國立臺灣大學生命科學院生化科學研究所 碩士論文
Graduate Institute of Biochemical Sciences College of Life Science
National Taiwan University Master Thesis
草原蝰(Vipera ursinii renardi)蛇毒促凝蛋白酶的基因選殖 與特性
cDNA cloning and protein characterization of the
pro-coagulant proteases from Vipera ursinii renardi venom
余信宏 Xin-hong Yu
指導教授:蔡蔭和 博士 Advisor: Inn-Ho Tsai, Ph.D.
中華民國 100 年 7 月
July, 2011
殖
誌謝
能順利完成這本研究論文,要感謝有以往大家的研究成果可以提供參考,並 感謝蔡蔭和老師的指導,尤其在本論文的修改方面更是勞煩老師費心,此外也感 謝朱善德博士、李明亭博士與林淑華博士能在百忙之中撥空審核我的論文並且指 導修正。
感謝實驗室的英敏學姐、鴻森及安惇兩位學長平時實驗技術上的解惑與幫忙,
還有感謝惠晴學妹的提醒,由其在畢業前夕手忙腳亂的時候雪中送炭,實在萬分 感謝,與大家相處了兩年時光後終須一別,保重了大家。還有碩士班的各位同學 們,祝大家都能找到嚮往的工作。
最後感謝一直在背後支持我的家人們,無論是遠在他鄉常打電話關心的爸媽,
還是陪在身邊的阿靜,亦或是常常找我玩電動的阿宅弟弟,感謝你們讓我的研究 生生涯更多彩,也衷心期盼自己不要辜負你們的期望。
Contents
中文摘要……….………... I Aabstrate………..…..II Lists of abbreviations………..……….IV Lists of Figures………...………...V Lists of Table……….………...…VI
1. Introduction………..………..1
2. Materials and methods……….…..…..13
2-1. Venoms and other materials………..…...….13
2-2. Cloning and sequencing………..……13
2-3. Protein quantification………...……15
2-4. SDS- polyacrylamide gel electrophoresis……….…….15
2-5. Purification of Vur-FVA……….….15
2-6. Peptide mass fingerprinting………...….…16
2-7. N-terminal sequencing………16
2-8. Hydrolysis of human coagulation factor V by Vur-FVA……….….…..17
2-9. Phylogenetic analysis of CTL and CTLLs………..……17
2-10. Chromogenic assay of venom factor X activator activity………..…..17
2-11. Purification of Vur-FXA………….………..………..…..18
3. Results……….…….19
3-1. cDNA cloning and the predicted sequence of Vur-FVA…………..….…….….19
3-2. Sequence alignment and comparison between related venom serine proteases………..………..20
3-3. Purification and characterization of Vur-FVA………..…..20
3-4. Proteolytic activity of Vur-FVA toward human coagulation factor V…….…...20
3-5. cDNA cloning and the predicted sequence of C-typr lectin and C-type lectin...21
3-6. Chromogenic assay of crude venom factor X activator activity……….…21
3-7. cDNA cloning and the predicted sequence of Vur-FXA……….22
3-8. Sequence alignment and comparison between the published snake venom FX activator heavy chains………...….22
3-9. Purification and characterization of Vur-FXA………23
4. Discussion………41
5. Reference……….45
6. Appendix………..…53
I
中文摘要
本篇研究目的在於發現並了解 Vipera ursinii renardi 蛇毒內的促凝蛋白,Vipera ursinii renardi 的蛇毒先前較少被研究,因此仍然存在著許多未知。利用前人已發 表的類似蛋白的 cDNA 序列可設計出特殊的 primer,並可經由 PCR 放大所需的基 因,在本研究中已利用此技術成功放大了 Vipera ursinii renardi 蛇囊內編碼 Factor X activator subunits 與 Factor V activator 的基因,其中在絲胺酸蛋白酶的部分,除 了 FV activator (本文稱之為 Vur-FVA)還發現了 chymotrypsin-like protease,前者 的氨基酸序列與 Vipera lebetina FV activator 的具有 93%的高度相似性,而後者與 V. lebetina 的 chymotrypsin-like protease 也具有 90%的相似性,Vur-FVA 可經由凝 膠層析(gel filtration)與陽離子交換(cation exchanger )管柱而被純化出來,並可經 由 SDS-PAGE 觀察到 Vur-FVA 具有將 FV 活化的能力。除了 Vipera ursinii renardi 蛇毒活化 FX 的能力外,本研究中亦測量了其他許多 Vipernae 的蛇毒,儘管 V. u.
renard 蛇毒活化 FX 的能力比 Russell`s viper 弱了上百倍,然而其蛇毒確實具有 FX activator 的活性,V. u. renardi FX activator 在本研究中被命名為 Vur-FXA,經 過凝膠層析(gel filtration)管柱的初步純化後我們觀察到 Vur-FXA 的分子量大概 為 95 kDa,且經由 SDS-PAGE 的分析可得知 Vur-FXA 具有多個 subunit。研究中 還經由基因選殖的技術,發現了 Vur-FXA 的 CTLL light chains 與 Vipera lebetina 和 Daboia russelli 的 CTLL light chains 具有高度的相似性,而針對 Vur-FXA heavy chain 的部分,我們得到了一個 pIII 金屬蛋白酶,然而卻發現其缺少了負責與 light chains 形成雙硫鍵的 Cysteine。由以上結果可發現 V. u. renardi 蛇毒中具有多種促 凝成份,這些成份可能會與前人在 V. u. renardi 蛇毒中所發現的抗凝成份具有協 同作用,共同造成凝血症狀或獵物的死亡,因此未來可針對此一現象做更深入的 研究。
II
Abstrate
The aim of this study is to identify and characterize the pro-coagulant proteins in the venom of a less studied Viperinae species, Vipera ursinii renardi. Using the specific primers designed based on previously published cDNA sequences of the pro-coagulant proteases from the other two Viperinae species, we have cloned the cDNA encoding all the subunits or proteins of Factor X and Factor V activators from the venom glands of V. u. renardi (Vur). Two distinct serine proteases have been cloned from Vur cDNAs. One encodes a FV activator, and the other encodes a chymotrypsin-like protease. The deduced amino acid sequence of the former enzyme revealed 93% identity with Vipera lebetina FV activator and was designated as Vur-FVA. Another was 90% identical to a chymotrypsin-like protease of V. lebetina.
Vur-FVA was purified from the crude venom by gel filtration and cation exchanger columns; its FV activating activity was confirmed by SDS-PAGE analysis of the produced FVa. FX activating activities of many Vipernae venoms were compared by assay the FXa generated. Although the activity is 2 order of magnitude weaker than Russell`s viper venom, V. u. renardi venom do contain the activity. The FX activator (designated as Vur-FXA) was partially purified by gel filtration and shown the molecular weight is about 95 kDa. We also could find Vur-FXA was a oligomer by comparing the fractions containing Vur-FXA on reducing and non-reducing SDS-PAGE gel. The CTLL light chains of Vur-FXA were cloned and the deduced amino acid sequences are highly similar to those of V. lebetina and Daboia russelli.
The amino acid sequence of a PIII metalloprotease was also cloned. However, the deduced protease sequence lacks the Cys residue which involved in disulfide linking to the light chain in RVV-X. These results characterized Vur venom pro-coagulant components. It has been reported anticoagulant components exist in Vur venom. The
III
anticoagulant components and procoagulant components may act synergistically, leading to coagulopathy symptom or the death of prey.
