient
reaction. The concentrations of CL reagent, oxidant, and catalyst dramatically affect the
CL emission. The enhanced CL systems were employed to determine iodide, dopamine, uric
acid, and antioxidants.
References:
1. Yeh, H. –C.; Lin, W. –Y. Anal. Chim. Acta,
2001, 442, 71-77.
2. Yeh, H. –C.; Lin, W. –Y. Anal. Bioanal.
Chem. 2002, 372, 525-531.
3. Yeh, H. –C.; Lin, W. –Y. Talanta, 2003, 59,
1029-1038.
4. Yeh, H. –C.; Lin, W. –Y. J. Chin. Chem.
Soc. 2003, 50, 81-88.
5. Yeh, H. –C.; Hsu, W. –T.; Lin. W. –Y. J.
Chin. Chem. Soc. 2005, 52, 657-664.
Fig. 7. FIA-CL emission for the oxidation of pyrogallol (1 mM) with KIO4 (10 mM), CTAC (40 mM) and