T OO L ATE TO Q UIT ? E FFECT OF S MOKING AND S MOKING C ESSATION ON M ORBIDITY AND M ORTALITY
A MONG THE E LDERLY IN A L ONGITUDINAL S TUDY
Hui-Chuan Hsu and Raoh-Fang Pwu1
Department of Health Care Administration, Taichung Healthcare and Management University, Taichung, and 1Research Director, iStat Healthcare Consulting Co Ltd, Taipei, Taiwan.
This prospective study of the elderly population estimated the risks of smoking for morbidity and mortality and identified whether cessation of smoking reduced the risk of disease. Data came from face-to-face interviews that used a population-based probability sample of those aged 60 years or over in Taiwan, provided by the Population and Health Research Center, Bureau of Health Promotion. In total, 4,049 subjects were included at the baseline year of 1989 and followed up in 1993 and 1996. Smoking-related variables included current smoking status, smoking history, daily consumption, and years since the cessation of smoking. Cox regression models were used to analyze the relative risks for morbidity and mortality, controlling for demographics, physical function, and comorbidities. The sample was made up of 50.2%
nonsmokers, 15.2% ex-smokers, and 34.6% current smokers in the baseline year. Current smokers were more likely to have lower respiratory tract diseases throughout the study. Current smokers had a higher risk of stroke from 1989 to 1993. No dose-response relationship for smoking exposure or impact of years since smoking cessation was found. Whether cessation of smoking is protective should be investigated for middle-aged adults followed to old age. An effective strategy for smoking cessation in the elderly is suggested, and people should be encouraged to quit smoking at any time.
Key Words: smoking, smoking cessation, longitudinal studies, elderly, relative risk, successful aging (Kaohsiung J Med Sci 2004;20:484–91)
Received: May 17, 2004 Accepted: August 27, 2004 Address correspondence and reprint requests to: Dr. Hui-Chuan Hsu, Department of Health Care Administration, Taichung Health- care and Management University, 500 Lioufeng Road, Wufeng Shiang, Taichung 413, Taiwan.
E-mail: gingerhsu@seed.net.tw
Smoking is an important health hazard in mortality. It is estimated that about 3 million people die each year from smoking-related diseases, and this will increase to over 10 million worldwide by 2025 [1]. Many prospective studies have identified cigarette smoking as an important risk factor for cardiovascular disease, chronic bronchitis, several types of cancer, and other diseases [2–13].
There is evidence that a reduction in risk occurs on quitting smoking. The reduction in risk for heart disease is linked to the duration of cessation [14]. Smoking cessation can reduce the risk of mortality, and smoking cessation af- ter acute myocardial infarction can reduce the relative risk (RR) of death by between 15% and 61% [15]. Smoking cessation may increase lung function and lower mortality for male smokers [16]; even an interrupted cessation of smoking is helpful and can improve lung function.
Only a few studies of the impact of smoking on health in Taiwan have been reported. A prospective cohort study explored the attributable risk of cigarette smoking to death from various diseases in township residents aged 40 or over [17]. The RR of mortality for male smokers was 1.3, and 1.5 in all cancers. The RRs for stomach disease, ischemic and
other heart diseases, and chronic obstructive pulmonary disease (COPD) ranged from 1.4 to 1.9, with higher RRs for females. The attributable risks for mortality associated with smoking were 21.3% and 2.9% for males and females, re- spectively. In another case-control study, the risk of lung cancer was significantly higher in smokers than in non- smokers [18]. Even for those who had stopped smoking, the RRs were higher at 6.39 and 4.00. The risk for every pack of cigarettes smoked was 1.04. The risk of a smoking history over 25 years for nasopharyngeal cancer in Taiwan was 1.7 compared to nonsmokers [19]. In Taiwan, smoking cessation projects and intervention programs have been developed [20–23]. However, evidence that cessation of smoking re- duces the risk of disease and mortality is limited [24].
The question of whether smoking cessation reduces health hazard risks for the elderly is important for the attractive strategy of health promotion as a way of achieving successful aging [25]. However, few studies have shown that smoking cessation has an impact on morbidity and mortality in old age. Also, there have been no prospective follow-up studies targeting the elderly population to esti- mate the risks of smoking and the effect of smoking cessation on morbidity and mortality. Therefore, to explore the effects of smoking cessation on the risk of disease will be useful as a basis for the development of a health promotion strategy for the elderly and to attain the goal of successful aging.