IV
List of abbreviations
BCA: Bicinchoninic acid BSA: Bovine serum albumin CTL: C-type lectins
CTLL: C-type lectin like protein FV: Coagulation factor V
FX: Coagulation factor X HC: Heavy chain
LC: Light chain
LVV-V: Factor V activator from Vipera lebetina ORF: Open reading frame
PAGE: Polyacrylamide gel electrophoresis PBS: Phosphate buffered saline
PCR: Polymerase chain reaction PMF: Peptide mass fingerprinting
RVV-V: Factor V activator from Russell`s viper venom RVV-X: Factor X activator from Russell`s viper venom SDS: Sodium dodecyl sulfate
SVMP: Snake venom metalloprotease UTR: Untranslated region
VLFXA: Factor X activator from Vipera lebetina venom
Vur-FVA: Factor V activator from Vipera ursinii renardi venom Vur-FXA: Factor X activator from Vipera ursinii renardi venom
V
List of Figures
I. The cascade model of coagulation….…..………..4 II. The cell-based model of coagulation…..………..6
III. The primary sequence and domain structure of blood coagulation factor X mature protein and active form………..…7 IV. Domain structire of factor V………..………...…8 V. The activation of factor X by RVV-X……….…..…..……...9 VI. Schematic structures of snake venom metalloproteases…………...…..……...…10 1. The cDNA nucleotide sequence and the deduced amino acid sequence of the Vur-FVA………...……24 2. Alignment of the amino acid sequences of V. u. renardi FV activator and related venom serine proteases.………...……….26 3. Purification of Vur-FVA.……….…….27 4. SDS-PAGE analyses of the activation of human factor V (FV) by Vur-FVA……..28 5. Phylogenetic tree analysis of of Vur-CTLLs………...……….29 6. Sequence alignments of venom FX activator light chains………...…33 7. Alignment of the amino acid sequences of PⅢ metalloprotease from Viperinae venom……...………...……….34 8. Sequence alignments of FX activator heavy chains form D. russelii, V. lebitina venom, and V. u. renardi PIII metalloprotease.……….…………..…….35 9. Partial purification of Vur-FXA………...…36 10. Sequence alignments of PII metalloproteases………..…..37
VI
List of Tables
I. Snake species with factor-X-activator in the venom………11 II. Structural and functional properties of purified factor-X-activator…………....…11 III. Snake species with factor-V-activator in the venom………..…12 1. Comparison between the peptides masses of calculated and experimental trypsin hydrolyzed peptides of Vur-FVA.………..………..…….38 2. The CTLL cDNA cloned from venom glands of V. u. renardi.………..……..……39 3. Factor X activating activities of various viperid venoms in the presence of
………..…40
1
1. Introduction
In generally, snake venom composition is varied with different species. Closely related species or species under the same genus usually have similar venom components.
Hemorrhagic and myonecrotic toxins are characteristic symptom elicited by the Viperidae (Crotalinae and Viperinae) snake venoms [1,2]. Many of the viperid toxins are enzymes, including: L-amino acid oxidase, acetylcholinesterase, metallo- and serine proteases, phospholipases A2, ADPase etc. Differences in composition or proportions of venom proteins result in different symptom by the envenoming.
Vipera ursinii is a widespread species in Europe. It was found not only in regions from France to west Turkey, but also distributes further east in southern Russia, central Asia and west Xinjiang (China). Besides the well studied Russell’s viper and Macrovipera lebitina systems, we aim to solve the full sequences of Vipera ursinii renardi venom FX and FV activator.
Clotting cascade model of coagulation system comprises a series of steps where an enzyme activates another proenzyme to produce next enzyme. Many viperid venom components affect mammalian blood coagulation system, resulting in pro-coagulant or anti-coagulant effects. The targets of these venom components not only include blood coagulation factors, such as factor IX, X, VII, and prothrombin, but also protein cofactors, such as factor V and VIII [3]. Basically, these venom activators or inhibitors affect coagulation factors in the middle and last stages of the clotting cascade [4].
Mammalian coagulation system is divided into three parts, namely intrinsic, extrinsic and common pathway. The intrinsic pathway and extrinsic pathway converge into the common pathway, which begins at the activation of FX. FXa will associate with, FVa and on phospholipid membrane to form prothrombinase complex which is required for the efficient production of activated thrombin and leads to coagulation (Fig.
2
I). Additionally, a cell-based model suggests that coagulation occurs in vivo in distinct overlapping phases, and requires the participation of tissues factor bearing cell, and platelets [6]. The distinct phases are initiation, amplification and propagation (Fig. II).
The coagulation FX and FV play an important role in the coagulation system. In human and animal blood, the mature FX protein circulates as a two-chain protein. A 16.2kDa light chain linked with a 42kDa heavy chain are by disulfide bond [8].
Activation of FX is brought about by cleavage of a single peptide bond at Arg194-Ile195 (Fig. III); factor V circulates in blood as a 330kDa glycoprotein zymogen (Fig. IV). Its activation involves three specific cleavages by thrombin, and result to the release of B- domain. FVa is composed of a 105 kDa heavy chain (A1-A2 domains) and a 71/74 kDa light chain (A3-C1-C2 domains) associated by non-covalent interaction in the presence of divalent metal ions (Fig. IV)[3,10].
The present study is focus on FX and FV activators from Viperinae venoms. FX activators and FV activators were found in some Viperinae venoms (Table I and III), but none has been shown definitely from Crotalinae, Elapidae, and Colubridae venoms [8].
Snake venom FX activators can be either metalloproteases or serine proteases (Table. II).
Efficient FX activation by snake venom requires the presence of .
Among all the identified venom FX activators, RVV-X of Daboia russelii venom is the best characterized. It consists of a heavy chain of 57,600 and two light chains of 19,400 and 16,400 , respectively. The molecule contains six N-linked oligosaccharides, four of which are located in the heavy chain and one in each light chain. The complete amino acid sequences of all the three subunits of RVV-X have been determined [11]. RVV-X binds to the Gla domain of factor X via two C-type lectin like light chains which bring the heavy chain to cleave the Arg-Ile bond at position 194 (Fig.
V). The binding of two light chains to FX needs to form salt bridge with the Gla
3
domain [11]. C-type lectin like proteins (CTLLs) are an important snake venom family.
It can be classified into C-type lectin with carbohydrate recognition domain (CRD) and CTLL with non-carbohydrate binding domain, the former binds with sugars and agglutinate erythrocytes but the latter does not [12]. CTLL are linked to PIII metalloprotease by a disulfide bond during post-translational processing to form heterotrimeric RVV-X [12]. Snake venom metalloproteases can be classified into four groups (Fig. VI). Recently, there has been another method to classify SVMP [13].
Russell’s viper venom contains not only RVV-X, but also a powerful factor V activator, which is a serine protease known as RVV-V. RVV-V activates FV by cleaving the peptide bonds that after Arg1545 (Fig. IV). There are three isotype of RVV-V, α, β and γ [14]. The amino acid sequences of two of them (RVV-Vα and RVV-Vγ) were determined. Their content weight ratio is approximately 2:1:6. And they have molecular masses of 29, 27.5 and 29kD, respectively [14]. Like RVV-V, the FV activator of Vipera lebetina venom is a 28.4kDa single-chain serine protease, and cleaves the FV molecule at Arg1545 [15]. Factor V activators in the venom of Crotalidae, Elapidae and Viperidae were also reported but not characterized (Table. III).
In this study, I have investigated FV and FX activator based on the cDNA prepared from venom-glands of two specimens of the V. ursinii renardi captured in southern Russia (Krasnodar region), and partially purified both factor V and factor X activator from the V. u. renardi venom.
4
Fig. I. The cascade model of coagulation [5]. Intrinsic pathway is initiated by the factors that are present within the blood. It is start when FXII contact with negative charged, such as membrane of activated platelet membrane, with the aid of high molecular weight kininogen (HMWK). HMWK also is an anchor when FXIIa convert prekallikrein to kallikrein, which can accelerate the formation of FXIIa. FXIIa and HMWK convert FXI to FXIa. FXIa, thrombin and FXa then convert FVIII to FVIIIa.
5
Finally, FIXa, FVIIIa and negative cell membrane form tenase complex in the presence of . Tenase complex then converts FX to FXa. Extrinsic pathway is initiated by injury. When injury occurs, the subendothelial cell expose to following blood.
Subendothelial cell is tissue factor bearing cell, the situation let FVII come into contact with tissue factor (TF). Although some circulating cells (eg, monocytes or tumor cells) and microparticals may also express TF on their membrane surface, but this TF under normal conditions is thought to be inactive or encrypted [6]. Once contact with TF, FVII autoactivates to FVIIa, then following by the formation of TF and FVIIa complex. TF and FVIIa complex have the ability to activate FX to FXa. The intrinsic pathway and extrinsic pathway converge into the common pathway, which begin at the activation of factor X. FXa will associate with FVa and on phospholipid membrane to form prothrombinase complex which activates prothrombin (FII) to thrombin FIIa. Finally fibrinogen (FI) is converted into fibrin (FIa) by thrombin, and further forming insoluble mesh by the mediation of FXIIIa, which is activated by thrombin
6
Fig. II. The cell-based model of coagulation [6]. (a) initiation phase was occurs on the surface of TF-bearing cell. Once injury occurs, TF-bearing cell exposes to flowing blood, FVII binds to exposed TF and autoactivates to FVIIa. TF-FVIIa complex then activates additional FVII, small amount of FIX and FX. FV then be activated slowly by FXa. FXa and FVa form prothrombinase complex, which cleave prothrombin (FII) to generate small amount of prothrombin (FIIa). Once FXa dissociated from TF-bearing cell, it will be incativated rapidly by TFPI or AT. (b) Thrombin diffuse away from TF-bearing cell and activate platelets that at the site of injury. Further more, Thrombin cleaves FXI to FXIa and activates FV to FVa on the platelet surface. Thrombin also cleaves von willebrand factor off of FVIII, then activae FVIII to FVIIIa. The releasing von willebrand factor then mediate platelet adhesion and aggregation. (c) The propagation phase occurs on the surface of the platelets that activate in amplification phase. FIXa (generated in initiation phase) bind to FVIIIa (generated in amplification phase) on the surface of activated platelet to form intrinsic tenase complex. It generate rapidly FXa on the activated platelet surface,then FXa binds with FVa and cleaves prothrombin to form thrombin.
