e
e
Department of Plant Pathology and Microbiology College of Bioresources and Agriculture
National Taiwan University Master Thesis
SAGA I
The roles of SAGA complex in drug tolerance and virulence in Candida glabrata
Sheng-Yung Yang
Advisor: Ying-Lien Chen, Ph.D.
108 11
November 2019
e 1
1 7 p 1
_
e 7 e
e (
、 V
。
7
e 、 、( 7 ((
7
7 ((×
) RNA 、 7
(( ((
× pe ×
7
,
e
1 7
p 7
: I SAGA
Ada2 Ada3 I Gcn5
a 1 ada2 I
Ada3
Gcn5 Ada2 I
ADA3 GCN5 I
(H3K9) e e
I:
gcn5 : I
ada2 ada3
ada3 gcn5
ada3 gcn5 、 (ada2 ada3
ada2 gcn5) I (ada2 ada3 gcn5) Ada3 I
Gcn5 Ada2 H3K9 :
SAGA :
Abstract
Candida glabrata is an opportunistic human fungal pathogen and one of the non- albicans Candida species frequently isolated from patients with candidiasis. C. glabrata has intrinsic tolerance to antifungal drugs and ability to adhere on mucocutaneous surfaces, invade into bloodstream and cause systemic infection. However, the regulation of drug tolerance and virulence of C. glabrata remains elusive. SAGA (Spt-Ada-Gcn5 acetyltransferase) complex controls gene expression by regulating histone acetylation through the histone acetylation module (HAT) Ada2-Ada3-Gcn5. Our previous study showed that ada2 mutant is hypervirulence but decreases tolerance to antifungal drugs (i.e., azoles, echinocandins and polyene) and cell wall perturbing agents (i.e., calcofluor white, Congo red and SDS). This study further characterizes the functions of Ada3 and Gcn5 in C. glabrata. We found that ada3, gcn5, double or triple mutants in HAT module resulted in decreased level of acetylation on H3K9, slower growth, decreased antifungal drugs tolerance and oxidative stress response, while gcn5 mutant exhibited intermediate growth between the wild type and ada2 or ada3 mutant. In addition, HAT mutants increased agar invasion and expression of virulence associated genes. The ada3 and gcn5 mutants exhibited marginal hypervirulence, while double mutant (ada3 gcn5) showed hypervirulence in a murine model of systemic infection. Meanwhile, HAT double mutants (ada2 ada3 and ada2 gcn5) and triple mutant (ada2 ada3 gcn5) were hypervirulence. In conclusions, C. glabrata Ada3 and Gcn5 play similar roles as Ada2, regulating acetylation of H3K9, drug and oxidative stress tolerance and virulence.
Abstract
1. Introduction 1
2. Materials and Methods 4
2.1 Strains, media and chemicals 4
2.2 Gene disruption and complementation in C. glabrata 4
2.3 Determination of H3K9 acetylation 7
2.4 Growth kinetics assay 8
2.5 Serial dilution spotting assay 8
2.6 Agar invasion assay 8
2.7 Determination of minimum inhibitory concentrations 9
2.8 Real-time qRT-PCR 9
2.9 Murine systemic infection model 10
2.10 Ethics statement 11
3. Results 12
3.1 Ada3 and Gcn5 regulate H3K9 acetylation in C. glabrata 12
3.2 ADA3 and GCN5 are required for growth in C. glabrata 12 3.3 C. glabrata Ada3 and Gcn5 play crucial roles in drug tolerance and stress
responses 13
3.4 C. glabrata Ada3 and Gcn5 negatively regulate agar invasion and virulence
associated genes 14
3.5 Deletion of both ADA3 and GCN5 resulted in hypervirulence in murine
systemic infection model 16
4. Discussions
4.1 The roles of C. glabrata Ada3and Gcn5 in growth 17 4.2 The roles of C. glabrata Ada3 and Gcn5 in stress responses 17 4.3 The roles of C. glabrata Ada3 and Gcn5 in drug tolerance 19 4.4 The roles of C. glabrata Ada3 and Gcn5 in virulence in murine systemic
infection model 20
5. Tables 22
6. Figures and figure legends 27
7. Supplementary 37
8. Future work 39
9. References 40
Table 1. Candida glabrata strains used in this study 22
Table 2. Plasmids used in this study 23
Table 3. PCR primers used in this study 24
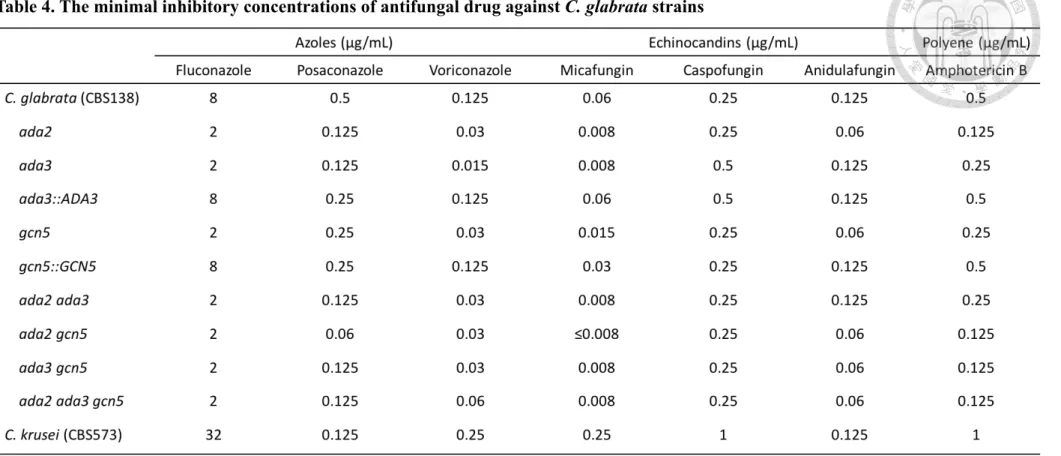
Table 4. The minimal inhibitory concentrations of antifungal drug against C. glabrata
strains 26
Table S1. Doubling time of the C. glabrata strains at 37°C 37
Figure 1. Deletion of ADA3 and GCN5 decreased the acetylation level of H3K9 in C.
glabrata 27
Figure 2. C . g l a b r a t a A d a 3 a n d G c n 5 w e r e c o n t r i b u t e d t o g r o w t h a t
37˚C 28
Figure 3. C. glabrata Ada3 and Gcn5 were involved in drug tolerance 29 Figure 4. C. glabrata Ada3 and Gcn5 were crucial in oxidative stress response and cell
wall integrity 30
Figure 5. C. glabrata Ada3 and Gcn5 were required for oxidative stress response by
regulating SOD1 and CTA1 31
Figure 6. D e l e t i o n o f C . g l a b r a t a A D A 3 o r G C N 5 e n h a n c e d a g a r
invasion 33
Figure 7. Defect of HAT module increased expression of virulence associated
genes 34
Figure 8. ada3 and gcn5 mutants exhibited marginal hypervirulence in murine systemic
infection model 35
Figure 9. Proposed roles of HAT module within SAGA complex in drug tolerance and
virulence in C. glabrata 36
Figure S1. The expression of two pseudohyphal regulation orthologs in C. glabrata were
affected by HAT module 38
1. Introduction
Candida species are one of the major cause of pathogenic fungi in nosocomial infection with high morbidity and mortality in immunocompromised patients with organ transplantation, iatrogenic immunosuppression or HIV infection. The incidence of candidiasis is 3-5 per 100,000 persons and the mortality can reach to 10-20% (1). In recent years with improved identification methods and intervention of drug, more patients were infected by non-albicans Candida species (NACs) rather than Candida albicans. In Taiwan, Candida tropicalis is the most frequently isolated NAC in clinical practice. Instead, C. glabrata is the most prevalent species among NACs from patients with candidiasis in most of countries (1, 2). The difference between countries is usually depending on the geographic regions, patients group and drug usage. However, C.
albicans is still the most prevalent species in clinical isolates. Consequently, extensive studies have already focused on virulence factors of C. albicans but how NACs threat to our health remain elusive. In addition, NACs were considered possessing the intrinsic antifungal drug resistance, indicating that our treatments are losing the ability to combat candidiasis efficiently.
Azoles, echinocandins and polyenes are three different actions of antifungal drugs, usually used in the first line of candidiasis treatment. However, the numbers of Candida species that have intrinsic antifungal drug resistance are increasing in decades which can be attributed to inherent problem that it is hard to find an effective target without being toxic to human (3, 4). Without a new strategy or target, it may facilitate the rising incidence of drug resistant isolates.