The purpose of this study was to use prospective elderly samples with 7-year follow-up to assess the impact of smoking status on morbidity and mortality, and examine whether cessation of smoking reduced the risks of diseases in old age.
M
ETHODSData and samples
Data came from a face-to-face interview, the “Study of Health and Living Status of the Elderly” a national repre- sentative elderly sample from a survey conducted by the Population and Health Research Center, Bureau of Health Promotion. Samples were of people aged 60 years or over and drawn randomly from the entire elderly population in Taiwan with 1989 as the baseline, and then followed up in 1993 and 1996. Sampling was made up of a three-stage probability sample. All interviews were conducted by senior interviewers under high quality control. In this study, we used the 1989 sample as the baseline (n = 4,049), together with follow-up data for 1993 (n = 3,155) and 1996 (n = 2,669, aged 67 years or over). Completion rate (excluding deaths)
for 1989, 1993 and 1996 were 91.8%, 91.0%, and 88.9%. There were no differences in age, sex, and smoking status among the completed cases and those missing through loss or death from the initial sample when examined for goodness- of-fit.
Variables
Dependent variables were self-reported chronic diseases and mortality. Diseases included heart disease, stroke, lower respiratory tract diseases (asthma, bronchitis, tuberculosis), diabetes, digestive system diseases (ulcer, gastrointestinal problems, liver/gallbladder diseases), kidney diseases, and cancer (only surveyed in 1993 and 1996).
The major independent variables related to smoking were current smoking status (nonsmokers, who had never smoked; ex-smokers, who quit smoking; and current smokers), smoking history (starting age of smoking, years of quitting), and smoking exposure (cumulative smoking years, amount smoked every day). The cumulative smoking years were defined from the age of starting smoking to the survey year. If the subject had quit smoking, their smoking history was also counted. The cumulative smoking years for those who never smoked was defined as zero.
Controlled risk factors included age (60–74, ≥ 75 years), gender, education (low = illiterate, no formal education, elementary school; medium = junior high school; high = senior high school or over), ethnic group (Ming-Nan, others including Hakka, mainland provinces, etc), disability in physical function in 1989 (any difficulties caused by health problems in shopping for personal items, management of money, making phone calls, and taking a bath alone).
Analysis
The incidence of each disease was estimated. The RRs and population-attributable risk (PAR) of smoking status on morbidity and mortality were estimated using Cox proportional hazards regression models. Three kinds of smoking effects were analyzed. First, the risk of smoking status (nonsmokers, ex-smokers, and current smokers) in all samples. Second, for current smokers, the risks of smok- ing exposure (daily cigarette consumption, age at starting smoking, cumulative smoking years) were analyzed. Final- ly, for ex-smokers, the risk of smoking history (cumulative smoking years, years after quitting) on morbidity and mortality were analyzed. We did not estimate the 7-year morbidity from cancer because of the absence of cancer data for the baseline year. Multiple Cox regression analysis of smoking status relative to morbidity and mortality, controlling for related factors, was also done.
R
ESULTSIn 1989, 36.6% of the initial sample were aged 60–64 years, 28.5% were aged 65–69 years, 17.9% were aged 70–
74 years, and 17.0% were aged 75 years or above. There were more men (57.1%, n = 2,311) than women (42.9%, n = 1,738). Half (50.3%) of the elderly were illiterate or without formal education, 30.7% had attended elementary school, and 18.9% were educated to junior high school or above. Of the sample, 60.9% were Ming-Nan, 15.0% were Hakka, 22.4% came from mainland provinces, and 1.7%
were of other ethnic groups. Overall, 18.2% were physically disabled.
Of the initial sample of 4,049, 21.7% had heart disease, 4.3% had had a stroke, 8.4% had diabetes, 18.5% had lower respiratory tract disease, 27.2% had digestive system disease, and 6.3% had kidney disease. In 1993, the prevalence of cancer was 1.7%.