7
Fig. III. The primary sequence and domain structure of blood coagulation factor X mature protein and active form [9]. The 16.2kDa light chain contains 11γ-carboxy glutamic acid residues (Gla domain), which are essential for the -dependent binding of factor X(a) to negatively charged procoagulant membranes. The Gla domain is followed by two epidermal growth factor-like domain (EGF-like domains), the Asp63 of the first EGF-like domains is ß-hydroxylated. The 42kDa heavy chain of factor X contains a catalytic triad ser195-His57-Asp102. These two chains linked by disulfide bond. Activation of factor X is brought about by cleavage of a single peptide bond at Arg194-Ile195, which removes the first 52 amino-terminal residues of the heavy chain and upon which the active site becomes fully exposed.
8
Fig. IV. Domain structire of factor V [3]. The doamins are indicated by letters A, B and C inside boxes. The A doamins corredpond to residue numbers of 1 to 307, 317 to 656, and 1546 to 1877. The C doamin correspond to residues numbers of 1878 to 2036 and 2037 to 2196. Thrombin cleavage sites are indicated by solid arrow. After activation by thrombin, the heavy chain 105kDa (A1, A2 doamin) and light chain 71/74 kDa (A3, C1, C2) are associated non-covalently in prepresece of divalent ion [14]. The snake venom FV activators from Daboia russelii and Daboia lebetina cleave FV at Arg1545, giving rise to a FVa molecule with a light chain of 71/74 kDa (A3, C1, C2) and a heavy chain of around 290 kDa (A1, A2, B doamin) [54].
9
Fig. V. The activation of factor X by RVV-X [8]. The heavy chain of RVV-X contains the metalloprotease domain responsible for the proteolytic cleavage of the target bond in factor X. The cleavage site Arg194 is indicated by allow. The two C-type lectin light chains (LC1 and LC2) together form a secondary binding site specific for interaction with the Gla domain of factor. The disulfide bonds linkage between each subunit of RVV-X are indicated by dash line.
10
Fig. VI. Schematic structures of snake venom metalloproteases [2]. Based on domain structures, SVMPs are classified into four groups. P-I enzymes comprise only the metalloprotease domain, P-II enzymes contain a disintegrin domain after the metalloprotease domain, and the disintegrin domain of P-II enzyme containing RGD sequence will release after proteolytic processing. P-III enzymes are usually glycoproteins that contain an additional Cys-rich C-terminal domain. And the RGD or KGD sequence of disinterin domain is instated by XXGD sequence, so it will not release. P-IV enzymes are P-III enzymes link with two C-type lectin-like proteins by disulfide bond. The preprosequence contain 18 residues signal peptide, and the prosequence (about 170 residues) with a cysteine-switch motif (PKMCGV), which inhibits catalytic activity. These domains will be removed, after that SVMP is activated.
The metalloproteinase domain (about 200 residues) has the -binding motif (HEXXHXXGXXH).
11
Table I. Snake species with factor X activator in the venom [8].
Table II. Structural and functional properties of purified factor X activator [8].
,
12
Table III. Snake venom factor V activator that have been reported [14].
13
2. Material and Method
2-1. Venoms and other materials
Lyophilized venom of Vipera ursinii renardi was a gift from Professors Yuri N. Utkin, Russia. Lyophilized venoms of Vipera ammodytes montandoni, Vipera latifi, Vipera bornmuelleri were purchased from Latoxan. Bothrops atrox, Daboia russselli (Pakistan), Daboia russselli pulchella, Daboia siamensis (Flores), Daboia siamensis (Java) were purchased from LaToxan, and Daboia siamensis (Sumatra) venom was from Yayasan bina karya kesatria herbal medical center. Daboia russselli (Kolkata) was a gift from Prof. Antony Gomes (Univ. of Calcutta, India) and Daboia russselli (Haffkine bio-pharmaceutical coporation, Bombay) was a gift from Prof. Anthony T. Tu (State Univ. of Colorado, USA).
2-2. Cloning and sequencing
After obtaining the fresh venom gland of V. u. renardi preserved in RNAlater solution (Sigma-Aldrich, U.S.A.), total RNA was extracted with RNA isolation kit (Invitrogen, USA). The mRNA was purified with poly (A) mRNA purification kit (Invitrogen, USA). Finally, cDNA was synthesized with cDNA synthesis system kit (Stratagene, USA). Specific primers corresponding to conserved 5’ signal peptide and 3’-UTR were designed based on the cDNA sequences of related venom serine proteases, C-type lectin like proteins and metalloprotease (MP), respectively. In addition to these primers, another sense primer for metalloprotease was designed to anneal the region encoding the pro-protein domain, and was used to amplify the Vur PIII-MP cDNAs by PCR.
For serine protease, the sense primer was: 5’-CAG, AGT, TGA, AGC, TAT, GGT, (G/T) (C/T)T, G-3’; antisense primer was 5’-G(A/G)T, T(A/T)(A/G), G(A/G)A, TAT,
14
AG(A/G), AGA, (C/G)AT, (A/C/G)T(A/C), C-3’. The PCR condition was: 35 cycles of denaturation (92℃, 1min), followed by annealing (52℃, 1min), extension (72℃, 1min).
For PII metalloprotease, the sense primer was: 5’-ATG, AT(A/T/C), CA(A/G), GTN, CTN, TT(A/G), GT-3’; antisense primer was 5’-C(G/T)T, GAC, AGC, AAA, TAA, GC-3’. The PCR condition was: 35 cycles of denaturation (94℃, 1min), followed by annealing (52℃, 1min), extension (72℃, 3min). For PIII metalloprotease, the sense primer was: 5’-TCT, CAG, TTA, GTT, (T/G)CT, ACT, TCT-3’; antisense primer was 5’-TAG, TAG, GAT, G(A/T)A, GGC, AAA, TG-3’. The PCR condition was: 35 cycles of denaturation (94℃, 1min), annealing (45℃, 1min), and extension (72℃, 2min). For C-type lectin like proteins, two pairs of primer and PCR condition are performed, the first: the sense primer was 5’-GGA, A(C/G)G, AAG, (A/G)CC, ATG, GGG, CG-3’;
antisense primer was 5’-CTT, C(C/T)T, TGC, TTC, TCC, A(A/G)A, CTT, C-3’. The PCR condition was: 35 cycles of denaturation (94℃, 1min), annealing (50℃, 1min), and extension (72℃, 1min). The second: the sense primer was 5`-GAA, CTA, GAG, CTG, ATT, TTG, ATT-3`; antisense primer was 5`-CTT, C(C/T)T, TGC, TTC, TCC, A(A/G)A, CTT, C-3`. The PCR condition was: 35 cycles of denaturation (94℃, 1min), annealing (45℃, 1min), and extension (72℃, 1min).
After PCR, the product was purified by PCR Centrifugal Filter Devices (Milipore, U.S.A.). The purified product was treated with polynucleotide kinase (Promega Biotech, Wisconsin, U.S.A.) then separated DNA fragment with correct size as the procedure of Gel Extration Kit (QIAGEN, Germany). The DNA fragment was inserted into
“pGEM-T easy vector” (Promega Biotech, Wisconsin, U.S.A.) and then transformed into E.coli strain JM109 (Promega Biotech, Wisconsin, U.S.A.). After proliferation at selected medium overnight, white colonies were selected and the plasmids were extracted using Miniprep Kit (QIAGEN, Germany). Finally, DNA sequencing was
15
conducted with DNA sequencing system (Model 3730) using the Taq-Dye-Deoxy terminator cycle sequencing kit (PE Applied Biosystems, U.S.A.).
2-3. Protein quantification
BCA protein assay kit (Pierce, USA) was used to quantify proteins. The protein concentrations were determined spectrophotometrically and calibration with standard solution of bovine serum albumin (BSA).