C. glabrata is an opportunistic fungal pathogen with intrinsic drug tolerance and
acts as a commensal organism under normal conditions. It can colonize onto mucosal membrane and skin and further invade to bloodstream during depression of immune system, causing inflammation of multi organs. Since C. glabrata can not form true hyphae as C. albicans does to get rid of immune cells, it must develop strategies to withstand harsh environment given by macrophages or neutrophils. To rapid response to the changing environment, regulations of gene expressions in a manner of histone modification plays a crucial role in C. glabrata (5, 6). Histone modification includes acetylation, ubiquitination, phosphorylation, methylation and sumoylation. Within histone acetylation, Gcn5 is a catalytic subunit of SAGA (Spt-Ada-Gcn5 acetyltransferase) complex that has been well studied in Saccharomyces cerevisiae, responsible for acetylation on lysine residue of histone. SAGA complex is a multifunctional gene regulator that comprises about 20 subunits, including histone acetylation module, TATA-box binding protein (TBP), cofactor for RNA polymerase II and deubiquitination module. Histone acetyltransferase (HAT) module can transfer an acetyl group from acetyl-CoA to a lysine residue, which can neutralize the positive charge of lysine and facilitate gene transcription (7-10). In previous studies, S.
cerevisiae Ada2 and Ada3 play a role as adapter, assisting the catalytic subunit, Gcn5, to transfer an acetyl group specifically as well as to enhance the acetyltransferase efficiency (11, 12), while Gcn5 bromodomain is contributed to site specificity of H3 lysine residue acetylation (13). In addition to the function in specific lysine acetylation, Ada2, Ada3 are involved in rapid response to glucose for G1 cyclin induction (14) and Gcn5 is essential for respiration and DNA replication (15, 16). Besides, S. cerevisiae ADA2 and GCN5 are required for the transcription of FLO1 (17) which ortholog in C.
glabrata is EPA1, controlling the adhesion of yeast to host epithelial cells (18). In pathogenic yeasts, the acetyltransferase within SAGA complex regulates growth, adaption to various environment and virulence factor, such as yeast-hypha switching and capsule formation of C. albicans and Cryptococcus neoformans, respectively (19- 22). Meanwhile, in filamentous fungus, Aspergillus fumigatus GcnE regulates conidiation and biofilm formation but not contributes to virulence in murine model (23).
Our previous study shows that deletion of ADA2 increases the drug tolerance and virulence in C. glabrata but the roles of Ada3 and Gcn5 in drug tolerance and virulence remain elusive (24, 25).
In present study, we demonstrate that deletion of ADA3 or GCN5 decreased the acetylation level of H3K9, growth rate, drug tolerance and cell wall integrity, indicating conserved roles as ADA2 in C. glabrata. Our results revealed new findings that Ada3 and Gcn5 involve in oxidative stress response. Interestingly, deletion of GCN5 has an intermediate effect to cell wall perturbing agents and reactive oxygen species compared to wild-type (CBS138) and ada2 or ada3 mutant. Deletion of ADA3 or GCN5 increased agar invasion, adhesion related gene expression and leaded to marginal hypervirulence while deletion both ADA3 and GCN5 resulted in hypervirulence in murine systemic infection model.
2. Materials and Methods
2.1 Strains, media and chemicals
C. glabrata strains used in this study are listed in Table 1. Yeast-peptone-dextrose (YPD, 1% yeast extract, 2%peptone, 2% glucose) liquid and agar (2%), synthetic complete (SC) medium (0.17% yeast nitrogen base without amino acid, 0.5%
(NH4)2SO4, 2% glucose, amino acids, and 2% agar), Luria-Bertani (LB, 1% tryptone, 0.5% yeast extract, 1% NaCl) liquid and agar (2%) were used in this study. YPD containing 100 µg/mL nourseothricin (Werner BioAgents, Jena, Germany) was used to select C. glabrata transformants. LB containing 34 µg/mL chloramphenicol (BioShop, Burlington, ON, Canada) or Ampicillin (BioShop, Burlington, ON, Canada) was used to select E. coli transformants. Sodium dodecyl sulfate (SDS; Bioman, New Taipei city, Taiwan), calcofluor white (CFW; fluorescent brighter 28, Sigma, St. Louis, MO, USA), Congo red (CR; Genzyme, Cambridge, MA, USA), fluconazole (FLC; selleckchem, Houston, TX, USA), posaconazole (PSC; Merck, Rahway, NJ, USA), voriconazole (VRC; sigma), micafungin (MCF; Astellas Pharma Inc., Deerfield, IL, USA), caspofungin (CSF; Merck), anidulafungin (ANF; Pfizer Inc., Groton, CT, USA) and amphotericin B (Sigma, St. Louis, MO, USA)were added to the media at the concentrations indicated below. Minimum inhibitory concentration was performed using Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma, St. Louis, MO, USA) buffered with MOPS (Sigma, St. Louis, MO, USA).
2.2 Gene disruption and complementation in C. glabrata
All the disruption cassettes and complementation cassettes were made by Shang-
Jie Yu. All deletion mutants were generated from the prototrophic wild-type CBS138 (26) using the SAT1-flipper system (27). All the plasmids used in this study are listed in Table 2. To disrupt the GCN5 (CAGL0F08283g) gene, we used homologous recombination method. We first amplified approximately 1kb of the 5’ and 3’ non- coding region (NCR) of the GCN5 open reading frame (ORF) with primer JC717/JC718 (for 3’ NCR) (Table 3) and JC715/JC716 (for 5’ NCR) from the genomic DNA of the wild-type CBS138. The PCR product of 3’ and 5’ GCN5NCR were double digested with two restriction enzymes SacII/SacI and KpnI/ApaI, respectively. The digested 3’
GCN5NCR PCR product was purified and cloned into plasmid pSFS2A, resulting in plasmid pYSJ59. The digested 5’ GCN5NCR PCR product was then purified and cloned into pYSJ59 to make the GCN5 disruption plasmid pYSJ61. The GCN5 disruption cassette 5’ GCN5NCR-SAT1-FLP-3’-GCN5NCR was excised from pYSJ61 using restriction enzymes KpnI and SacI, then transformed by modified lithium acetate transformation method (28) into wild-type CBS138 to obtain the nourseothricin- resistant gcn5 mutant YSJ65. To generate GCN5 complementation strain, we first removed the nourseothricin-resistant marker SAT1-FLP from gcn5 mutant YSJ65 by culturing YSJ65 in YPD medium for 4 days then spread on YPD plates for two days and replicated colonies onto nourseothricin-containing plate to confirm the loss of SAT1-FLP, resulting in nourseothricin-sensitive gcn5 mutant YSJ104. Second, we amplified 5’ GCN5NCR – GCN5 from CBS138 genomic DNA using primer JC1307/JC1308, double digested with XhoI and HindIII and cloned into pYSJ59 make plasmid pYSJ101. The complementation cassette 5’ GCN5NCR-GCN5-SAT1-FLP-3’
GCN5NCR was excised from pYSJ101 using XhoI and SacI and transformed into
YSJ104 to obtain the GCN5 complementary strain YSJ108.
A similar approach was used to disrupt ADA3 (CAGL0E00693g) gene. The 3’ and 5’ NCR of ADA3 were amplified with JC906/907 and JC904/905, and the PCR products were double digested with NotI/SacI and ApaI/XhoI, respectively. The digested 3’
ADA3NCR PCR product was purified and cloned into plasmid pSFS2A, resulting in plasmid pYSJ58. The digested 5’ ADA3NCR PCR product was then purified and cloned into pYSJ58 to make the ADA3 disruption plasmid pYSJ63. To generate ada3 mutant, we digested pYSJ63 with ApaI and SacI to release the disruption cassette and transformed into the wild-type CBS138, resulting in ada3 mutant (YSJ68). The SAT1- FLP was removed from YSJ68 using the same approach mentioned above to obtain nourseothricin-sensitive ada3 mutant, YSJ115. To make complementation cassette, we amplified 5’ ADA3NCR–ADA3 from CBS138 genomic DNA using primer JC904/JC1309, double digested with ApaI and XhoI and cloned into pYSJ58 to make plasmid pYSJ103. The complementation cassette 5’ ADA3NCR-ADA3-SAT1-FLP-3’
ADA3NCR was excised from pYSJ103 using ApaI/SacI and transformed into YSJ115 to obtain the ADA3 complementary strain YSJ126.
For generating ada2 ada3, ada2 gcn5 and ada3 gcn5 double mutants, we used the nourseothricin-sensitive ada2 mutant (YSJ43) from our previous study (29) and ada3 mutant (YSJ115) in this study as background strains. The enzyme digested ADA3 or GCN5 disruption cassette from pYSJ63 or pYSJ61 was transformed into YSJ43 and YSJ115 respectively to obtain double mutants ada2 ada3 (YSJ74), ada2 gcn5 (SY26) and ada3 gcn5 (SY20).
For generating ada2 ada3 gcn5 triple mutant, we used the nourseothricin-sensitive
ada2 ada3 double mutant (YSJ131) which nourseothricin-resistant marker SAT1-FLP was removed from YSJ74 as background strain. The enzyme digested GCN5 disruption cassette from pYSJ61 was transformed into YSJ131 to obtain triple mutant ada2 ada3 gcn5 (SY16). All strains were confirmed by PCR amplification of ORF, 5’ and 3’ NCR integration of the disruption cassette.
2.3 Determination of H3K9 acetylation
The procedure of total protein extraction was modified from previous study (29).