In 1989, there were 2,033 (50.2%) nonsmokers, 616 (15.2%) ex-smokers, and 1,399 (34.6%) current smokers (1 case was missing). In 1996, 670 of 2,669 (25.1%) complete respondents still smoked. Most smokers were male. In 1989, 55.3% of men were current smokers, 24.4% were ex- smokers, and 20.4% were nonsmokers. Of the women, only 7.0% were current smokers, 3.0% were ex-smokers, and 90.0% were nonsmokers. The younger elderly had a higher rate of smoking. In 1989, the current smoking rate was 36.8% for those aged 60–74 and 24.0% for those aged 75 or above.
Of the current smokers, 1,326 of 1,385 (95.7%) complete cases were long-term smokers (smoking history > 20 years);
483 of 594 (81.3%) ex-smokers had smoked for more than 20 years. Among the 1,390 current smokers (9 cases were missing), the daily cigarette consumption was less than 10 cigarettes for 44.1% and 11–20 cigarettes for 50.9%, while only 5.0% smoked more than 20 cigarettes per day. For ex-smokers, about 40% had quit smoking within the past 5 years, while 25.5% had quit for 20 years or more.
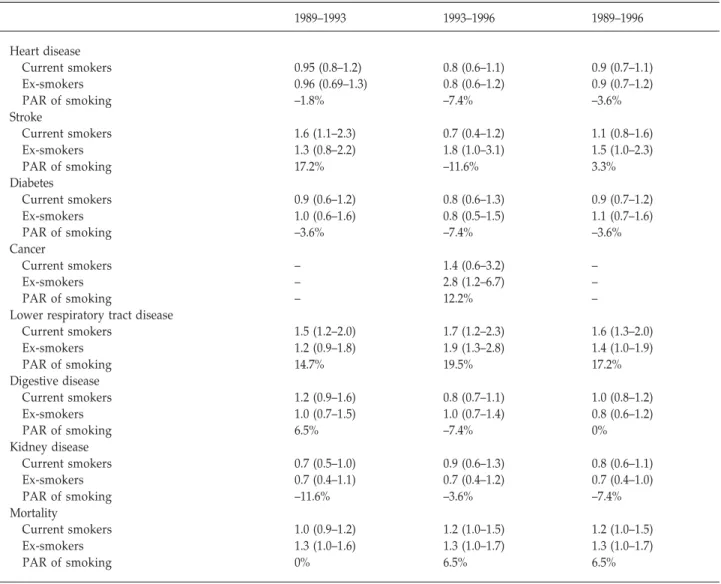
The univariate RR of morbidity and mortality for dif- ferent smoking status is shown in Table 1. Smokers were more likely to have lower respiratory tract disease (RR, 1.6 for current smokers, 1.4 for ex-smokers; PAR, 17.2% from 1989 to 1996). Current smokers also had a higher risk over the two intervals 1989–1993 and 1993–1996. In addition, current smokers had a higher risk of stroke from 1989 to 1993 (RR, 1.6; PAR, 17.2%). The 1993–1996 RR of cancer was higher for ex-smokers.
For current smokers, univariate Cox regression was used to analyze the risk of daily cigarette consumption, age
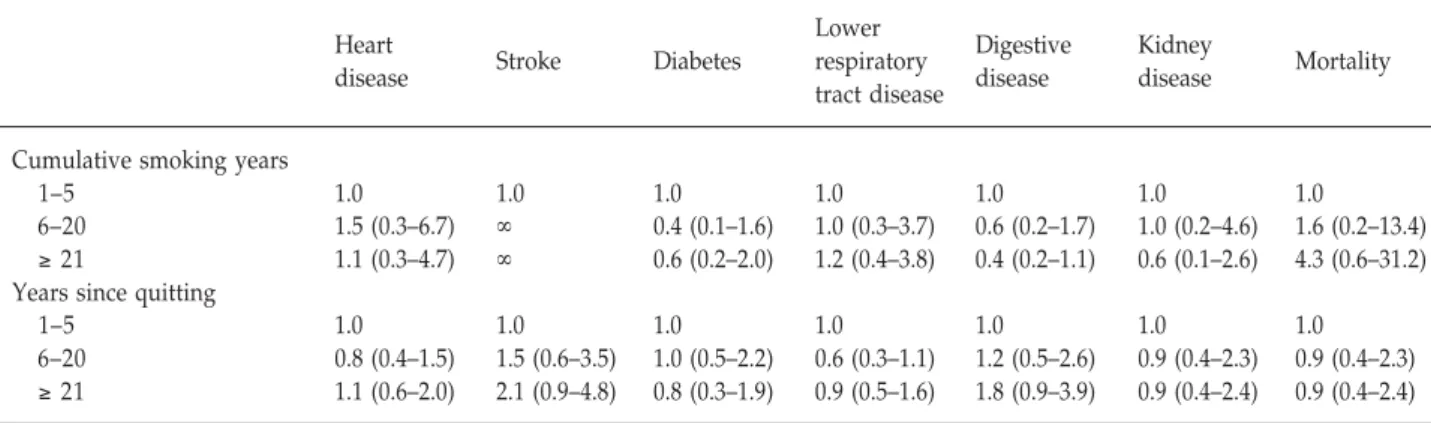
at starting smoking, and cumulative smoking years on the 7-year incidence of disease and mortality (Table 2). How- ever, no dose-response relationship was found. We also analyzed the risk of cumulative smoking years and the years since quitting on the 7-year incidence of disease and mortality for ex-smokers (Table 3). Smoking history did not have a significant impact on the relative risks of mor- bidity or mortality.