2-4. SDS-polyacrylamide gel electrophoresis
Protein under reduced condition was mixed with reduced sample buffer (containing 2% (V/V) β-mercaptoethanol) and incubate at 95℃ for five minutes. Protein under non-reduced condition was mixed with sample buffer (not containing reducing agent).
The processed samples were loaded to a 4-12% gradient SDS-polyacrylamide gel (Invitrogen, USA) and electrophoresed at a constant voltage of 160 V. Two running buffers were used. MES buffer (Invitrogen, USA) was for separating low molecular weight protein and MOPS buffer (Invitrogen, USA) was for separating high molecular weight protein. After electrophoresis, the gels were stained with RAPIDstain reagent (Bioman, Taiwan).
2-5. Purification of Vur-FVA
Approximately 15mg of the crude venoms of V. u. renardi was dissolved in 150ul of 0.1 M ammonium acetate (pH6.5) and centrifuged at 12,000g for 5min. The supernatant was then loaded to Superdex 75 column (10/300 GL; GE healthcare, Germany) on an FPLC apparatus (Pharmacia). The column was eluted with 0.1 M ammonium acetate (pH6.5) at a flow rate of 1.0 ml/min, and fractions of 0.5ml were collected. After assay
16
for the ability to cleave chromogenic substrate p-Tosyl-Gly-Pro-Arg p-nitroanilide (Sigma-Aldrich, U.S.A.), the active fractions were pooled, and lyophilized. After rehydration, the pooled fraction was further loaded into a Mono S column (5/50 GL; GE healthcare, Germany) which had been pre-equilibrated with 20mM MES buffer (pH 6.0), and eluted with the same buffer under a gradient of NaCl (0-1 M). After assay with the chromogenic substrate, active fractions were pooled and desalted using a centrifugal filter device (Amicon ultra, U.S.A.).
2-6. Peptide mass fingerprinting
For peptide mass fingerprinting (PMF), the gel bands of target proteins were excised separately and cut into pieces after electrophoresis. The in-gel trypsin digestion kit of Thermo Scientific Co. (USA) was used. Each sample was reduced with ~50mM Tris [2-carboxyethyl]phosphine at 60 ℃ for 10 minutes, and alkylated with ~100mM iodoacetamide at room temperature for 1 hour in the dark. The sample was washed twice with 50% acetonitrile at 37℃ for 15 minutes each time, dehydrated with acetonitrile for 25 minutes, and then air dried. Finally, it was hydrolyzed with ~25ng modified trypsin. For further extract peptide, 1% formic acid was added to gel pieces and incubate for 5 minutes. The resultant peptides were analyzed using MALDI-TOF/TOF with a detection mass range of 800-4000 Da. The results and the calculated masses of the predicted fragments were matched.
2-7. N-terminal sequencing
Purified Vur-V (10-20 ug/well) electrophoresed on a 1.0mm thick 4-12% SDS PAGE under reducing condition. The protein bands were blotted to a PVDF membrane. After the gel was stained with Amido Black (0.2% in 7% acetic acid), the protein bands were
17
cut out, and the extracted protein was sequenced by a gas-phase sequencer (Procise 492;
Applied Biosystems).
2-8. Hydrolysis of human coagulation factor V by Vur-FVA
Human factor V and factor Va were purchased from Haematologic Technologies Inc.
0.3ug factor V and 0.3ug Vur-FVA were mixed and incubated at 37℃ for 30 minutes.
The product was analyze by SDS-polyacrylamide gel electrophoresis along with 0.3ug FV, 0.3ug FVa and 0.3ug Vur-FVA as controls, under reduced and non-reduced conditions. The protein bands were stained by silver stain kit (Invitrogen, USA).
2-9. Phylogenetic analysis of CTLs and CTLLs
Amino acid sequences of a total of 27 snake venom CTLs and CTLLs were retrieved using Blast search in addition to our newly determined sequence. Their sequences were aligned using the Vector NTI program (Invitrogen Corp.). A phylogenetic tree was generated using the neighbor-joining methodology of the PHYLIP program, while the Bothrops jararacussu CTL (BjcuL) sequence was used as an out-group. The degree of confidence was determined using bootstrap analysis of 1000 replicates [16].
2-10. Chromogenic assay of venom factor X activator activity
The assay was conducted in 96 well-plate filled with 80ul buffer containing 150 mM NaCl, 20 mM Tris/HCl, 5 mg/ml BSA, 2.5 mM Ca (pH 7.4), 5µl appropriately diluted snake venom was added, each well was then added 1.5µl of 0.06 ug/ul factor X.
After incubation at 37℃ for 20 min, 20ul of 2 mM Spectrozyme FXa substrate MeO-CO-D-CHG-Gly-Arg-pNA (American diagnostic inc.) was added. The path length is about 3.5mm. The amount of factor Xa generated by the venom was quantified by
18
measuring the initial velocity of p-nitroaniline liberation at 405nm with a kinetic plate reader (Molecular Devices. SpectraMax 3). The unit was defined as liberation of 1mM p-nitroaniline per min per mg venom, under the experimental condition.
2-11. Partial purification of Vur-FXA
Approximately 15mg of the crude venoms of V. u. renardi was dissolved in 150µl of 0.1 M ammonium acetate (pH6.5) and centrifuged at 12,000g for 5 min. The supernatant was loaded to a Superdex 75 column (10/300 GL; GE healthcare, Germany) on an FPLC apparatus (Pharmacia). The column was eluted with 0.1 M ammonium acetate (pH6.5) at a flow rate of 1.0 mL/min, and fractions of 0.25mL were collected.
Each fraction was diluted up to thirty folds (V/V) during the FX activator assay as described in Method section 2-10. The Unit was defied as liberation of 1mM p-nitroaniline per min per mg protein under the experimental condition. Proteins in the fractions were analysis by gradient SDS-PAGE under reduced and non-reduced condition. Fractions that contain FX activator were collected, pooled and lyophilized.
19
3. Results
3-1. cDNA sequences and the predicted protein sequence of Vur-FVA
PCR amplification and cloning of Vur-FVA were carried out using the cDNA prepared from the venom glands of V. u. renardi as the template. After PCR, a DNA of about 0.9 kb was amplified. Totally twenty clones were selected for sequencing, 20 clones encoded Vur-FVA while two encoded another serine protease which is similar to Chymotrypsin like V. lebetina protease. The amino sequences were deduced from the nucleotide sequence (Fig. 1). The cDNA of Vur-FVA encodes an ORF of 260 amino acids including 24 amino acids of signal peptide. Structurally, it is most similar to the factor V activator of V. lebitina [17] and with 93% identity. Moreover, the mature 236 residue protease contains the catalytic triad ( , , and ) of the trypsin-family, and 12 conserved Cys residues of venom serine proteases. The isoelectric point calculated from Vur-FVA amino acid sequence was 8.46. There are two potential N-glycosylation sites (Asn-X-Ser/Thr) at residues N100 and N229 in Vur-FVA.
In order to confirm the protein sequence deduced from the cDNA is same with purified Vur-FVA protein, we conducted the N-teminal sequencing and the “Peptide Mass Fingerprinting” analysis. The N-terminal sequence was determined as VTGGD by automatic sequencer (Fig. 1). It was same as the deduced amino acid sequence but some of its N-terminal Val residue could be removed during the purification. The purified Vur-FVA protein was digested in gel with trypsin, and the resulting peptides were analyzed by MALDI-TOF/TOF. I found five peptide fragments matched those predicted.
The total coverage is more than 40% of the entire mature Vur-FVA sequence (Fig. 1 and Table 1).
20
3-2. Sequence comparison between related venom serine proteases
Protein sequences of mature Vur-FVA and related FV activating serine proteases were aligned in Fig. 2. These sequences are highly similar (more than 83%), and the putative catalytic site residues ( , disulfide bonds (Cys7, 141; Cys28, 44;
Cys76, 234; Cys120, 188; Cys152, 167; Cys178, 203) and N-glycosylation sites ( are all conserved. Notably, they are basic proteins with isoelectric point from eight to nine.
3-3. Purification and characterization of Vur-FVA
Vur-FVA was purified from the crude venom of V. u. renardi by two steps of chromatography. The venom was separated into about ten peaks by using Superdex 75 column (Fig. 3A). The third peak (indicated by bar) exhibiting the ability to cleave chromogenic substrate N-(p-Tosyl)-Gly-Pro-Arg p-nitroanilide was collected.