Briefly, cells were grown overnight at 30˚C with shaking at 200 rpm, then adjust to 0.2 OD600 with 50 mL fresh YPD broth and incubated for 4 h at 37˚C with shaking at 200 rpm. Then cells were centrifuged, washed once with distilled H2O and resuspended in 1 mL extraction buffer (25 mM Tris-HCl, pH7.4, 0.5 mM EDTA, 0.5 mM EGTA, 10 mM β-mercaptoethanol, 1 µg/mL leupeptin, 1 µg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.94 mM sodium orthovanadate). Total proteins were isolated using the glass beads method with 0.5 mm diameter glass beads, samples were homogenized five times for 30 seconds by vortex and placed on ice between each homogenization. Crude extractions were quantified using Bradford method. The 10 µg of proteins were separated on SDS-PAGE gels and transferred to nitrocellulose membrane. The membranes were blocked in 5% silk milk (Fonterra, Auckland City, New Zealand) in Tris-buffered saline with Tween 20 (TBST; 20 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Tween 20) and incubated with primary anti-actin antibody (#MAB1501; Merck Millipore) or anti-H3K9ac antibody (#07-352; Millipore, Temecula, CA, USA) overnight at 4°C with shaking. The membranes were then washed
three times with TBST buffer and incubated with secondary horseradish peroxidase- conjugated goat anti-mouse IgG antibody (#AP124P; Merck Millipore, Billerica, MA, USA) or goat anti-rabbit IgG antibody (#ab205718; Abcam, Cambridge, MA, USA).
Chemiluminescence signals were detected using an enhanced chemiluminescence system (T-Pro Biotechnology, New Taipei City, Taiwan).
2.4 Growth kinetics assay
Cells were grown overnight at 30˚C with shaking at 200 rpm, then washed twice with ddH2O and diluted to 0.01 OD600 with 50 mL YPD broth. Optical measurements were conducted at 0, 3, 6, 9, 12, 24 and 48 hours at 37˚C using SpectraMax 190 microplate reader. To calculate doubling times, cells were diluted to 0.01 OD600 with 200 µL YPD broth and the optical measurements were monitored every 15 min during 48 hours at 37˚C. The doubling times were calculated at exponential phase (3-6 h) and using the formula as dT = ln2 (T2- T1) / lnOD2-lnOD1. dT, OD2 and OD1 represents doubling time, optical density at final time (T2) and optical density at initial time (T1), respectively.
2.5 Serial dilution spotting assay
Cells were grown overnight at 30˚C with shaking at 200 rpm, then washed twice with ddH2O and diluted to 1 OD600 with ddH2O, then 3 µl of 5-fold serial dilution of cell suspensions were spotted onto YPD plate in the absence or presence of anti-fungal drug, reactive oxygen species (ROS) or cell wall perturbing agents, then incubated at 37˚C for 24 or 48 h.
2.6 Agar invasion assay
Cells were grown overnight at 30˚C with shaking at 200 rpm, then washed twice with ddH2O. The cell suspensions were diluted to 1 OD600, 3 µL were spotted on YPD plate and incubated at 37°C for 3 days, then colonies were removed using swab and washed with dH2O.
2.7 Determination of minimum inhibitory concentrations
To determine the minimum inhibitory concentrations, we followed CLSI guideline M27-A3 (30). Fluconazole (FLC), posaconazole (PSC), voriconazole (VRC), micafungin (MCF), caspofungin (CSF), anidulafungin (ANF) and amphotericin B (AmB) representing three different actions of antifungal drug were used in this study.
Briefly, 100 µL of 2-fold serial diluted drugs and 100 µL cell suspensions were added into 96-well polystyrene plate. The final concentration of cell suspensions was 3 103 cells/mL. The plates were incubated at 35°C for 24 h without shaking. The quality control strain Candida krusei CBS573 was used to ensure that the drug, medium and procedures were reliable.
2.8 Real-time qRT-PCR
The procedure of RNA extraction was modified from previous study (29). Cells were grown overnight in YPD broth at 30˚C with shaking at 200 rpm, then adjusted to 0.2 OD600 with 50 mL fresh YPD broth containing 2 mM H2O2, incubated at 37˚C for 3 h with shaking at 200 rpm. Cells were centrifuged at 3500 rpm for 10 min and poured
off the supernatant, cells were immediately placed in liquid nitrogen and homogenized with beads. After homogenization, cells were added with 1 mL TRIzol immediately and centrifuged at 4˚C, 12,000×g. Supernatant was treated with chloroform (supernatant:
chloroform = 5:1) then centrifuged for 10 min at 4˚C, 12,000×g. Supernatant was added with isopropanol (v: v = 1: 1) and placed on ice for 10 min. Then centrifuged for 10 min at 4˚C, 12,000×g. Pellet was washed with 200 µL 75% EtOH twice, then dried the pellet and resuspended with 200 µL DEPC-treated water. Using Turbo DNA-free kit (Invitrogen, Carlsbad, CA, USA) to eliminate DNA, then 1 µg DNA-free RNA were reverse transcribed to cDNA using high capacity reverse transcription kit (Thermo Fisher Scientific Baltics, UAB). 10 µL qPCR reaction mixtures included 2 µL cDNA (1 ng/µL), 5 µL 2× quantitative PCR master mix, 0.5 µL forward and reverse primers (5 µM) and 2 µL distilled water. Quantitative PCR running program were: 95°C 7 min for denaturation, 95°C 10 sec; 60°C 30 sec (40 cycles) and 95°C 15 sec; 60°C 60 sec and 95°C 15 sec (milting curve). StepOnePlus system and StepOne (v2.2) system were used to determine cycle threshold (CT) value and the transcription level were quantified using 2-∆∆Ct method. C. glabrata ACT1 was used to normalize the relative quantity. P values were determined using one way ANOVA.
2.9 Murine systemic infection model
Five to six-week-old male ICR mice (BioLasco Taiwan Co., Ltd.) were used in this study. 10 mice as one group and all groups were administrated with 150 mg/kg cyclophosphamide (Sigma, St. Louis, MO, USA) at day -3, 0 and 1. Cells were grown in liquid YPD overnight at 30°C with shaking at 200 rpm, washed twice with
phosphate-buffered saline (PBS), then adjusted to 3.5 × 108 cells/mL. 200 µL (7 ×107 cells) cell suspension was used for systemic infection in a lateral tail vein injection manner. The course of infection was monitored for 15 days. Statistical analysis was conducted using Mantel-Cox log-rank test and the P value was used to determine the significant difference of virulence between wild type and HAT mutants.
2.10 Ethics statement
All experimental procedures were carried out according to NIH guidelines and were approved by the Institutional Animal Care and Use Committee at National Taiwan University (approval number NTU108-EL-106005).
3. Results
3.1 Ada3 and Gcn5 regulate H3K9 acetylation in C. glabrata
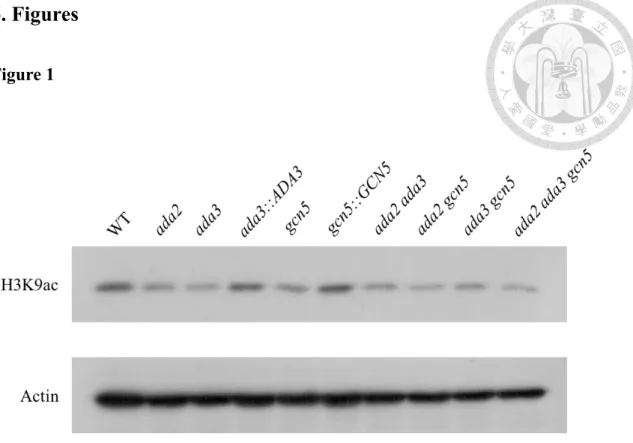
Chromatin-mediated expression is one of the strategies for Candida species to adapt the various environment. Changing the structure of nucleosome and distinct gene expressions are determined by specific lysine acetylation (31). In our previous study, deletion of ADA2 in C. glabrata results in decreased acetylation level of H3K9 but not H3K14 (29). However, the roles of C. glabrata ADA3 and GCN5 in H3K9 acetylation are unknown. To test whether ADA3 and GCN5 are required for H3K9 acetylation, we performed western blots. Results showed that deletion of ADA3 and GCN5 decreased the H3K9 acetylation level (Fig. 1). We did not observe the difference of acetylated H3K9 among ada2 mutant, ada3 mutant, gcn5 mutant, double mutants (ada2 ada3, ada2 gcn5 and ada3 gcn5) and triple mutant (ada2 ada3 gcn5), indicating that the HAT module (ADA3, GCN5 and ADA2) acts in concert to regulate H3K9 acetylation.
Interestingly, H3K9 was still acetylated in triple mutant (ada2 ada3 gcn5), indicating that other components might also involve in H3K9 acetylation in C. glabrata.