Table 4 shows the multiple Cox regression models for smoking status relative to morbidity and mortality, controlling for demographic and related health factors.
Smoking status influenced only lower respiratory tract disease: current (RR, 1.7) and ex-smokers (RR, 1.4) were more likely to have respiratory disease. Comorbidity with other diseases was also more likely to increase the incidence of morbidity. The older elderly with comorbidity and physical disability had a higher risk of mortality.
D
ISCUSSIONThis study used a 7-year national longitudinal dataset from Taiwan to explore the RRs of morbidity and mortality with respect to smoking status, smoking history, and the cessation of smoking among the elderly. The smoking elderly had a higher RR of lower respiratory tract disease (1.6; PAR, 17.2%), as did ex-smokers (RR, 1.4). Ex-smokers had a higher risk of cancer morbidity. However, for both current smokers and quitters at the 7-year follow-up, the dose- response relationship of smoking consumption, smoking history, and years of cessation was not associated signifi- cantly with morbidity and mortality risks.
The prevalence of elderly smoking in Taiwan was stable from 1989 to 1996 in this study. According to the 1989 survey, smoking prevalence among the elderly was 34.6%, with 15.2% who had quit smoking and 50.2% who had never smoked. The 1996 prevalence was similar to that in 1989. Prospective panel data suggest that there may have been changes in smoking behavior during the 7 years of follow-up. However, the three surveys did not all include the complete set of variables for smoking exposure, so we could only estimate the exposure according to the baseline year.
Current smoking status was associated with the incidence of stroke, lower respiratory tract disease, and cancer using univariate Cox models, and the effect on respiratory disease persisted in the multivariate model.
Respiratory disease can be very sensitive to smoking behavior, as other case-control studies and prospective
Table 1. Incidence of disease and mortality by smoking status among the elderly between 1989 and 1996: relative risk (95%
confidence interval)
1989–1993 1993–1996 1989–1996
Heart disease
Current smokers 0.95 (0.8–1.2) 0.8 (0.6–1.1) 0.9 (0.7–1.1)
Ex-smokers 0.96 (0.69–1.3) 0.8 (0.6–1.2) 0.9 (0.7–1.2)
PAR of smoking –1.8% –7.4% –3.6%
Stroke
Current smokers 1.6 (1.1–2.3) 0.7 (0.4–1.2) 1.1 (0.8–1.6)
Ex-smokers 1.3 (0.8–2.2) 1.8 (1.0–3.1) 1.5 (1.0–2.3)
PAR of smoking 17.2% –11.6% 3.3%
Diabetes
Current smokers 0.9 (0.6–1.2) 0.8 (0.6–1.3) 0.9 (0.7–1.2)
Ex-smokers 1.0 (0.6–1.6) 0.8 (0.5–1.5) 1.1 (0.7–1.6)
PAR of smoking –3.6% –7.4% –3.6%
Cancer
Current smokers – 1.4 (0.6–3.2) –