N-(p-Tosyl)-Gly-Pro-Arg p-nitroanilide is a chromogenic substrate for thrombin and other serine proteases. The amount of proteins in third peak is approximately 9.2% of total proteins loaded. From the elution volume, the molecular weight of Vur-FVA was estimated to be 58.8 kDa. The third peak was further purified by cation exchange chromatography Mono S column (Fig. 3B). The putative Vur-FVA was eluted at 0.4M NaCl, approximately, its yield was about 0.15% of the crude venom. SDS-PAGE analysis of Vur-FVA revealed one major bane with an apparent mass of 30 kDa under non-reducing and 37 kDa under reducing conditions (Fig. 3B).
3-4. Proteolytic activity of Vur-FVA toward human coagulation factor V
I have studied whether Vur-FVA can activate FV by analyzing its reaction product.
After 0.3 ug Vur-FVA and 0.3 ug human FV were mixed and incubated for 30 min at
21
37°C, the product was studied by SDS-PAGE analysis under reduced condition. As expected, almost all human FV (330kDa) was cleaved, and it may transform into FVa containing 290 kDa and 74 kDa subunits (Fig. 4).
3-5. cDNA cloning and sequencing of CTL and CTLL of V. u. renardi
We identified totally six distinct nucleotide sequences for the CTL and CTLL clones obtained by PCR amplification and DNA sequencing. Since they exist as disulfide bond linked heterodimer, I speculate a total of three Vur-CTLLs exist. We attempted to monitor their proteins functions by molecular phylogenetic analysis along with other CTL and CTLL family members (Fig. 5). It was found that two of the CTLL were closely related to α and β chain of dabocetin [18], so we designed them as vipercetin α and β. Another pair of sequences closely related to Dr-FGbp α and β, so they were named as Vur-FGbp α and β putatively. Remarkably, two of the CTLL amino acid sequences were similar to the light chains of FX activator (designated as Vur-FXA) and they were designated as Vur-FXA-LC1 and -LC2. Each of the subunits has one potential N-glycosylation site.
The amino acid sequences of light chains of the FX-activators, Vur-FXA, RVV-X [19] and VLFXA [20] were aligned in Fig. 6. They all shared conserved residues , which are responsible for interacting with Gla domain of FX [21].
All of the Cys residues of CTLLs are conserved, including the Cys at the C-terminus of LC2 is responsible for forming disulfide bond with the heavy chain [22]. The successful cloning of Vur-FXA light chains from the cDNA of V. u. renardi venom glands supports the presence of RVV-X like FX activator in this venom species.
3-6. cDNA cloning and the predicted sequence of Vur-FXA heavy chain
22
After PCR amplifying using designed specific primers, a 1400 bp product was obtained. The amino sequence of the venom metalloprotease was deduced from the amplified DNA fragment. It was found that the Vur-PIII protease are 74% and 73%
similar to the pIII metalloproteses of Echis ocellatus [23], and 72% similar to PIII metalloprotese of Echis carinatus sochureki [24] but less similar to factor X activator heavy chain (Fig. 7). Additionally, I had used many other designs of the primers, trying to amplify and clone other metalloproteses from this venom species. In the process, we also solved two PII metalloprotease sequences and they were highly (>80% ) similar to that of lebetase-2 [58] (Fig. 10). Lebetases are fibrinolytic metalloprotease from Macrovipera lebetina venom [59].
3-7. Sequence alignment and comparison between FX-activator heavy chains The tentative Vur-FXA PIII sequence is 56% identical to the HC of Daboia siamensis RVV-X, and a 67% identical to that of HC of V. lebetina FXA (Fig. 8). It has four potential N-glycosylation site and 17 Cys residues. The putative disulfide bonds were predicted based on those in RVV-X (D. siamensis). Like the FXA-HC of V. lebetina the first disulfide bond was disappeared in Vur-FXA-HC. The isoelectric point (pI) predicted for Vur-FXA is 6.8 or lower, which is slightly lower than the pI values of the FXA-HC of D. siamensis and V. lebetina. Remarkably, a Cys- residue responsible for the disulfide bonding with the light chain 2 of both HCs of RVV-X and V. lebetina FXA [25] was missing in Vur-FXA-HC.
3-8. Chromogenic assay of FX activator activity in crude venoms
I have studied factor X activator activities in various venoms, including: V. u. renardi, Vipera latifi, Vipera ammodytes montandoni, Vipera bornmuelleri, Vipera ursinii
23
renardi, Vipera xanthina palestinae, Daboia russselii (Pakistan), Daboia russselii (Bombay), Daboia russselii (Kolkata), Daboia russselli pulchella (Sri Lanka), Daboia siamensis (Flores), Daboia siamensis (Java and Sumatra), Bothrops atrox, and Echis ocellatus. From this assay, I found V. u. renardi venom has factor X activator activity but which is 20-50 folds weaker than D. russselii or D. siamensis (Table. 3). Consistent with previous reports, Daboia russelii and D.siamensis venoms contain the highest levels of FX-activating activity [26]. Notably, Daboia shows geographic variations in their venom FX-activator activities.
3-9. Purification and characterization of Vur-FXA
The Vur-FXA was partially purified by Superdex 75 column (Fig. 9A). Fractions of 0.25 mL were collected and assayed for the FX-activator activity. FX-activator activities were detected in fractions eluted from 8.25 to 10 ml from the column. The calculated molecular weight of Vur-FXA is about 95.9 kDa. After this purification, the specific activity has increased from 256 to 6902 (Unit/mg). Parts of fractions were analyzed by a gradient SDS-PAGE (Fig. 9B). A 94 kDa protein band showed increasing concentrations which were associated with the increasing FX-activator activities, and this 94 kDa protein band disappeared in the gel under reducing condition.
24
25
Fig. 1. The cDNA nucleotide sequence and the deduced amino acid sequence of the Vur-FVA. The signal peptide and the primers used in PCR were underlined. The N-terminus sequence and tryptic peptides confirmed by PMF are boxed. The coding region is shown in uppercase and untranslated regions in lowercase letters. Potential N-glycosulated asparagines are high-lighted in bold.
26
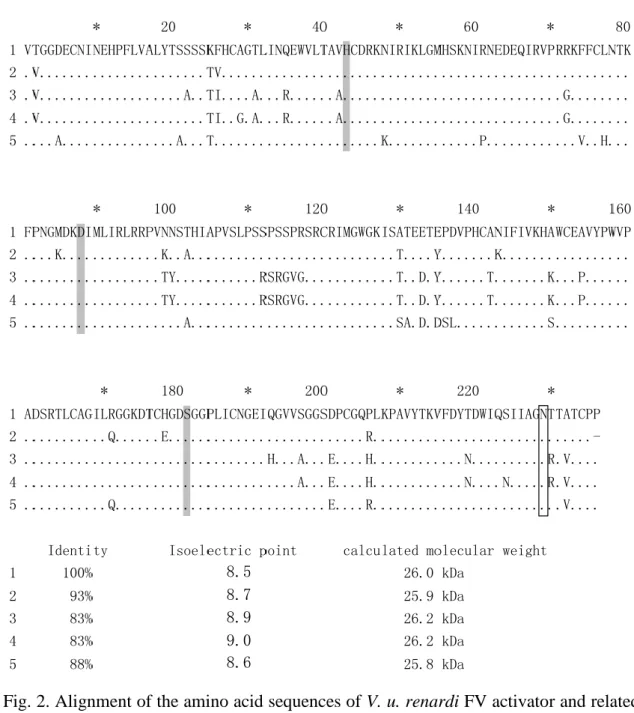
Fig. 2. Alignment of the amino acid sequences of V. u. renardi FV activator and related venom serine proteases. 1. Vur-FVA; 2. V. lebetina FV activator (AAF03233) [17]; 3.
D. siamensis RVV-V gamma (ADP88558); 4. D. siamensis RVV-V alpha (P18964) [50]; 5. Echis coloratus protease (ADI47574) [24]. Residues identical to those of Vur-FVA are denoted by dots, and gaps are marked with hyphens. Tentative catalytic triad residues and N-glycosylation sites are showing in gray and box, respectively.
8.5 8.7 8.9 9.0 8.6
27
Fig. 3. Purification of Vur-FVA. (A) Superdex 75 gel filtration of V. u. renardi venom (about 15mg) dissolved in pure water. SP fraction (indicated by bar) capable of hydrolyzing the chromogenic substrate N-(p-Tosyl)-Gly-Pro-Arg p-nitroanilide was pooled and lyophilized. (B) Subsequent separation of the Superdex 75 gel filtration SP fraction on a Mono S column. The elution was achieved by a gradient of NaCl (dashed line) in 20mM MES, pH6.0. Active fractions were pooled (indicated by bar), desalted and lyophilized. The inset shows the SDS-PAGE analysis of the purified Vur-FVA under reducing (R) and non-reducing (NR) conditions.