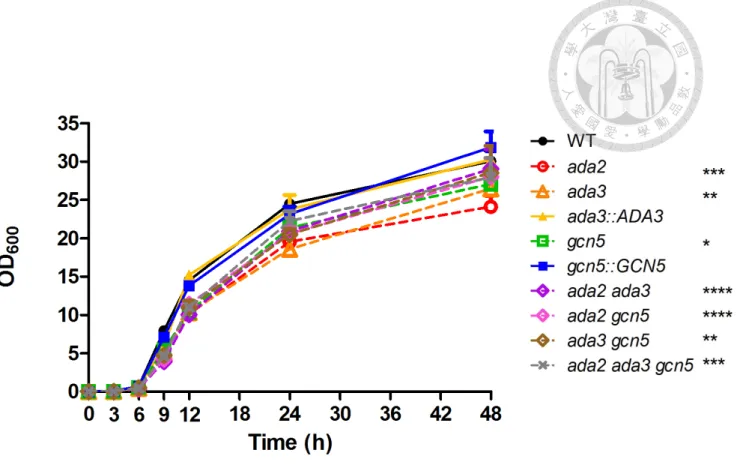
3.2 Ada3 and Gcn5 are required for growth in C. glabrata
Approximately 65% of Candida species can not grow well at human body temperature (4). Loss of ADA2 or ADA3 in S. cerevisiae is sensitive to grow at 37˚C (32), and the loss of ADA2 in Cryptococcus neoformans and C. glabrata reduced the growth rate at 37˚C (19, 29). It is unclear whether C. glabrata ADA3 and GCN5 are required for growth at 37°C. C. glabrata strains were incubated in YPD broth at 37°C and evaluated growth kinetics using SpectraMax 190 microplate reader. Loss of ADA3
or GCN5 exhibited reduced growth rate compared to the wild type after 9 h (P < 0.05, two way ANOVA) (Fig. 2), similar reduced growth was observed in double mutants (ada2 ada3, ada2 gcn5 and ada3 gcn5) and triple mutant (ada2 ada3 gcn5). There is no significant difference among ada3 mutant, gcn5 mutant, double mutants and triple mutant. The ada3::ADA3 and gcn5::GCN5 complementary strains had a similar growth to the wild type.
3.3 C. glabrata Ada3 and Gcn5 play crucial roles in drug tolerance and stress responses
To investigate the roles of ADA3 and GCN5 in drug tolerance, we determined minimum inhibitory concentration (MIC) and performed serial dilution spotting assay.
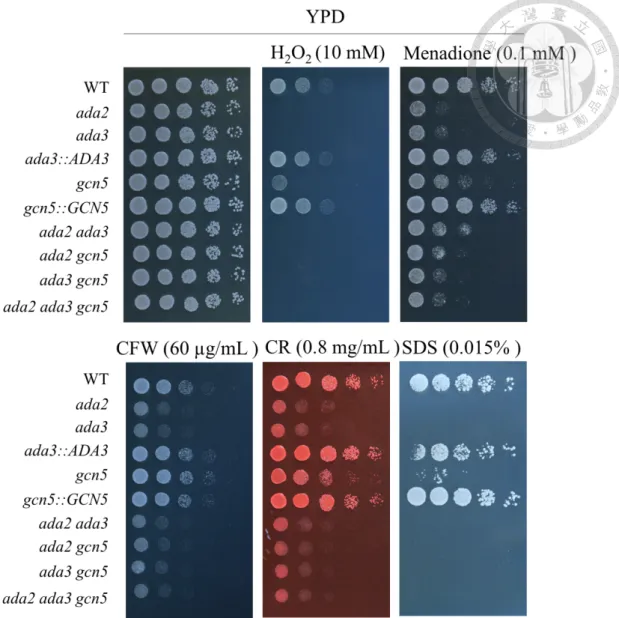
We found that ada3 mutant, gcn5 mutant, double mutants (ada2 ada3, ada2 gcn5 and ada3 gcn5) and triple mutant (ada2 ada3 gcn5) decreased tolerance to azoles (FLC, PSC and VRC), echinocandin (MCF) and polyene (AmB) (Table 4). Interestingly, gcn5 mutant exhibited intermediate viability under the stress from CSF compared to the wild type and other HAT mutants (Fig. 3). In addition to drug tolerance, the relationship between ADA3/GCN5 and stress responses is still unclear in C. glabrata.
Three actions of antifungal drugs function at cell wall or components within the cell wall leading to osmotic instability and cell death. To determine whether deletion of ADA3 or GCN5 decreases the cell wall integrity and the response to oxidative stress, strains were serial diluted and spotted onto YPD in the absence or presence of cell wall perturbing agents or reactive oxygen species. We found that ada3 mutant, gcn5 mutant, double mutants (ada2 ada3, ada2 gcn5 and ada3 gcn5) and triple mutant (ada2 ada3
gcn5) were more sensitive to cell wall perturbing agents, such as calcofluor white (CFW), Congo red (CR), sodium dodecyl sulfate (SDS), and reactive oxygen species (ROS), such as H2O2 and menadione (Fig 4). Interestingly, gcn5 mutant exhibited intermediate responses under the cell wall perturbing agents and oxidative stress compared to the wild type and other HAT mutants. Gene encoding superoxide dismutase (SOD), catalase (CTA) or glutathione peroxidase (GPX) are three major actions to detoxify the ROS. To clarify which gene was contributed to oxidative response regulated by ADA3 and GCN5, real-time qRT-PCR was performed. Under the oxidative stress (H2O2), SOD1 was downregulated in mutants except double mutants (ada2 ada3 and ada2 gcn5) and CTA1 was down-regulated in HAT mutants. However, GPX2 was slightly upregulated in HAT mutants (Fig. 5). C. glabrata oxidative stress response is regulated by Yap1, Msn2, Msn4 and Skn7 (33).We further assessed the expression of transcription factors, YAP1, MSN2, MSN4 and SKN7. Under the oxidative stress (H2O2), YAP1, MSN2 and MSN4 were not upregulated in HAT mutants (Data not shown); however HAT mutants increased the transcription of SKN7 (Fig. 5). In addition, we found the complex lacking one of the genes (ADA2, ADA3 or GCN5) enhanced the expression of other two HAT genes (Fig. 5), indicating compensation effect of the complex.
3.4 C. glabrata Ada3 and Gcn5 negatively regulate agar invasion and virulence associated genes
Adherence onto host surface is an important trait for colonization and infection.
Deletion of ADA2 in C. glabrata enhanced agar invasion (29) but whether Ada3 and
Gcn5 have conserved or divergent function in agar invasion is unknown. Deletion of ADA3, GCN5, ADA2 ADA3, ADA2 GCN5 ADA3 GCN5 and ADA2 ADA3 GCN5 showed robust agar invasion compared to the wild type and complementary strains (ada3::ADA3 and gcn5::GCN5) (Fig. 6). Agar invasion is correlated to adhesion and it is mediated by Flo11 in S. cerevisiae (34, 35), whereas C. glabrata adherence to epithelial cells is mediated by Epa1 adhesin, which is a well-defined virulence factor (18). Loss of GCN5 decreases virulence in C. albicans (21, 22), while Aspergillus fumigatus GcnE is not involved in virulence in murine models (23). Nevertheless, whether Ada3 and Gcn5 involve in regulation of virulence factors in C. glabrata remain elusive. Thus, we performed the real-time qRT-PCR to analyze the expression level of EPA genes. Under the oxidative stress, the expression level of EPA1 were upregulated in ada2 mutant, ada3 mutant, gcn5 mutant, double mutants (ada2 ada3, ada2 gcn5 and ada3 gcn5) and triple mutant (ada2 ada3 gcn5) (Fig. 7). Two putative adhesins, EPA20 and EPA23 which are ScFLO1 ortholog, were also upregulated in ada2 mutant, ada3 mutant, gcn5 mutant, double mutants (ada2 ada3, ada2 gcn5 and ada3 gcn5) and triple mutant (ada2 ada3 gcn5). In addition to adhesins, the other virulence factor, aspartyl proteases, encoded by YPS gene cluster are required for virulence and suppression of the host immune response (36, 37). Hence, we assessed the transcription level of YPS genes. YPS1, YPS7 and YPS (2-6 and 8-11) are localized on different chromosome. Here we chose YPS1 and YPS7 as these genes are crucial in disseminated infection and chose YPS4 and YPS10 since these genes are induced by macrophages (37). Under oxidative stress, the transcription of YPS7, YPS4 and YPS10 were upregulated in ada2 mutant, ada3 mutant, gcn5 mutant, double mutants (ada2 ada3, ada2 gcn5 and ada3 gcn5) and
triple mutant (ada2 ada3 gcn5); however, YPS1 was not upregulated in those mutants.