Ex-smokers – 2.8 (1.2–6.7) –
PAR of smoking – 12.2% –
Lower respiratory tract disease
Current smokers 1.5 (1.2–2.0) 1.7 (1.2–2.3) 1.6 (1.3–2.0)
Ex-smokers 1.2 (0.9–1.8) 1.9 (1.3–2.8) 1.4 (1.0–1.9)
PAR of smoking 14.7% 19.5% 17.2%
Digestive disease
Current smokers 1.2 (0.9–1.6) 0.8 (0.7–1.1) 1.0 (0.8–1.2)
Ex-smokers 1.0 (0.7–1.5) 1.0 (0.7–1.4) 0.8 (0.6–1.2)
PAR of smoking 6.5% –7.4% 0%
Kidney disease
Current smokers 0.7 (0.5–1.0) 0.9 (0.6–1.3) 0.8 (0.6–1.1)
Ex-smokers 0.7 (0.4–1.1) 0.7 (0.4–1.2) 0.7 (0.4–1.0)
PAR of smoking –11.6% –3.6% –7.4%
Mortality
Current smokers 1.0 (0.9–1.2) 1.2 (1.0–1.5) 1.2 (1.0–1.5)
Ex-smokers 1.3 (1.0–1.6) 1.3 (1.0–1.7) 1.3 (1.0–1.7)
PAR of smoking 0% 6.5% 6.5%
Reference group is nonsmokers. PAR = population-attributable risk.
Table 2. Incidence of disease and mortality by cigarette consumption and smoking history among elderly current smokers during 7-year follow-up, 1989–1996: relative risk (95% confidence interval)
Heart Lower
Digestive Kidney
disease Stroke Diabetes respiratory
disease disease Mortality
tract disease
Daily cigarette consumption
≤ 10 1.0 1.0 1.0 1.0 1.0 1.0 1.0
11–20 0.7 (0.5–1.0) 0.8 (0.5–1.3) 1.0 (0.6–1.6) 1.2 (0.8–1.6) 0.9 (0.6–1.3) 0.6 (0.4–1.0) 1.0 (0.7–1.4) ≥ 21 0.9 (0.5–1.7) 1.0 (0.3–2.8) 1.0 (0.3–2.8) 1.2 (0.6–2.4) 1.4 (0.7–2.7) 0.7 (0.3–2.0) 0.6 (0.3–1.4) Age of starting smoking (yr)
< 20 1.0 1.0 1.0 1.0 1.0 1.0 1.0
≥ 20 0.8 (0.6–1.2) 0.9 (0.5–1.5) 0.8 (0.5–1.3) 0.9 (0.7–1.3) 1.2 (0.8–1.8) 0.9 (0.5–1.4) 0.8 (0.6–1.1) Cumulative smoking years
0–20 1.0 1.0 1.0 1.0 1.0 1.0 1.0
≥ 21 0.9 (0.4–2.0) 1.0 (0.2–3.9) 0.7 (0.2–2.2) 0.9 (0.4–2.1) 1.3 (0.4–4.0) 0.6 (0.2–1.6) 1.7 (0.5–5.4) Only those who were smokers according to their 1989 smoking status are included in this table.
Table 3. Incidence of disease and mortality by smoking history among ex-smokers during 7-year follow-up, 1989–1996:
relative risk (95% confidence interval)
Heart Lower
Digestive Kidney
disease Stroke Diabetes respiratory
disease disease Mortality
tract disease
Cumulative smoking years
1–5 1.0 1.0 1.0 1.0 1.0 1.0 1.0
6–20 1.5 (0.3–6.7) ∞ 0.4 (0.1–1.6) 1.0 (0.3–3.7) 0.6 (0.2–1.7) 1.0 (0.2–4.6) 1.6 (0.2–13.4) ≥ 21 1.1 (0.3–4.7) ∞ 0.6 (0.2–2.0) 1.2 (0.4–3.8) 0.4 (0.2–1.1) 0.6 (0.1–2.6) 4.3 (0.6–31.2) Years since quitting
1–5 1.0 1.0 1.0 1.0 1.0 1.0 1.0
6–20 0.8 (0.4–1.5) 1.5 (0.6–3.5) 1.0 (0.5–2.2) 0.6 (0.3–1.1) 1.2 (0.5–2.6) 0.9 (0.4–2.3) 0.9 (0.4–2.3) ≥ 21 1.1 (0.6–2.0) 2.1 (0.9–4.8) 0.8 (0.3–1.9) 0.9 (0.5–1.6) 1.8 (0.9–3.9) 0.9 (0.4–2.4) 0.9 (0.4–2.4)
Only those who had quit smoking according to their smoking status in 1989 are included in this table. Some cells show “∞” because of non- convergence of parameters in iterations.