28
Fig. 4. SDS-PAGE analyses of the activation of human factor V (FV) by Vur-FVA.
4-12% of acrylamid gel and MES running buffer were used. Protein bands were silver-stained. Lane 1, 0.3ug FV (330 kDa); lane 2, 0.3ug standard FVa (two subunits, 94 and 74 kDa); lane 3, 0.3ug FV plus 0.3ug Vur-FVA, incubated 30 min at 37°C; lane 4, 0.3ug Vur-FVA (35 kDa); lane M, molecular weight markers.
29
30
Fig. 5A. Phylogenetic analysis of Aα subunits of snake venom CTLLs. The BjcuL Aα chain is the out-group. Bootstrap values are shown at each node. The CTLL (accession number) and the venom origin are: Aggretin (AAF79952) [27] and Rhodocetin (P81397) [28], Calloselasma rhodostom; Agkicetin (AAG42040) [29]and ACP (BAA99281) [30], Agkistrodon actus; AHP-IX-BP (AAQ24215) [31], A. halys pallas; Alboaggregin A2 (P81112) [32] and B (ABS12076) [33], Trimeresurus albolabris; Bitiscetin (1UEX_A) [34], Bitis arietans; BjcuL (p83519) [35], Bothrojaracin (AAX68503) [36] and Botorocetin (P22029) [37], Bothrops jararaca; Convulxin (CAA76181) [38], Crotalus durissus terifficus; Dabocetin (ABA86561) [4], Dr-FGBP (Chen H.S. Ph.D. thesis result), Dr-P31 (Chen H.S. Ph.D. thesis result) and RVV-X-LC (Q4PRD2) [6], D.
siamensis; Echicetin (P81017) [39], Echis carinutus leucogaster; EMS16 (BAC77706) [40], E. multisquamatus; Flavocetin-A (AAN72438) [41], Habu IX-BP (1BJ3A) [42]
and Habu IX/X-BP (P23806) [43], T. flavoviridis; Halyxin (AAG17178) [44], A. halys brevicaudus; LmsL (Q9PSM4) [45], Lachesis muta stenophyrs; Mamushigin (BAA34424) [46], A. halys blomohoffi; TMVA (AAM43808) [47], T. mucrosquamatus;
TSV-IX-BP (AAQ15153) [48], T. stejnegri; X-BP (1IOD_A) [49], Deinagkistrodon acutus. VLFXA-LC (Q696W1) [7],Vipera lebetina. Newly determined sequences are bolded.
31
Vur-FXA-LC
Vipercetin Vur-FGBP
32
Fig. 5B. Phylogenetic analysis of Bβ subunits of snake venom CTLLs. The BjcuL Bβ chain is the out-group. Bootstrap values are shown at each node. The CTLL (accession number) and the venom origin are: Aggretin (AAF79953) [27] and Rhodocetin (P81398) [28], Calloselasma rhodostom; Agkicetin (AAG42041) [29] and ACP (BAB20441) [30], Agkistrodon actus; AHP-IX-BP (AAQ24216) [31], A. halys pallas; Alboaggregin A3 (P81113) [32] and B (ABS12077) [33], Trimeresurus albolabris; Bitiscetin (1UEX_B) [34], Bitis arietans; BjcuL (p83519) [35], Bothrojaracin (AAX68504) [36] and Botorocetin (P22030) [37], Bothrops jararaca; Convulxin (CAA76182) [38], Crotalus durissus terifficus; Dabocetin (AAY63876) [4], Dr-FGBP (Chen H.S. Ph.D. thesis result), Dr-P31 (Chen H.S. Ph.D. thesis result) and RVV-X-LC (Q4PRD1) [6], D.
siamensis; Echicetin (P81996) [39], Echis carinutus leucogaster; EMS16 (BAC77707) [40], E. multisquamatus; Flavocetin-A (AAN72439) [41], Habu IX-BP (1BJ3B) [42]
and Habu IX/X-BP (P23807) [43], T. flavoviridis; Halyxin (AAG28522) [44], A. halys brevicaudus; LmsL (Q9PSM4)[45], Lachesis muta stenophyrs; Mamushigin (BAA34425) [46], A. halys blomohoffi; TMVA (AAM43809)[47], T. mucrosquamatus;
TSV-IX-BP (AAQ15154) [48], T. stejnegri; X-BP (1IOD_B) [49], Deinagkistrodon acutus. VLFXA-LC (Q7T045) [7], Vipera lebetina. Newly determined sequences are bolded.
33
Fig. 6. Sequence alignments of venom FX activator light chains: (1) RVV-X LC1:
Q4PRD1 [11] and LC2: Q4PRD2 [19]; (2) VLFXA LC1: Q7T045 [20] and LC2:
Q696W1 [20]) (3) Vur-FXA LC1 and LC2. Residues identical to those of RVV-X light chains are denoted by dots, and gaps are marked with hyphens. Potential N-glycosylation sites are boxed and the percent identities to Vur-FXA light chain are shown at the end. Cys residues are highlighted in gray.
34
Fig. 7. Alignment of the amino acid sequences of PⅢ metalloprotease from Viperinae venom. 1. V. u. renardi PⅢ; 2. Echis carinatus sochureki PIII (ADI47580) [24]; 3.
Echis ocellatus PIII (ADW54352); 4. Echis ocellatus PIII (CAJ01687) [25]; 5. Echis coloratus PIII (ADI47619) [24]. Residues identical to those of V. u. renardi PⅢ are denoted by dots, and gaps are marked with hyphens. Zinc binding site and hypervariable regions are boxed. Percent identities to the first sequence are shown at the end.
35
Fig. 8. Sequence alignments of FX activator heavy chains form D. russelii, V. lebitina venom, and V. u. renardi PIII metalloprotease. 1. RVV-X heavy chain (Q7LZ61) [52]; 2.
VLFXA heavy chain (Q7T046) [20]; 3. V. u. renardi PIII. Residues identical to those of RVV-X HC are denoted by dots, and gaps are marked with hyphens. Conserved zinc-binding site, methionine-turn and hypervariable regions are boxed. Cys residues are shown in gray, and known disulfide bond based on RVV-X are expressed as solid line.
36
(A)
(B)
Elution volume Unit 8.25-8.5ml (lane 1,6) 0.5 8.5-8.75ml (lane 2,7) 1.6 9.0-9.25ml (lane 3,8) 2.4 9.5-9.75ml (lane 4,9) 0.7 10.0-10.25ml (lane 5,10) 0.2
Fig. 9. Partial purification of Vur-FXA. (A) Gel filtration of crude venom of V. u.
renardi (about 15mg) on a Superdex 75 column, which was equilibrated and eluted with 0.1 M ammonium acetate, pH6.5. (B) The factor X activator activities in the eluted fractions were assayed and shown in the table; the unit was defined as liberation of 1mM p-nitroaniline per min per 0.17ul fractions under the experimental condition.
Selected fractions were also analyzed by SDS-PAGE (shown at right). Lane 1-5 were under non-reduced condition and lane 6-10 were under reduced condition.
0 0.5 1 1.5 2 2.5
0 10 20 30
Absorbance, 280nm
Elution volume (mL)
Activity (Unit)
0 1 2 3 4 5
FXA
37
Fig. 10. Sequence alignments of PII metalloproteases: (1) lebetase-2 (accession No.
Q98995) of V. lebetina, (2) PIIa and (3) PIIb of V. u. renardi. Residues identical to those of lebetase are denoted by dots, and gaps are marked with hyphens. The zinc-chelating sequences and KGD/VGD domain are bolded.
38
Table 1. Comparison between the peptides masses of calculated and experimental trypsin hydrolyzed peptides of Vur-FVA.
Amino acid sequence position No. of Cys-CAM
FHCAGTLINQEWVLTAVHCDR 50-70 2 2527.1969 2527.2048 ISATEETEPDVPHCANIFIVK 152-172 1 2370.1645 2370.1685 HAWCEAVYPWVPADSR 173-188 1 1943.8857 1943.8893 VFDYTDWIQSIIAGNTTATCPP 239-260 1 2470.1595 2470.1204 FPNGMDKDIMLIR 105-117 0 1549.7865 1549.749 HAWCEAVYPWVPADSR 173-188 0 1886.8642 1886.84
Cys-CAM : S-carbamidomethylated
: calculated massed of Vur-FVA fragments : experimental masses of Vur-FVA fragments
The was based on the monoisotopic massed of amino acids, assuming the peptide mass as [M+ ]
39
Table 2. The CTLL cDNA cloned from venom glands of V.u.renardi.