3.5 Deletion of both ADA3 and GCN5 resulted in hypervirulence in murine systemic infection model
In previous study, deletion of ADA2 increased virulence in murine systemic infection model (29). Based on the fact that deletion of ADA3 or GCN5 enhanced agar invasion and transcription of virulence associated genes, we hypothesize that ada3 or gcn5 mutant will exhibit hypervirulence similar to ada2 mutant in murine systemic infection model. Interestingly, we found the deletion of ADA3 or GCN5 resulted in marginal hypervirulence (WT vs. ada3, P = 0.1707; WT vs. gcn5, P = 0.0566), while deletion of both ADA3 and GCN5 resulted in hypervirulence (WT vs. ada3 gcn5, P = 0.0038) in murine systemic infection model (Fig. 8). Meanwhile, ada2 ada3, ada2 gcn5 and ada2 ada3 gcn5 exhibited hypervirulence (Fig. 8), indicating a critical role of ADA2 in negatively regulate virulence. The virulence was similar between the wild type and complementary strains (WT vs. ada3::ADA3, P = 0.3495; WT vs. gcn5::GCN5, P
= 0.9536).
4. Discussion
4.1 The roles of C. glabrata Ada3 and Gcn5 in growth
SAGA complex is a general cofactor in transcription by recruiting the RNA polymerase II and general transcription factors that regulates global gene expressions through histone post modification (38, 39). Previous studies show that ADA2, ADA3 and GCN5 play a role in response to cell cycle progression, while GCN5 regulates the respiratory in S. cerevisiae (14, 15, 40). Deletion of ADA2 in C. albicans results in a mild growth defect while C. glabrata without ADA2 decreased growth rate at the elevated temperatures (29, 41). However, whether deletion of ADA3 or GCN5 have a convergent or divergent role in growth in C. glabrata is still unknown. In this study, deletion of ADA3 and GCN5 exhibited a slower growth rate after 9 h. Strains lack of ADA2 ADA3, ADA2 GCN5, ADA3 GCN5 and ADA2 ADA3 GCN5 grew similar to single mutants (ada2, ada3 or gcn5), indicating that there is no dominant role in growth among Ada2, Ada3 and Gcn5, provided the evidence that ADA2, ADA3 and GCN5 regulate growth within a similar pathway (32, 42). Gcn5 plays a role in DNA replication rate (16) and deletion of ADA2 and ADA3 in S. cerevisiae reduces CLN3 expression which regulates the cell progression (14). This suggests that deletion of ADA3 and GCN5 resulted in a longer S phase and G1 phase that delay the cell progression in C.
glabrata.
4.2 The roles of C. glabrata Ada3 and Gcn5 in stress responses
In the initiation of Candida infection, the first line of immune system, phagosomes (macrophages and neutrophils), will be attracted and engulf Candida, and uses ROS
generated by NADPH oxidase, low pH value, deprivation of nutrients and hydrolytic activity to kill Candida (43-45). However, C. glabrata can not form true hyphae to escape from phagosomes as C. albicans does for immune evasion. Owing to that, C.
glabrata has developed an unusual stress response to survive and replicate inside the phagosomes, behaves like Trojan horse (44, 46). CgADA2 is critical to against oxidative stress in Drosophila larvae model (24) while CaADA2 involves in gene regulation which is recruited to about 200 promoters related to stress response including oxidative stress (47). Our results revealed that loss of ADA3 or GCN5 in C. glabrata increased susceptibility to ROS and cell wall perturbing agents, indicating ADA3 and GCN5 have conserved function similar to ADA2. Interestingly, C. glabrata without GCN5 grew intermediate under ROS (i.e., H2O2 and menadione) compared to the wild type and ada2 or ada3 mutant. Furthermore, the expression of oxidative stress response genes, SOD1 and CTA1, were down-regulated in ada2 mutant, ada3 mutant, gcn5 mutant, double mutant (ada3 gcn5) and triple mutant (ada2 ada3 gcn5) when cells were grown with H2O2. YAP1, MSN2, MSN4 and SKN7 are involved in CTA1 regulation in C. glabrata (48). We found that YAP1, MSN2 and MSN4 were not regulated in HAT mutants while SKN7 was slightly upregulated. The regulation of oxidative response genes (i.e., CTA1) depends on co-operate by Yap1 and Skn7 in S. cerevisiae (49, 50), suggesting that HAT mutants can not respond to ROS without the upregulation of YAP1 although the expression of SKN7 was subtle increased. Deletion of ADA3 and GCN5 increased susceptibility to oxidative stress and cell wall perturbing agents may due to several dimensions, not only the lower transcription of antioxidative stress response gene but also defect the fitness of growth. Besides, the compensation effect that deletion of
GCN5 in Fusarium fujikuroi increased the expression of HAT-encoding genes, partially explain the upregulation of ADA2 in ada3 and gcn5 mutants, upregulation of ADA3 in ada2 and gcn5 mutants and upregulation of GCN5 in ada2 and ada3 mutants.
4.3 The roles of C. glabrata Ada3 and Gcn5 in drug tolerance
Antifungal drug resistance is being a large concern in recent years. The emerging drug resistance strains is an inevitable consequence of constraining treatment. Deletion of ADA2 in C. glabrata decreased antifungal drug tolerance (29). Whether C. glabrata ADA3 and GCN5 have conserved or divergent function in antifungal drug tolerance remain elusive. ada3 mutant, gcn5 mutant, double mutants (ada2 ada3, ada2 gcn5 and ada2 ada3 gcn5) and triple mutant (ada2 ada3 gcn5) decreased tolerance to FLC, PSC, VRC, MCF, CSF, ANF and AmB. The MIC results indicated that Ada2, Ada3 and Gcn5 play a similar role to against antifungal drug. The histone acetyltransferase activity is required for antifungal drug tolerance or resistance. For example, disruption of ADA3 in S. cerevisiae decreases tolerance to FLC (51) while loss of GCN5 in C. albicans or Cryptococcus neoformans increases susceptibility to caspofungin (21, 41) or FK506 (20), respectively. Multiple efflux pump Mdr1 is crucial in drug resistance. Previous studies have indicated that Ada2 and Gcn5 are involved the regulation of MDR1 in C.
albicans (21, 52). Interestingly, gain of function mutation in CgPDR1 is common in clinical with an increased antifungal drug tolerance. However it is synthetically lethal in the absence of GCN5 (53), implied that target of Gcn5 or even Ada3 and Ada2 can reduce the emergence of antifungal drug tolerance.
4.4 The roles of C. glabrata Ada3 and Gcn5 in virulence in murine systemic infection model
The relationship between the agar invasion and virulence is not clear but indeed provides a high throughput screen to determine the virulence (54, 55). Deletion of ADA3, GCN5, ADA2 ADA3, ADA2 GCN5, ADA3 GCN5 and ADA2 ADA3 GCN5 increased agar invasion which is similar to ada2 mutant. Whereas in fission yeast, Schizosaccharomyces pombe, deletion of GCN5 reduced invasive growth (56), suggesting the divergent role in invasive growth between S. pombe and C. glabrata. In addition, several studies have indicated that SAGA complex involves in virulence in yeasts and filamentous fungi (19-22, 57, 58). For example, deletion of ADA2, ADA3 or GCN5 in C. albicans attenuates virulence in Caenorhabditis elegans infection model and murine systemic infection model, respectively (21, 22, 58, 59), while deletion of ADA2 in C. glabrata resulting in hypovirulence in Drosophila model (24) or hypervirulence in murine systemic infection model (29). Nevertheless, despite defect in growth, cell wall integrity and oxidative stress response, deletion of ADA3 or GCN5 increased transcription level of virulence associated genes (i.e., EPA1, EPA20, EPA23, YPS4, YPS7 and YPS10) and resulted in a marginal hypervirulence, but deletion of both ADA3 and GCN5 resulted in hypervirulence in murine systemic infection model. The robust invasive growth and increased expression of virulence associated genes of HAT mutants might partially explain the hypervirulence.
Sir proteins including Sir2, Sir3 and Sir4 are NAD+-dependent histone deacetylase which involved in subtelomeric silencing. Disruption of SIR3 and RIF1 increases the transcription of C. glabrata EPA1, EPA6 and EPA7 due to the defect of subtelomeric
silencing (60, 61). In addition, S. cerevisiae without ADA2 and GCN5 resulted in Sir2 and Sir3 moving to subtelomeric DNA resulting in hypoacetylation of H4K16 and elevation of interaction between histone and DNA (62, 63). However, loss of ADA3 and GCN5 in C. glabrata increased the transcription of EPA1, indicating Sir proteins are not moving to subtelomeric regions and suppressing gene expressions. Thus suggests the divergent function of HAT module between C. glabrata and S. cerevisiae that components of HAT module might not involve in SIR-dependent telomere silencing Besides the regulation of adhesins, three yapsins (YPS4, YPS7 and YPS10) were upregulated in HAT mutants. There are two possibilities that deletion of ADA3 and GCN5 increased the transcription of YPS genes. One is YPS genes are regulated by Ada3 and Gcn5 negatively and the other one is deletion of ADA3 and GCN5 decreased the cell wall integrity which might be a signal for compensation effect since YPS family genes are involved in cell wall metabolism and induced by various environmental cues.
For example, C. glabrata YPS1 is induced by SDS and growth at 37˚C (64). C. glabrata YPS4, YPS5, YPS8 and YPS10 play a crucial role in intracellular survival (36), indicating that deletion of ADA3 and GCN5 might enhance intracellular survival and virulence in murine systemic infection model.