Table 4. Incidence of disease and mortality by smoking status and other risk factors among the elderly by Cox multiple regression models, 1989–1996: relative risk (95% confidence interval)
Heart Lower
Digestive Kidney
disease Stroke Diabetes respiratory
disease disease Mortality
tract disease
Smoking status
Current smoker 1.1 (0.8–1.5) 0.8 (0.5–1.3) 1.2 (0.8–1.8) 1.7 (1.2–2.3) 0.7 (0.5–1.0) 0.8 (0.6–1.2) 1.2 (0.9–1.6) Ex-smoker 1.1 (0.8–1.5) 1.0 (0.6–1.7) 1.4 (0.9–2.3) 1.4 (1.0–2.1) 0.8 (0.6–1.1) 0.7 (0.4–1.1) 1.2 (0.9–1.6)
Nonsmoker 1.0 1.0 1.0 1.0 1.0 1.0 1.0
Age (yr)
≥ 75 1.0 (0.7–1.3) 1.0 (0.6–1.7) 0.5 (0.3–0.96) 1.3 (1.0–1.8) 0.9 (0.6–1.3) 0.6 (0.4–1.0) 2.8 (2.3–3.4)
60–74 1.0 1.0 1.0 1.0 1.0 1.0 1.0
Gender
Male 0.7 (0.6–1.0) 1.7 (1.1–2.6) 0.6 (0.4–0.96) 1.1 (0.8–1.5) 1.3 (1.0–1.8) 1.0 (0.7–1.4) 1.3 (1.0–1.7)
Female 1.0 1.0 1.0 1.0 1.0 1.0 1.0
Education level
High 1.0 (0.7–1.2) 0.8 (0.6–1.3) 0.8 (0.6–1.2) 0.5 (0.4–0.7) 1.1 (0.8–1.4) 0.8 (0.5–1.1) 0.8 (0.6–1.0)
Low 1.0 1.0 1.0 1.0 1.0 1.0 1.0
Ethnic group
Ming-Nan 1.0 (0.8–1.2) 0.7 (0.5–0.98) 0.9 (0.7–1.2) 0.8 (0.7–1.0) 1.2 (1.0–1.5) 1.2 (0.9–1.5) 1.2 (1.0–1.4)
Others 1.0 1.0 1.0 1.0 1.0 1.0 1.0
Comorbidity 1.3 (1.2–1.5) 1.4 (1.2–1.6) 1.1 (0.95–1.3) 1.2 (1.1–1.4) 1.2 (1.0–1.3) 1.4 (1.2–1.6) 1.3 (1.2–1.4) Physical function
Disabled 1.0 (0.7–1.3) 1.2 (0.7–1.9) 0.8 (0.5–1.3) 1.2 (0.8–1.6) 0.9 (0.6–1.3) 0.9 (0.6–1.4) 1.8 (1.4–2.3)
Normal 1.0 1.0 1.0 1.0 1.0 1.0 1.0
Education level: low = illiterate, no formal education, elementary school, junior high school; high = senior high school and over. Ethnic group: others include Hakka, mainland provinces, and others. Incidence of disease models, comorbidity = cumulative prevalent numbers of other 5 diseases in 1989; mortality model, comorbidity = cumulative numbers of 6 diseases in 1989. Smoking status, age, and physical function according to 1989 status.
studies have shown [8,18,26]. Ex-smokers had a lower risk of morbidity than current smokers. Smoking cessation may thus be a protective factor. A previous study did not find a dose-response relationship between smoking ex- posure and mortality in the elderly [17], and the effect of
smoking cessation is not significant [14–16]. This can possibly be explained as follows.