CTLL of clones encoding Quterary the CTLL structure Vur-FXA-LC1 3 αβ/ metalloprotease Vur-FXA-LC2 3 αβ/ metalloprotease Vur-FGBP β 3
Vipercetin α 2 αβ Vipercetin β 2 αβ
Vur-FGBP α sequence was obtained from another clone with different PCR primers.
Totally fifteen selected clones were sequenced.
40
Table 3. Factor X activating activities of various viperid venoms in the presence of .
Venom species Unit
Daboia russelii (Bombay) 42.3
Daboia russelii (Pakistan) 11.3 Daboia russelii (Kolkata) 2.16 Daboia russelii (Srilanka) 1.99 Daboia siamensis (Flores) 9.62 Daboia siamensis (Sumatra, Java) 7.67, 8.41 Vipera ursinii renardi 0.25 Vipera ammodytes montandoni 0.21
Vipera bornmuelleri 0.07
Vipera latifi 0.07
Vipera xanthina palestinae 0.00
Echis ocellatus 0.07
Bothrops atrox 0.20
Unit is defined as liberation of 1mM p-nitroaniline per min per mg venom in the condition described in material and method.
41
4. Discussion
In this study, I have identified and characterized the factor X and favtor V activators of Vipera ursinii renardi venom. My study started from cDNA cloning of procoagulating proteases. I cloned two serine proteases and deduced their amino acid sequences. More than twenty clones encode the serine protease whose amino acid sequence is highly similar to those of the FV-activators of V. lebetina and Daboia venoms (Fig. 2). The sequence is most similar to that of factor V activator of V. lebetina, and with 93% identity. Just two clones encode the protease with 90% identity to the chymotrypsin-like serine protease of V. lebetina [60].
Using SDS-PAGE analysis, we found that the molecular weight of Vur-FVA was close to 29kDa (Fig. 3B), which is higher than the value predicted from the protein sequence (26kDa), possibly due to N-glycosylation. Glycosylation may reduce protein’s isoelectric point (depending on the charged carbohydrate groups) and increase the molecular weight. Two potential N-glycosylation sites (N124, N229) of Vur-FVA exist, but possibly, only one (N229) of them is glycosylated, like the case of the V. lebetina FVA [17]. The tertiary structure may hinder the glycosylation of N124.
I used Superdex 75 column to purify Vur-FVA (Fig. 9A), the column not only partially purified the proteins but also can estimate its molecular weight by using protein markers for calibration. By using a calibration curve based on four markers (Appendix. 1), the molecular weight of Vur-FVA was estimate to be 58.8 kDa.
Therefore, it is likely that Vur-FVA is present as dimmers if the gel-filtration results are reliable.
Based on its deduced protein sequence, I estimated the pI value of Vur-FVA to be 8.46 or lower. Thus at pH6.0, Vur-FVA should bear positive charges although glycosylation possibly reduces its positive charges. Mono S column is a strong cation
42
exchanger, which was used to purify Vur-FVA fraction obtained from Superdex 75 (Fig.
3B). The charged group (- - ) will bind the positive charged protein. By measuring absorbance of 280nm, the yield of Vur-FVA was estimated to be about 0.15%
of the crude venom.
The mechanism of the activation of factor V by Vur-FVA may be the same as VLFVA and RVV-V. It has been reported that VLFVA will cleave the peptide bond between Arg 1545 and Ser 1546 of factor V [53, 54], and generate a heavy chain of about 290 kDa and a light chain of 74kDa [54]. Coomassie Brilliant Blue R stains can usually detect a 0.5ug protein band, but silver staining increases the sensitivity up to 100 fold and more bands were detected in silver staining than in Coomassie Brilliant Blue R stains. From Fig. 4, we found the band of 330kDa greatly diminished, suggesting that FV was cleaved and new bands of 290 kDa and 74 kDa appeared.
In the coagulation system, FVa work together with FXa to activate prothrombin. If snake venom contains factor V activator, it is likely to contain factor X activator, too.
The venoms of V. lebitina and D. siamensis are the best examples. The structure of Vur FX activator may similar with that of V. lebitina and D. siamensis. It was consisted by one PIII heavy chain and two CTL light chain. Thus, I cloned the genes that encoded C-type lectin like proteins of V. u. renardi and I got six amplified cDNA fragments. By phylogenetic analyses of the CTLL sequences, we can identify three pairs of CTLL proteins with possibly distinct functions. Their functions were predicted based on previous finding from Russell’s viper research (HS Chen, Ph.D. thesis). They are named as Vipercetin α / β, Vur-FGbp α / β, and Vur-FXA-LC1 / LC2, respectively, according to the names of homologous CTLLs from Russell’s viper venom. Vipercetin α / β are similar to α and β subunits of dabocetin, which is a potent antiplatelet agent from D.
siamensis venom [54]. Vur-FGbp α and β are similar to those of Dr-FGbp (fibrinogen
43
binding protein). Vur-FXA-LC1 / LC2 are similar to the light chains of RVV-X and VLFXA. Vur-FXA-LC1 and Vur-FXA-LC2 show more than 84% similarity to the light chains of VLFXA (Fig. 6). I speculate that not only the light chains but also the heavy chain of Vur-FXA could be rather similar to that of VLFXA.
In addition, I cloned a PIII metalloprotease (designated as Vur-PIII) from the cDNA of V. u. renardi and deduced its amino acid sequence. Vur-PIII contain a metalloprotease domain (residue 1-205), and a disintegrin domain (residue 206-298), and a Cys-rich domain (residue 209-428) (Fig. 7). In order to evaluate whether Vur-PIII is the heavy chain of FX activator, its amino acid sequence was compared with those of the RVV-X and VLFXA heavy chains (Fig. 8), the similarities were only 55.6% and 67.1%, respectively. It have been reported that of heavy chain of RVV-X and VLFXA link with C- terminal residue of light chain 2 [51, 52]. residue presents in RVV-X and VLFXA heavy chains seem to be important for the quaternary structure for RVV-X and VLFXA. Missing of this Cys residue in the deduced amino acid sequence of Vur PIII casts doubt about its role. However, it has been reported that carinactivase-1, a potent prothrombin activator from Echis carinatus, consists of a metalloprotease subunit linking with two CTLL light chains non-covalently [61]. The deduced PIII sequence may follow the mode as carinactivase-1, link with two CTLL light chains by non-covalent bond. Thus, more purification and biochemical analyses are underway to verify the heavy chain structure of Vur-FXA.
As shown in Table 6, factor X activating activity of V. u. renardi venom was proved.
It was much weaker than all of Daboia venoms. Interestingly, factor X activator contents differ by more than twenty fold between D. r. russselli (Bombay) and D. r.
pulchella (Srilanka). Not only geographical variations [55], ontological variations of venom have also been reported [56, 57].
44
When crude venom was separated by Superdex 75 column (Fig. 9A), the second peak has factor X activator activity. Vur-FXA is eluted out at elution volume of 9.0 ml. We can estimate the molecular weight by using a calibration standard curve (Appendix. 1).
The measured value is about 96 kDa. It is close to the value of 94 kDa estimated from SDS-PAGE of the fractions containing the FXA activities (Fig. 9B). This 94 kDa protein band disappeared under reducing condition of SDS-PAGE. The results suggest Vur-FXA, like RVV-X and VLFXA, probably is a heterotrimer composed of one heavy chain and two light chains. However, Vur-FXA heavy chain and light chains on the SDS-PAGE gel (under reducing condition), are difficult to confirmed before Vur-FXA is purified, because the interference of other proteins with similar molecular weight.
I also cloned two PII metalloprotease (named Vur-PIIa and PII-b) and determined its sequence. Their sequences are 83-84% similar to that of lebetase-2 (Fig. 10), and composed a protease followed by a disintegrin domain. They are probably fibrinolytic enzyme of V. u. renardi. Interestingly, the N-terminal three residues of the major one (Vur-PIIa) is KKK, quite different from that of lebetase-2, while the N-terminal sequence of minor one (Vur-PIIb) is same as lebetase-2. We need to purify the small metalloproteases from V. u. renardi venom and determine the N-terminal sequence of it.