5. Tables
Table 1. Candida strains used in this study
Strain Genotype Parent Reference
C. glabrata (CBS138)
Prototrophic wild type Clinical isolate (26)
YSJ39 ada2Δ::SAT1-FLP CBS138 (25)
YSJ43 ada2Δ::FRT YSJ39 (25)
YSJ68 ada3Δ::SAT1-FLP CBS138 This study
YSJ115 ada3Δ::FRT YSJ68 This study
YSJ126 ada3Δ::FRT::ADA3-SAT1-FLP YSJ115 This study
YSJ65 gcn5Δ::SAT1-FLP CBS138 This study
YSJ104 gcn5Δ::FRT YSJ65 This study
YSJ108 gcn5Δ::FRT::GCN5-SAT1-FLP YSJ115 This study
YSJ74 ada2Δ::FRT ada3Δ::SAT1-FLP YSJ43 This study
SY26 ada2::FRT gcn5Δ::SAT1-FLP YSJ43 This study
YSJ131 ada2Δ::FRT ada3Δ::FRT YSJ74 This study
SY20 ada3Δ::FRT gcn5Δ::SAT1-FLP YSJ115 This study
SY16 ada2Δ::FRT ada3::FRT gcn5::SAT1-FLP YSJ131 This study C. krusei
(CBS573)
Prototrophic wild type Clinical isolate (65)
Table 2. Plasmids used in this study
Plasmid Relevant insert Parent
pSFS2A (27)
pYSJ59 3’ GCN5NCR pSFS2A
pYSJ61 5’ GCN5NCR pYSJ59
pYSJ101 5’ GCN5NCR – GCN5 pYSJ59
pYSJ58 3’ ADA3NCR pSFS2A
pYSJ63 5’ ADA3NCR pYSJ58
pYSJ103 5’ ADA3NCR – ADA3 pYSJ58
Table 3. PCR primers used in this study
Primer Use Sequence (5’ to 3’)
JC717 3’ GCN5NCR AAACCGCGGCTATTCTTCTGAGTTTGTAAC
JC718 3’ GCN5NCR AAAGAGCTCTGGGACTTTAGAGGCTTTTG
JC715 5’ GCN5NCR AAAGGTACCGCATAATTTCATACAAACG
JC716 5’ GCN5NCR AAAGGGCCCCCTTTGTTGTTTCTTGCTAGT
JC639 GCN5 ORF TACGAGGAGGAGATTGCATCA
JC640 GCN5 ORF AGACCAGTCAAGACCATCATA
JC48 5’ SAT1-FLP ACAATCAAAGGTGGTCCT
JC81 3’ SAT1-FLP AACTTCCTCGAGGGGGGGCC
JC1076 5’ integration (gcn5) AACCATTGAGAAGTTGCCTTG JC1077 3’ integration (gcn5) ATCCAGGAACGGATGATGAGT
JC1307 GCN5 complement AAACTCGAGGGTTTTCATGAATAAATGAGG JC1308 GCN5 complement AAAAAGCTTCTAATCGATCAAATGTGAGTA
JC906 3’ ADA3NCR AAAGCGGCCGCCACAGCCTACATAAGCACCAA
JC907 3’ ADA3NCR AAAGAGCTCACTCGAACTCCTTGACGATCT
JC904 5’ ADA3NCR AAAGGGCCCTAGGGTGCTCTTCACCCACAT
JC905 5’ ADA3NCR AAACTCGAGTCTTTAGCCCATTCACCACG
JC908 ADA3 ORF GGAAGAAAACGAAGGCACAGA
JC909 ADA3 ORF GCATTGTCATTATCCTTTGAG
JC910 5’ integration (ada3) TTGCAGTAGCCTCTTCAGGAT JC911 3’ integration (ada3) ATAATGGGCGTGTTAGGTGA
JC1309 ADA3 complement AAACTCGAGCTAGTCTTCCAGTCCTTCTTC JC873 qPCR ADA2 ORF GCCTCCGTGCCGTCTTG
JC874 qPCR ADA2 ORF CCAAAGGTTGATCATCTGGTTCA JC1889 qPCR ADA3 ORF TGCGGATCAAAGAGGAGGAA JC1890 qPCR ADA3 ORF GCCTCGGGACTTTCAGCTTT JC1891 qPCR GCN5 ORF GAACAATCCACCGAGGACCA JC1892 qPCR GCN5 ORF TCCGTGCCTTCATCATTCGT JC1933 qPCR EPA1 ORF CCATCTGGGGCTCAAAAACA JC1934 qPCR EPA1 ORF GCAGCCCTCCTCTGTGTCAT
Table 3. continued
Primer Use Sequence (5’ to 3’)
JC1046 qPCR EPA20 ORF TGTCAAGCCATCCAGTTCAGTT
JC1047 qPCR EPA20 ORF TAACCGTCTGTACATATCGTTGCA
JC1042 qPCR EPA23 ORF TGATACTTCCCCCCAAAACG
JC1043 qPCR EPA23 ORF TGGTTCACTTGATATGGCTGATG
JC1994 qPCR YPS1 ORF TGAGCTAAAGAGGGCGCTTG
JC1995 qPCR YPS1 ORF AGTTGGCTGAGCGGAGTCTG
JC1899 qPCR YPS4 ORF GTGGTCCATGGGGAGTTGAT
JC1900 qPCR YPS4 ORF TTCTAGAGCCATTGGCAGCA
JC1996 qPCR YPS7 ORF ACGGATTCGCAGACACCAGT
JC1997 qPCR YPS7 ORF CCCATAGGCACCACAGGGTA
JC1048 qPCR YPS10 ORF GGACACGGGTTCGTCTGATT
JC1049 qPCR YPS10 ORF TGCATCTAAGGAGGCAATGCT
JC1875 qPCR SOD1 ORF CCTCCGAACAGGACCCTACC
JC1876 qPCR SOD1 ORF CACCGACGTGTCTGTTCTCG
JC1877 qPCR CTA1 ORF AAGGTCTGGCCACACAAGGA
JC1878 qPCR CTA1 ORF GAGGCGTATGGGCAGTTGAC
JC1879 qPCR GPX2 ORF AGGGCTTGGTCATCCTTGGT
JC1880 qPCR GPX2 ORF ACCCAGCAGGCCACTCTTTT
JC1881 qPCR SKN7 ORF CCGGTGACAGTTTTGTGGTG
JC1882 qPCR SKN7 ORF CGAACTCCCAGCTTTGTTCG
JC1901 qPCR CAGL0M07634g ORF GCACGAAGCTGCTGAATGTC JC1902 qPCR CAGL0M07634g ORF GCCATTGCTCTATGCCTTCC JC853 qPCR CAGL0L01771g ORF CAGTCGCTGGAGATGGTAAGG JC854 qPCR CAGL0L01771g ORF ACCACCGACACGCCATTAG 1. Restriction enzyme enzyme cutting site are underline.
Table 4. The minimal inhibitory concentrations of antifungal drug against C. glabrata strains
C. krusei as reference
6. Figures Figure 1
Figure 1. Deletion of ADA3 and GCN5 decreased the acetylation level of H3K9 in C. glabrata.
C. glabrata strains were grown at 37˚C and total proteins were extracted. 10 µg of crude extracted proteins were separated by protein electrophoresis and transferred onto nitrocellulose, then probed with anti-H3K9ac antibody to determine the acetylation level. The anti-actin antibody was used as an internal control. Three biological repeats were conducted and the bolts were analyzed using ImageJ software. Asterisks indicate statistically significant difference compared to the wild type using one way ANOVA (*P < 0.05).
Figure 2. C. glabrata Ada3 and Gcn5 were contributed to growth at 37°C.
C. glabrata strains were grown overnight at 30˚C, washed twice with ddH2O and then diluted to 0.01 OD600 with fresh YPD broth. Measurements were conducted at 0, 3, 6, 9, 12, 24 and 48 h after incubation at 37˚C using SpectraMax 190 microplate reader.
Plot was drawn using Prism software (v5.03). The error bars represent standard deviations from three technical repeats. Asterisks indicate statistically significant difference compared to the wild type using two way ANOVA (*P < 0.05; **P < 0.01;
***P < 0.001; ****P < 0.0001).
Figure 3. C. glabrata Ada3 and Gcn5 were involved in drug tolerance.
C. glabrata strains were grown overnight at 30˚C with shaking at 200 rpm, washed twice with ddH2O, diluted to 1 OD600 with ddH2O, and then 3 µl of 5-fold serial dilution of cell suspensions were spotted onto YPD plate in the absence or presence of antifungal drug. The plates were incubated at 37˚C for 24 h and photographed.
Fluconazole (FLC); Posaconazole (PSC); Voriconazole (VRC); Micafungin (MCF);
Caspofungin (CSF); Anidulafungin (ANF); Amphotericin B (AmB).
Figure 4. C. glabrata Ada3 and Gcn5 were crucial in oxidative response and cell wall integrity.