First, all subjects in our study were aged 60 years and over. Some elderly might have had diseases attributed to smoking before they entered their old age, or smoking
might have shortened their life. These persons with early morbidity or immature mortality were lost or not eligible for our analysis at baseline. Thus, the different results ob- tained here may be attributed to a different study design and sample. Our study was based on a national represen- tative and prospective elderly population, rather than a case-controlled one or targeted at specific patients. Using such a longitudinal study design limited the incidence and case numbers and may have made the observation of a dose-response relationship difficult. Thus, the risk of smoking might have been underestimated.
Second, smoking behavior is changeable. Patients with severe diseases often have stronger motivation to quit smoking [27]. The elderly might have sensed their frailty or the presence of disease and quit smoking before the sur- veys. Cessation of smoking might thus be combined with other changes in health behavior, which might create a survival advantage. Further, quitting smoking might re- duce risks immediately (such as after surgery or myocar- dial infarction) for some patients [14,15]. The effect of ces- sation over the long-term thus cannot be observed.
Third, the variation in smoking exposure among these elderly was limited (elderly smokers were mostly long- term smokers and also low-consumption smokers), so the dose-response relationship might have been masked.
Subjects were divided into two ethnic groups, Ming- Nan and others, in the multivariate model. Initially, it had been separated into Ming-Nan, Hakka, mainland provinces, and others, but because of the limited cases in this longi- tudinal study and the many parameters in the multivari- ate model, we categorized independent variables into fewer groups to avoid convergence problems in Cox regression estimation. We considered the sample numbers in each ethnic group, the lower incidence of morbidity in the Ming- Nan group, and because the purpose of using ethnic groups was only for control variables, we conducted the analysis with these two groups. Readers should be careful to note that this might mingle the effects of Hakka, mainland province or aboriginal ethnic origin.
This study is subject to a number of limitations. First, the data came from a face-to-face self-report survey, and the accuracy of disease prevalence and recall bias about smoking history should be considered. In addition, the perception of hypertension, heart disease, stroke, and so on, might be in- accurate. Second, cancer prevalence was not available in the 1989 survey, and the different kinds of cancers could not be differentiated in the 1993 and 1996 data; therefore, the relative risks of cancer over the 7 years could not be estimated.
Measures of physical function were not consistent across
the three surveys: in 1993 and 1996, physical function was measured using six activities of daily living (ADL) (eating, dressing, walking indoors, transferring from bed or chair, using the toilet, taking a bath), but in 1989, the physical function included only one ADL and three instrumental ADL items. The physical function variable was a more advanced activity. Third, the variables in the surveys for each year were not the same, and some information was not available. For example, there was no quitting smoking and cigarette consumption data in the 1993 survey, so the dynamic changes in years since quitting, cumulative smok- ing years, and daily consumption of cigarettes during these 7 years were unavailable. Fourth, some cases were lost to follow-up or died during the longitudinal study. Al- though we compared the demographic and smoking sta- tus of the lost cases and the initial cases and found no dif- ference, the morbidity and mortality of the lost cases is unknown. Additionally, if smoking shortened the time from morbidity to death or increased the mortality from diseases, or smoking induced cancer during the subject’s middle age, or individuals were dead before their old age, then the RR of smoking for such a longitudinal study might be underestimated.
In this study, we analyzed the RRs of smoking on morbidity and mortality among the elderly population in Taiwan. Smoking has its greatest effect on morbidity from respiratory disease in the elderly. The effect of years since cessation was not clear. We suggest that the protective effect of smoking cessation should be investigated from middle age to old age. Further studies should emphasize the observation of dynamic smoking behavior and be based on a life-course perspective because health status and health behavior is changeable. In terms of promotion of successful aging, we suggest that health promotion for the elderly should start at an earlier age and continue throughout old age.
A
CKNOWLEDGMENTSThis study was supported by the project “Attributable risks of smoking to multiple diseases of the elderly in Taiwan”
(BHP-92-Anti-Tobacco-E204) and grants from the Bureau of Health Promotion, Department of Health, Taiwan, ROC.
This study is based on data from the “Study of Health and Living Status of the Elderly”, provided by the Population and Health Research Center, Bureau of Health Promotion.
The interpretation and conclusions contained herein do not represent those of the Bureau of Health Promotion.
R
EFERENCES1. Emmons K. Smoking cessation and tobacco control: an over- view. Chest 1999;116:490S–2S.
2. Roget E, Murray JL. Smoking and cause of death among U.S.
veterans: 16 years of observation. Public Health Rep 1980;95:
213–22.
3. Carstensen JM, Pershagen G, Eklund G. Mortality in rela- tion to cigarette and pipe smoking: 16 years observation of 25,000 Swedish men. J Epidemiol Community Health 1987;41:
166–72.
4. Freund KM, Belanger AJ, D’Agostino RB, et al. The health risk of smoking. The Framingham study: 34 years of follow-up.
Ann Epidemiol 1993;3:417–24.
5. McLaughlin JK, Hrubez Z, Blot WJ, et al. Smoking and cancer mortality among U.S. veterans: a 26-year follow-up. Intl J Cancer 1995;60:190–3.
6. Suminori A, Hirayama T. Cigarette smoking and cancer mortality risk in Japanese men and women: results from reanalysis of the six-prefecture cohort study data. Environ Health Perspect 1990;87:19–26.
7. Yuan JM, Ross RK, Wang XL, et al. Morbidity and mortality in relation to cigarette smoking in Shanghai, China: a prospective male cohort study. JAMA 1996;275:1646–50.
8. Wald NJ, Watt HC. Prospective study of effect of switching from cigarettes to pipes or cigars on mortality from three smoking related diseases. BMJ 1997;314:1860–3.
9. Christen WG, Glynn RJ, Ajani UA, et al. Smoking cessation and risk of age-related cataract in men. JAMA 2000;284:713–6.
10. He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I Epidemiologic Follow-up Study. Arch Intern Med 2001;161:996–1002.
11. Hu SC, Lanese RR. The applicability of the theory of planned behavior to the intention to quit smoking across workplaces in southern Taiwan. Addict Behav 1998;23:225–37.
12. Ruigrok Y, Buskens E, Rinkel GJE. Attributable risk of common and rare determinants of subarachnoid hemorrhage. Stroke 2001;32:1173–5.
13. Sauer W, Berlin JA, Strom BL, et al. Cigarette yield and the risk of myocardial infarction in smokers. Arch Intern Med 2002;162:
300–6.
14. Rea T, Heckbert SR, Kaplan RC, et al. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med 2002;137:494–500.
15. Wilson P, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:
1837–47.
16. Pelkonen M, Notkola IL, Tukiainen H, et al. Smoking cessation, decline in pulmonary function and total mortality: a 30 year follow up study among the Finnish cohorts of the Seven Countries Study. Thorax 2001;56:703–7.
17. Liaw KM, Cheng CJ. Mortality attributable to cigarette smoking in Taiwan: a 12-year follow-up study. Tobacco Control 1998;7:
141–8.
18. Lee LT, Chen CJ, Suo J, et al. Pulmonary tuberculosis and lung cancer: a case-control study. Formosan J Med 1997;2:176–84.
19. Cheng YJ, Hildesheim A, Hsu MM, et al. Cigarette smoking, alcohol consumption and risk of nasopharyngeal carcinoma in Taiwan. Cancer Causes Control 1999;10:201–7.
20. Lee L, Tsai Y. A Community-Based Intervention Study on Smoking Cessation. Project of Department of Health, DOH89-TD-1180, 1999.
21. Lee WC, Lin RS, Chen WJ. An Epidemiology Study on Health Hazard of Smoking. Project of Department of Health, DOH84- HP-015-3M24, 1995.
22. Tsen CH. The impact of cigarette smoking and smoking ces- sation on cardiovascular disease in diabetic patients. Chinese J Public Health (Taiwan) 1999;18:241–6.
23. Yang SC, Yang SP. Bronchial responsiveness and lung function related to cigarette smoking and smoking cessation. Chang Gung Med J 2002;25:645–55.
24. Pan LY, Lee L. Adult smoking prevalence and its relationships with education and occupation in Taiwan: conditions before the implementation of the Tobacco Hazards Control Act (1993–
1996). Chinese J Public Health (Taiwan) 1999;18:189–98.
25. Rowe JW, Kahn RL. Successful aging. Gerontologist 1997;37:
433–40.
26. Almirall J, Gonzalez C, Balanzo X, et al. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest 1999;116:375–9.
27. Bak S, Sindrup SH, Alslev T, et al. Cessation of smoking after first-ever stroke: a follow-up study. Stroke 2002;33:2263–9.
– –