Remarkably, it has been reported that there are anti-coagulant PL exist in the venom of V. u. renardi [62]. In the present study, most of the found venom components are pro-coagulant, although they are relatively weak. May be these proteins that have sequential and synergistic effects, but the details should be further studied.
45
References
1. Toshiaki N., Yumik K. Kinin-releasing and kinin-degrading enzyme, in: Enzymes from snake venom. Bailey G. S. (Ed), Alaken, Colorado, 1998; pp.287-316
2. Matsui T, Fujimura Y, Titani K. Snake venom proteases affecting hemostasis and thrombosis. Biochim Biophys Acta. 2000;1477:146-56.
3. Fuminori T, Sadaaki I. Proteases activating factor V, in: Enzymes from snake venom.
Bailey GS, (Ed), Alaken, Colorado, 1998; pp.209-226
4. Morita T. Protease which activate factor X, in: Enzymes from snake venom. Bailey G. S. (Ed), Alaken, Colorado, 1998; pp.179-208
5. Riddel JP Jr, Aouizerat BE, Miaskowski C, Lillicrap DP. Theories of blood coagulation. J Pediatr Oncol Nurs. 2007;24:123-31.
6. Smith SA. The cell-based model of coagulation. J Vet Emerg Crit Care (San Antonio). 2009;19:3-10.
7. Kini RM, Rao VS, Joseph JS. Procoagulant proteins from snake venoms.
Haemostasis. 2001;31:218-24.
8. Tans G, Rosing J. Snake venom activators of factor X: an overview. Haemostasis.
2001;31:225-33.
9. Krupiczojc MA, Scotton CJ, Chambers RC. Coagulation signalling following tissue injury: focus on the role of factor Xa. Int J Biochem Cell Biol. 2008;40:1228-37.
10. X J Yang, M A Blajchman, S Craven, L M Smith, N Anvari, and F A Ofosu
Activation of factor V during intrinsic and extrinsic coagulation. Inhibition by heparin, hirudin and D-Phe-Pro-Arg-Ch2Cl. Biochem J. 1990; 272: 399–406.
11. Takeya H, Nishida S, Miyata T, Kawada S, Saisaka Y, Morita T, Iwanaga S.
Coagulation factor X activating enzyme from Russell's viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type
46
lectin-like domains. J Biol Chem. 1992;267:14109-17.
12. Clemetson KJ, Lu Q, Clemetson JM Snake C-type lectin-like proteins and platelet receptors. Pathophysiol Haemost Thromb. 2005;34:150-5.
13. Takeda S, Takeya H, Iwanaga S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim Biophys Acta. 2011. Epub ahead of print
14. Rosing J, Govers-Riemslag JW, Yukelson L, Tans G. Factor V activation and inactivation by venom proteases. Haemostasis. 2001;31:241-6.
15. Siigur J, Aaspollu A, Tonismagi K, Trummal K, Samel M, Vija H, Subbi J, et al.
Proteases from Vipera lebetina venom affecting coagulation and fibrinolysis.
Haemostasis 2001;31:123–132.
16. Felsenstein J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 39, 1985; pp.783-791
17. Siigur E, Aaspõllu A, Siigur J. Molecular cloning and sequence analysis of a cDNA for factor V activating enzyme, a Coagulant Protein from Vipera lebetina Snake Venom. Biochem Biophys Res Commun. 1999;262:328-32.
18. Zhong SR, Jin Y, Wu JB, Chen RQ, Jia YH, Wang WY, Xiong YL, Zhang Y.
Characterization and molecular cloning of dabocetin, a potent antiplatelet C-type lectin-like protein from Daboia russelii siamensis venom. Toxicon. 2006;47:104-12.
19. Gowda DC, Jackson CM, Hensley P, Davidson EA. Factor X-activating glycoprotein of Russell's viper venom. Polypeptide composition and characterization of the carbohydrate moieties. J Biol Chem. 1994;269:10644-50.
20. Siigur E, Aaspõllu A, Trummal K, Tõnismägi K, Tammiste I, Kalkkinen N, Siigur J.
Factor X activator from Vipera lebetina venom is synthesized from different genes.
Biochim Biophys Acta. 2004;1702:41-51.
21. Mizuno H, Fujimoto Z, Atoda H, Morita T. Crystal structure of an anticoagulant
47
protein in complex with the Gla domain of factor X. Proc Natl Acad Sci U S A.
2001;98:7230-4.
22. Siigur E, Aaspõllu A, Trummal K, Tõnismägi K, Tammiste I, Kalkkinen N, Siigur J.
Factor X activator from Vipera lebetina venom is synthesized from different genes.
Biochim Biophys Acta. 2004;1702:41-51.
23. Wagstaff SC, Laing GD, Theakston RD, Papaspyridis C, Harrison RA.
Bioinformatics and multiepitope DNA immunization to design rational snake antivenom. PLoS Med. 2006;3:e184.
24. Casewell NR, Wagstaff SC, Harrison RA, Wüster W. Gene tree parsimony of multilocus snake venom protein families reveals species tree conflict as a result of multiple parallel gene loss. Mol Biol Evol. 2011;28:1157-72.
25. Takeda S, Igarashi T, Mori H, Araki S. Crystal structures of VAP1 reveal ADAMs' MDC domain architecture and its unique C-shaped scaffold. EMBO J.
2006;25:2388-96.
26. Yamada D, Sekiya F, Morita T. Prothrombin and factor X activator activities in the venoms of Viperidae snakes. Toxicon. 1997;35:1581-9.
27. Chung CH, Au LC, Huang TF. Molecular cloning and sequence analysis of aggretin, a collagen-like platelet aggregation inducer. Biochem Biophys Res Commun.
1999;263:723-7.
28. Wang R, Kini RM, Chung MC. Rhodocetin, a novel platelet aggregation inhibitor from the venom of Calloselasma rhodostoma (Malayan pit viper): synergistic and noncovalent interaction between its subunits. Biochemistry. 1999;38:7584-93
29. Chen YL, Tsai KW, Chang T, Hong TM, Tsai IH. Glycoprotein Ib-binding protein from the venom of Deinagkistrodon acutus--cDNA sequence, functional characterization, and three-dimensional modeling. Thromb Haemost. 2000;83:119-26.
![Fig. I. The cascade model of coagulation [5]. Intrinsic pathway is initiated by the factors that are present within the blood](https://thumb-ap.123doks.com/thumbv2/9libinfo/9607193.632977/15.892.163.736.128.860/cascade-model-coagulation-intrinsic-pathway-initiated-factors-present.webp)
![Fig. II. The cell-based model of coagulation [6]. (a) initiation phase was occurs on the surface of TF-bearing cell](https://thumb-ap.123doks.com/thumbv2/9libinfo/9607193.632977/17.892.171.732.136.839/based-model-coagulation-initiation-phase-occurs-surface-bearing.webp)
![Fig. III. The primary sequence and domain structure of blood coagulation factor X mature protein and active form [9]](https://thumb-ap.123doks.com/thumbv2/9libinfo/9607193.632977/18.892.173.735.130.782/primary-sequence-domain-structure-coagulation-factor-mature-protein.webp)
![Fig. IV. Domain structire of factor V [3]. The doamins are indicated by letters A, B and C inside boxes](https://thumb-ap.123doks.com/thumbv2/9libinfo/9607193.632977/19.892.264.629.146.361/domain-structire-factor-doamins-indicated-letters-inside-boxes.webp)
![Fig. V. The activation of factor X by RVV-X [8]. The heavy chain of RVV-X contains the metalloprotease domain responsible for the proteolytic cleavage of the target bond in factor X](https://thumb-ap.123doks.com/thumbv2/9libinfo/9607193.632977/20.892.235.659.152.391/activation-factor-contains-metalloprotease-domain-responsible-proteolytic-cleavage.webp)
![Fig. VI. Schematic structures of snake venom metalloproteases [2]. Based on domain structures, SVMPs are classified into four groups](https://thumb-ap.123doks.com/thumbv2/9libinfo/9607193.632977/21.892.161.732.129.883/schematic-structures-metalloproteases-based-domain-structures-svmps-classified.webp)
![Table I. Snake species with factor X activator in the venom [8].](https://thumb-ap.123doks.com/thumbv2/9libinfo/9607193.632977/22.892.115.799.524.895/table-i-snake-species-factor-x-activator-venom.webp)
![Table III. Snake venom factor V activator that have been reported [14].](https://thumb-ap.123doks.com/thumbv2/9libinfo/9607193.632977/23.892.191.668.189.321/table-iii-snake-venom-factor-v-activator-reported.webp)