C. glabrata strains were grown overnight at 30˚C with shaking at 200 rpm, washed twice with ddH2O, diluted to 1 OD600 with ddH2O, and then 3 µl of 5-fold serial dilution of cell suspensions were spotted onto YPD plate in the absence or presence of reactive oxygen species (H2O2 or menadione) or cell wall perturbing agents (CFW, CR or SDS).
The plates were incubated at 37˚C for 24 h except SDS plate for 48 h and photographed.
Hydrogen peroxide (H2O2); Menadione ; Calcofluor white (CFW); Congo red (CR)
Figure 5
Figure 5. C. glabrata Ada3 and Gcn5 were required for oxidative stress response by regulating SOD1 and CTA1.
C. glabrata strains were grown overnight in YPD broth at 30˚C, adjusted to 0.2 OD600/mL with fresh YPD broth with 2 mM H2O2, and incubated at 37˚C for 3 h. Total RNA was extracted using TRIzol and Turbo DNA-free kit to eliminate DNA, then 1 µg DNA-free RNA was reverse transcribed to cDNA using high capacity reverse transcription kit. Transcription levels were analyzed by SYBR® Green PCR Master Mix.
StepOnePlus system and StepOne (v2.2) system were used to determine cycle threshold (CT) value. C. glabrata ACT1 expression was used to normalize the relative quantity for comparing with wild type. P values were determined using one way ANOVA. (*P
< 0.05; **P < 0.01; ***P < 0.001).
Figure 6. Deletion of C. glabrata ADA3 or GCN5 enhanced agar invasion.
C. glabrata strains were grown overnight at 30˚C with shaking at 200 rpm, washed twice with ddH2O. Cell suspensions were diluted to 1 OD600 and 3 µL were spotted on YPD, incubated at 37°C for 3 days, then colonies were removed using swab and washed with dH2O.
Figure 7. Defect of HAT module increased expression of most virulence associated genes.
C. glabrata strains were incubated in fresh YPD broth with 2mM H2O2 at 37˚C. Total RNA was extracted and reverse transcribed into cDNA. Two virulence related gene family, EPA gene family and YPS gene family, were upregulated in HAT mutants.
Figure 8. Deletion of both ADA3 and GCN5 exhibited hypervirulence in murine systemic infection model.
Five to six-week-old male ICR mice were used in this study. 10 mice as one group and all groups were administrated with 150 mg/kg cyclophosphamide at day -3, 0, 1. C.
glabrata strains were grown in liquid YPD overnight at 30°C, washed twice with phosphate-buffered saline then adjusted to 3.5 × 108 cells/mL. 200 µL (7 ×107 cells) cell suspension was used for lateral tail vein injection and mice were monitored for 14 days.
Statistical analysis was conducted using Mantel-Cox log-rank test.
Figure 9. Proposed roles of HAT module within SAGA complex in drug tolerance and virulence in C. glabrata.
C. glabrata Ada3 and Gcn5 have conserved roles in oxidative stress response, cell wall integrity and drug tolerance. Interestingly, loss of GCN5 in C. glabrata have an intermediated response to oxidative stress, cell wall perturbing agents and drug tolerance (i.e., FLC, PSC, MCF). In addition, we found ADA3 and GCN5 might play as negative regulators in virulence that deletion of ADA3 and GCN5 enhance agar invasion, upregulate transcription of adhesion associated genes (EPA1, EPA20 and EPA23) and intracellular survival associated genes (YPS4 and YPS10), leading to hypervirulence in murine systemic infection model.
7. Supplementary
Table S1. Doubling time of the C. glabrata strains at 37°C
Doubling time are shown with mean ± standard error of the mean for three technical replicates. Statistical analyzed by unpaired t test with Welch’s correction. The P values between two measurements with the same letters were as follows: A1, P = 0.2935; A2, P = 0.1028; A3, P = 0.0829; A4, P = 0.2479; A5, P = 0.2446; A6, P = 0.0855; A7, P
= 0.3035; A8, P = 0.0745; A9, P = 0.2529; B1, P = 0.8557; B2, P = 0.6425; B3, P = 0.3697; B4, P = 0.6583; B5, P = 0.5649; B6, P = 0.7239; C1, P = 0.6737; C2, P = 0.333; C3, P = 0.6992; C4, P = 0.194; C5, P = 0.7706; D1, P = 0.7193; D2, P = 0.9654;
D3, P = 0.9233; D4, P = 0.8938; E1, P = 0.7935; E2, P = 0.5255; E3, P = 0.5971; F1, P = 0.8974; F2, P = 0.8724; G1, P = 0.9351.
Figure S1. The expression of two pseudohyphal regulation orthologs in C. glabrata were affected by HAT module.
CAGL0M07634g and CAGL0L01771g are SOK2 and PHD1 ortholog in S. cerevisiae, respectively. Loss of ADA2, ADA3 or GCN5 in C. glabrata increased CAGL0M07634g expression level but decreased CAGL0L01771g expression level.
8. Future work
a. Using GFP or other reporter genes to better understand the subcellular localization of Gcn5 in the absence of Ada2 and Ada3.
b. To investigate whether defect of HAT module affects the function of other modules in SAGA complex.
c. Using protein-protein interaction to elucidate whether other proteins function as Gcn5 will interact with SAGA complex in the absence of Gcn5.
d. To find which transcription factor is regulated by SAGA complex against oxidative stress in C. glabrata.
9. References
1. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. 2018.
Invasive candidiasis. Nature Reviews Disease Primers 4:18026.
2. Tan BH, Chakrabarti A, Li RY, Patel AK, Watcharananan SP, Liu Z, Chindamporn A, Tan AL, Sun PL, Wu UI, Chen YC, Xu YC, Wang H, Sun ZY, Wang LL, Lu J, Yang Q, Zhang QQ, Shao HF, Liao K, Woo PCY, Marak RSK, Kindo AJ, Wu CL, Ho MW, Lu PL, Wang LS, Riengchan P. 2015. Incidence and species distribution of candidaemia in Asia: a laboratory-based surveillance study. Clinical Microbiology and Infection 21:946.
3. Wiederhold NP. 2017. Antifungal resistance: current trends and future strategies to combat. Infection and drug resistance 10:249.
4. Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. 2012.
Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiology Reviews 36:288.
5. Kumar K, Askari F, Sahu MS, Kaur R. 2019. Candida glabrata: A Lot More Than Meets the Eye. Microorganisms 7:39.
6. De Las Peñas A, Juárez-Cepeda J, López-Fuentes E, Briones-Martín-del-Campo M, Gutiérrez-Escobedo G, Castaño I. 2015. Local and regional chromatin silencing in Candida glabrata: consequences for adhesion and the response to stress. FEMS Yeast Research 15.
7. Bannister AJ, Kouzarides T. 2011. Regulation of chromatin by histone modifications. Cell Research 21:381.
8. Verdin E, Ott M. 2015. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nature Reviews Molecular Cell Biology 16:258.
9. Suganuma T, Workman JL. 2011. Signals and combinatorial functions of histone modifications. Annual Review of Biochemistry 80:473.
10. Hong L, Schroth GP, Matthews HR, Yau P, Bradbury EM. 1993. Studies of the DNA binding properties of histone H4 amino terminus. Thermal denaturation studies reveal that acetylation markedly reduces the binding constant of the H4
"tail" to DNA. Journal of Biological Chemistry 268:305.
11. Sun J, Paduch M, Kim S-A, Kramer RM, Barrios AF, Lu V, Luke J, Usatyuk S, Kossiakoff AA, Tan S. 2018. Structural basis for activation of SAGA histone acetyltransferase Gcn5 by partner subunit Ada2. Proceedings of the National Academy of Sciences 115:10010.
12. Balasubramanian R, Pray-Grant MG, Selleck W, Grant PA, Tan S. 2002. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. Journal of Biological Chemistry 277:7989.
13. Cieniewicz AM, Moreland L, Ringel AE, Mackintosh SG, Raman A, Gilbert TM, Wolberger C, Tackett AJ, Taverna SD. 2014. The bromodomain of Gcn5 regulates site specificity of lysine acetylation on histone H3. Molecular & Cellular Proteomics 13:2896.
14. Wu M, Newcomb L, Heideman W. 1999. Regulation of gene expression by glucose in Saccharomyces cerevisiae: a role for ADA2 and ADA3/NGG1. Journal of Bacteriology 181:4755.
15. Canzonetta C, Leo M, Guarino SR, Montanari A, Francisci S, Filetici P. 2016.
SAGA complex and Gcn5 are necessary for respiration in budding yeast.
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1863:3160.
16. Kurat CF, Yeeles JTP, Patel H, Early A, Diffley JFX. 2017. Chromatin controls DNA replication origin selection, lagging-strand synthesis, and replication fork rates. Molecular Cell 65:117.
17. Church M, Smith KC, Alhussain MM, Pennings S, Fleming AB. 2017. Sas3 and Ada2(Gcn5)-dependent histone H3 acetylation is required for transcription elongation at the de-repressed FLO1 gene. Nucleic Acids Research 45:4413.
18. Cormack BP, Ghori N, Falkow S. 1999. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285:578.
19. Haynes BC, Skowyra ML, Spencer SJ, Gish SR, Williams M, Held EP, Brent MR, Doering TL. 2011. Toward an integrated model of capsule regulation in Cryptococcus neoformans. PLoS Pathog 7:e1002411.
20. Meara TR, Hay C, Price MS, Giles S, Alspaugh JA. 2010. Cryptococcus neoformans histone acetyltransferase Gcn5 regulates fungal adaptation to the host.
Eukaryotic Cell 9:1193.
21. Shivarathri R, Tscherner M, Zwolanek F, Singh NK, Chauhan N, Kuchler K. 2019.
The fungal histone acetyl transferase Gcn5 controls virulence of the human pathogen Candida albicans through multiple pathways. Scientific Reports 9:9445.
22. Chang P, Fan X, Chen J. 2015. Function and subcellular localization of Gcn5, a histone acetyltransferase in Candida albicans. Fungal Genetics and Biology
81:132.
23. Lin C-J, Hou Y-H, Chen Y-L. 2019. The histone acetyltransferase GcnE regulates conidiation and biofilm formation in Aspergillus fumigatus. Medical Mycology doi:10.1093/mmy/myz043.
24. Kounatidis I, Ames L, Mistry R, Ho H-l, Haynes K, Ligoxygakis P. 2018. A host- pathogen interaction screen identifies ada2 as a mediator of Candida glabrata defenses against reactive oxygen species. G3: Genes|Genomes|Genetics 8:1637.
25. Yu S-J, Chang Y-L, Chen Y-L. 2018. Deletion of ADA2 Increases antifungal drug susceptibility and virulence in Candida glabrata. Antimicrobial Agents and Chemotherapy 62:e01924.
26. Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuvéglise C, Talla E. 2004. Genome evolution in yeasts.
Nature 430:35.
27. Reuß O, Vik Å, Kolter R, Morschhäuser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119.
28. Gietz D, St Jean A, Woods RA, Schiestl RH. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic acids research 20:1425.
29. Yu S-J, Chang Y-L, Chen Y-L. 2018. Deletion of ADA2 increases antifungal drug susceptibility and virulence in Candida glabrata. Antimicrobial agents and chemotherapy:AAC. 01924.
30. Wayne P. 2008. Reference methods for broth dilution antifungal susceptibility testing of yeasts: approved standard, 2nd ed. Document M27-A3.
31. Workman JL, Kingston RE. 1998. Alternation of nucleosome structure as a
mechanism of transcriptional regulation. Annual Review of Biochemistry 67:545.
32. Piña B, Berger S, Marcus GA, Silverman N, Agapite J, Guarente L. 1993. ADA3:
a gene, identified by resistance to GAL4-VP16, with properties similar to and different from those of ADA2. Molecular and Cellular Biology 13:5981.
33. Briones-Martin-Del-Campo M, Orta-Zavalza E, Juarez-Cepeda J, Gutierrez- Escobedo G, Cañas-Villamar I, Castaño I, De Las Peñas A. 2014. The oxidative stress response of the opportunistic fungal pathogen Candida glabrata. Revista Iberoamericana de Micología 31:67.
34. Lo W-S, Dranginis AM. 1998. The cell surface flocculin Flo11 is required for pseudohyphae formation and Invasion by Saccharomyces cerevisiae. Molecular Biology of the Cell 9:161.
35. Guo B, Styles CA, Feng Q, Fink GR. 2000. A Saccharomyces gene family involved in invasive growth, cell–cell adhesion, and mating. Proceedings of the National Academy of Sciences 97:12158.
36. Rasheed M, Battu A, Kaur R. 2018. Aspartyl proteases in Candida glabrata are required for suppression of the host innate immune response. Journal of Biological Chemistry 293:6410.
37. Kaur R, Ma B, Cormack BP. 2007. A family of glycosylphosphatidylinositol- linked aspartyl proteases is required for virulence of Candida glabrata.
Proceedings of the National Academy of Sciences 104:7628.
38. Helmlinger D, Tora L. 2017. Sharing the SAGA. Trends in Biochemical Sciences 42:850.
39. Baptista T, Grünberg S, Minoungou N, Koster MJE, Timmers HTM, Hahn S,
Devys D, Tora L. 2018. SAGA is a general cofactor for RNA polymerase II transcription. Molecular Cell 70:1163.
40. Vernarecci S, Ornaghi P, Bâgu A, Cundari E, Ballario P, Filetici P. 2008. Gcn5p plays an important role in centromere kinetochore function in budding yeast.
Molecular and Cellular Biology 28:988.
41. Shih P-Y, Liao Y-T, Tseng Y-K, Deng F-S, Lin C-H. 2019. A potential antifungal effect of chitosan against Candida albicans is mediated via the inhibition of SAGA complex component expression and the subsequent alteration of cell surface integrity. Frontiers in Microbiology 10.
42. Georgakopoulos T, Gounalaki N, Thireos G. 1995. Genetic evidence for the interaction of the yeast transcriptional co-activator proteins GCN5 and ADA2.
Molecular and General Genetics MGG 246:723.
43. Brown AJP, Haynes K, Quinn J. 2009. Nitrosative and oxidative stress responses in fungal pathogenicity. Current Opinion in Microbiology 12:384.
44. Kasper L, Seider K, Hube B. 2015. Intracellular survival of Candida glabrata in macrophages: immune evasion and persistence. FEMS Yeast Research 15.
45. Segal AW. 2004. How neutrolphils kill microbes. Annual Review of Immunology 23:197.
46. Galocha M, Pais P, Cavalheiro M, Pereira D, Viana R, Teixeira MC. 2019.
Divergent approaches to virulence in C. albicans and C. glabrata: two sides of the same coin. International journal of molecular sciences 20:2345.
47. Sellam A, Askew C, Epp E, Lavoie H, Whiteway M, Nantel A. 2009. Genome- wide Mapping of the Coactivator Ada2p Yields Insight into the Functional Roles
of SAGA/ADA Complex in Candida albicans. Molecular Biology of the Cell 20:2389.
48. Cuéllar-Cruz M, Briones-Martin-del-Campo M, Cañas-Villamar I, Montalvo- Arredondo J, Riego-Ruiz L, Castaño I, De Las Peñas A. 2008. High resistance to oxidative stress in the fungal pathogen Candida glabrata is mediated by a single catalase, Cta1p, and is controlled by the transcription factors Yap1p, Skn7p, Msn2p, and Msn4p. Eukaryotic Cell 7:814.
49. Lee J, Godon C, Lagniel G, Spector D, Garin J, Labarre J, Toledano MB. 1999.
Yap1 and Skn7 Control two specialized oxidative stress response regulons in yeast. Journal of Biological Chemistry 274:16040.
50. Morgan BA, Banks GR, Toone WM, Raitt D, Kuge S, Johnston LH. 1997. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. The EMBO Journal 16:1035.
51. Kontoyiannis DP. 1999. Genetic analysis of azole resistance by transposon mutagenesis in Saccharomyces cerevisiae. Antimicrobial Agents and Chemotherapy 43:2731.
52. Ramírez-Zavala B, Mogavero S, Schöller E, Sasse C, Rogers PD, Morschhäuser J. 2014. SAGA/ADA Complex Subunit Ada2 Is Required for Cap1- but Not Mrr1-Mediated Upregulation of the Candida albicans Multidrug Efflux Pump MDR1. Antimicrobial Agents and Chemotherapy 58:5102.
53. Usher J, Haynes K. 2019. Attenuating the emergence of anti-fungal drug resistance by harnessing synthetic lethal interactions in a model organism. PLoS genetics 15:e1008259.
54. Zupan J, Raspor P. 2008. Quantitative agar-invasion assay. Journal of Microbiological Methods 73:100.
55. Santos R, Costa C, Mil-Homens D, Romão D, de Carvalho CCCR, Pais P, Mira NP, Fialho AM, Teixeira MC. 2017. The multidrug resistance transporters CgTpo1_1 and CgTpo1_2 play a role in virulence and biofilm formation in the human pathogen Candida glabrata. Cellular Microbiology 19:e12686.
56. Dodgson J, Avula H, Hoe K-L, Kim D-U, Park H-O, Hayles J, Armstrong J. 2009.
Functional genomics of adhesion, invasion, and mycelial formation in Schizosaccharomyces pombe. Eukaryotic Cell 8:1298.
57. Hua SST, Beck JJ, Sarreal SBL, Gee W. 2014. The major volatile compound 2- phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Research 30:71.
58. Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E. 2009. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryotic Cell 8:1750.
59. Li D-D, Fuchs BB, Wang Y, Huang X-W, Hu D-D, Sun Y, Chai D, Jiang Y-Y, Mylonakis E. 2017. Histone acetyltransferase encoded by NGG1 is required for morphological conversion and virulence of Candida albicans. Future Microbiology 12:1497.
60. De Las Peñas A, Pan S-J, Castaño I, Alder J, Cregg R, Cormack BP. 2003.
Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent