DOI: 10.6165/tai.2014.59.119
RESEARCH ARTICLE
Vegetative and Reproductive Growth of an Invasive Weed Bidens pilosa L.
var. radiata and its Noninvasive Congener Bidens bipinnata in Taiwan
Hsiao-Mei Hsu(1) and Wen-Yuan Kao(1,2*)
1. Institute of Ecology and Evolutionary Biology, College of Life Science, National Taiwan University.No. 1, Sec. 4, Roosevelt Road, Taipei, 10617 Taiwan.
2. Department of Life Science, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei, 10617 Taiwan. * Corresponding author. Email: wykao@ntu.edu.tw
(Manuscript received 14 October 2013; accepted 10 March 2014)
ABSTRACT: To gain a better understanding of traits and mechanisms underlying the fast spreading of an invasive plant, B. pilosa var. radiata, in Taiwan, we compared vegetative and reproductive growth of this invasive plant with its sympatric congener Bidens bipinnata L., a naturalized species. The two species had similar photosaturated photosynthetic rate and apparent quantum yield. However, both species differed in the temperature response of seed germination, in traits associated with life history, and in biomass allocation pattern. At winter temperature (18°C) seed germination was inhibited in B. bipinnata but not in B. pilosa var. radiata. Compared to B. bipinnata, B. pilosa var. radiata had higher specific leaf area, allocated more resource to leaves and roots in early growth stage, consequently, had a better growth and accumulated more biomass for an extended growth period. Laboratory experiment showed that shoot segments of B. pilosa var. radiata were capable of growing adventitious roots while those of B. bipinnata had no such ability. Thus, differences in specific leaf area, pattern of biomass allocation, seed germination response and vegetative reproduction between these two species explained why B. pilosa var. radiata outcompeted B. bipinnata in the field. KEY WORDS: Bidens pilosa L. var. radiata Sch. Bip., Bidens bipinnata L, biomass allocation, invasive plant, reproductive growth, vegetative growth.
INTRODUCTION
Invasion of exotic plants is a global phenomenon which often has highly impact on the environment and economics of regions being invaded (Pimentel et al., 2000; D’Antonio and Kark, 2002; Xu et al., 2006). For example, invasion by nonindigenous species has been recognized as second only to loss of habitat as a threat to global biodiversity (Walker and Steffen, 1997). Hence, preventing the spread of and facilitating remediation efforts of invasive species are of critical importance. Through increasing our understanding of the biology of invasive plants, we will have a better chance to find the most effective ways to accomplish the mission.
One of the most important questions been asked about the invasive plants is what makes them invasive. Traits such as reproductive and dispersal capabilities and growth-related characteristics may all contribute to the success of exotic species (Pattison et al., 1998; Rejmanek, 2000; Ridenour and Callaway, 2001). An effective approach to assess the characteristics that contribute to the competitive ability of an invasive species is to make comparisons between sympatric invasive and non-invasive congeners (Schierenbeck et al., 1994; Mack, 1996; McDowell, 2002; Feng et al., 2008; Bryson et al., 2012). The advantage of comparing
congeners rather than unrelated species is that it provides more insight into which traits actually play a role in the invasiveness of a species and which are merely coincidental (Mack, 1996; McDowell, 2002; Daehler, 2003; Deng et al., 2004; Schmidt et al., 2008).
Two exotic Bidens species, belonging to Asteraceae, were reported in Taiwan (Peng et al., 1998). Bidens pilosa L. var. radiata Sch. Bip., a native plant of North America (Peng et al., 1998), was first recorded in Taiwan in 1984 (Wu et al., 2004). It is now widely distributed and listed as one of the twenty most noxious invasive weeds in Taiwan (Chang et al., 2003). Bidens bipinnata, the other exotic Bidens species in Taiwan, is also a native of North America and was first recorded in Taiwan in 1904 (Wu et al., 2004). In contrast to B. pilosa var. radiata, B. bipinnata has become a naturalized plant and mainly distributed in Southern Taiwan (Peng et al., 1998). However, probably due to its less competitive than B. pilosa var. radiata, very few populations of B. bipinnata could be found in Taiwan now (personal obs). Local farmers used to make herb tea from B. bipinnata, however, owing to less and less B. bipinnata being found in the field they use B. pilosa var. radiata now. The first question prompted to us was what traits contributing to the superiority of B. pilosa L. radiata over B. bipinnata resulting in the diminishing of the later species in the southern region of Taiwan?
Could these traits also make B. pilosa var. radiata invasive in Taiwan?
In a previous study, we found that the germination percentage of seeds of B. bipinnata was significantly reduced by aqueous tissue extracts from B. pilosa var. radiata but the growth of seedlings of B. bipinnata was not affected (Hsu and Kao, 2009). Thus, the alleopathic effect could only partially explain the overwhelming dominance of the invasive species over its congener when they are sympatric. To gain a better understanding of traits and mechanisms underlying the fast spreading of B. pilosa var. radiata in Taiwan, in this study we compared traits related to carbon gain, growth and reproduction of these two species.
Studies comparing invasive versus non-invasive species growing in the same area generally have found that exotic invasive species have higher relative growth rates , specific leaf areas (leaf area per unit leaf weight), leaf area ratio (total leaf area per plant biomass) and maximal photosynthetic rates (Amax) as well as lower
respiratory costs than native species or non-invasive congeners (Durand and Goldstein, 2001; Smith and Knapp, 2001; Grotkopp et al., 2002; Pyšek and Richardson, 2007; Schlaepfer et al., 2010; van Kleunen et al., 2010). In addition, life history traits are also considered important in determining the success of invasive plants (Sutherland, 2004). B. pilosa L. has been reported as 1- to 2- years herb in Hawaiian rainforest (Pattison et al., 1998). No information about the life span of these two species in Taiwan is available. Also, there is evidence that invasive plants could change life history in the introduced range (Müller-Schärer et al., 2004).
Accordingly, we compared a subset of leaf-level and whole-plant traits related to carbon gain, growth, and reproduction between the invasive plant B. pilosa var. radiata and its noninvasive congener B. bipinnata. Field and greenhouse experiments were conducted. Photosynthetic rates and specific leaf area of field growing plants were measured. Physiological traits related to carbon gain (photosynthesis-irradiance response curve, photosaturated photosynthetic and dark respiration rates), morphological traits related to biomass allocation and growth, and life history traits related to spreading capabilities were measured on the two congeners grown in a greenhouse. Invasive plants are predicted to have traits that favor efficient use of resources and better ability for dispersal than non-invasive congeners. Thus, we tested the hypothesis that traits, such as biomass allocation to leaves, specific leaf area, and maximum photosynthetic rates, which are indicators of efficient use of resources (Vitousek, 1986) would be greater and the spreading capabilities would be better for the invasive B. pilosa var. radiata when compared to the non-invasive B. bipinnata.
MATERIALS AND METHODS
Field site description and measurements
Field study was conducted in an abandoned orchard, ca. 1500 m2, located in Madou town (120°15’ E, 23°10’
N), Tainan, in Sourthern Taiwan, where B. pilosa var. radiata and B. bipinnata growing sympatrically. By the time we started the experiment, about 99% of the ground inside the orchard was covered by B. pilosa L. var. radiata (ca. 40 plants m-2), while only about 20
plants of B. bipinnapa (covered less than 10 m2) were
found.
Photosaturated photosynthetic rates (Amax) were
measured with an LI-6400 infrared gas exchange system (LI-Cor, Lincoln, Nebraska, USA) on the most recently expanded leaves of field-growing plants in August, 2005. Measurement conditions within the cuvette were controlled as photon flux density (PFD) of 1500 μmol m-2 s-1, cuvette temperature 30°C, leaf-to-air
water vapor pressure difference (VPD) 1.2‒1.5 kPa, and ambient CO2 concentration 360 ± 5 cm3 m-3 (Kao et al.,
2003). After steady-state rates of Amax had been
recorded, light was turned off for the measurement of dark respiration rate (LI-COR, 2004). Finally, leaves were removed from the cuvette and excised, leaf area was measured using a leaf area meter (LI-3100, Licor, Lincoln, NE). The leaf was then dried at 60°C for at least 48 h and its dry mass weighted with an electronic balance (Mettler AB104). Specific leaf area (cm2 g-1)
was then calculated as leaf area/leaf dry mass.
Greenhouse study
To compare growth and pattern of biomass allocation during different growth stages, two experiments were conducted in a greenhouse in National Taiwan University, Taipei, Taiwan.
After being germinated in petri dishes, seedlings with one pair of leaves were transplanted into 2L plastic pots. One seedling was planted in each pot, which was filled with a mixture of 1 : 1 vermiculite: soil by volume. Plants were grown in a glasshouse in natural daylight, watered every day, and fertilized using inorganic fertilizer (Hyponex with N : P : K = 20 : 20 : 20, 1g L-1) 100 ml once every week.
In the first experiment (conducted from July to September, 2005), plants were harvested 64 days after being transplanted to the pots. At the time of harvesting, B. bipinnata had just stated flowering (flowers arranged in capitula) while B. pilosa var. radiata was still at vegetative stage. In the second experiment (conducted from Jan. to May, 2005), plants were harvested when both species had capitula, about 113 days after being transplanted to the pots. Following growth parameters were measured and calculated during and after the
experiments.
The relative growth rate of plant height (RGR), was calculate as RGR = [ln (plant height at harvest) – ln (initial plant height)] / time in growth period (days) (Hoffmann and Poorter, 2002). At harvest, the soil was cleaned from the roots, and plants were divided into roots, stems, leaves plus petiole, and reproductive components (capitula and seeds). Leaf areas of fresh leaves were measured with an area meter (LI-3100, Li-Cor). The harvested components were dried at 60°C for at least 48 hours before weighted with an electronic balance. Following variables were calculated from these measurements. Root mass ratio (RMR = root mass/total biomass), stem mass ratio (SMR = stem mass/total biomass), leaf mass ratio (LMR = leaf mass/total biomass), reproductive part mass ratio (RPMR = (capitula+seed)/total biomass), root: shoot ratio and leaf area ratio (LAR = total leaf area/total biomass) were determined.
In the second experiment, on days 83 after transplanting photosynthetic response of the most recently expanded leaves were measured with a LI-6400 infrared gas exchange system. For measuring the photosaturated photosynthetic rate (Amax), quantum
yield and dark respiration rate (Rd), the PFD was
adjusted from 2000 μmol m-2 s-1 to darkness in steps.
Other conditions within the cuvette were ambient temperature of 25°C, VPD 1.1‒1.5 kPa, and ambient CO2 concentration 380 cm3 m-3. After the response
curve had been recorded, leaves were removed from the cuvette and excised and specific leaf area was measured. The slope of the relationship between net CO2 assimilation rate (A) and PFD (< 100μmol m-2 s-1)
was taken to represent apparent photosynthetic quantum yield (AQY).
Laboratory experiments
To investigate if their seed germination rate differs at different seasons, we germinated seeds of both species at two different temperatures representing temperature for summer (28°C) and winter (18°C) months of the southern Taiwan. Seeds collected from field were placed on water-saturated filter paper in a petri dish (diameter × height = 90 mm × 15 mm), with 25 seeds per petri dish and 5 petri dishes per species per treatment. These petri dishes were then transferred to two growth chambers controlled as relative humidity of 70%, light/dark cycle of 12/12h and PFD at light period of 100‒150 μmol m-2 s-1 and air temperature of 28 and
18°C, respectively. Seed germination was recorded everyday for 10 days. Seeds were considered germinated when radicles could be observed by naked eyes (Reddy and Singh, 1992).
Field observation reveals that the vertical plant height of both species was similar, about 90 cm.
However, individuals of B. pilosa var. radiata extend horizontally and can reach 2‒3 m long. In addition, it is not rare to find adventitious roots growing from stems of B. pilosa var. radiata. Thus, in addition to reproduce sexually B. pilosa var. radiata seems to be able to reproduce vegetatively. Accordingly, we compared the ability of both species to produce adventitious roots in the laboratory. A segment of shoot between node 7 and 15 bearing 1‒2 nodes and pair of leaves was cut from plants of 4 months old, 3 replicates for each species. The tip of the segment was immersed into water and the growth of adventitious roots and lateral branch was recorded.
Statistical analysis
Means were analyzed by unpaired t-test (double-tailed, alpha = 0.05) The significant difference was defined as P < 0.05. All statistical tests were performed using SAS (SAS Software V8.1, USA) software package.
RESULTS
Field measurements
No significant difference was found in Amax between
the two species (Table 1). However, B. pilosa var. radiata had significantly higher SLA than B. bipinnata (P < 0.05).
Greenhouse study
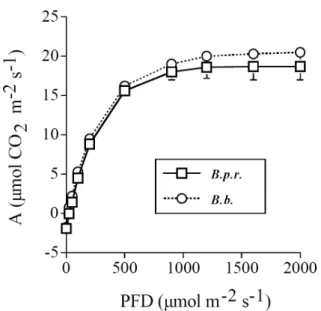
For plants growing in greenhouse, leaves of both species responded to increasing PFD with increases in net CO2 assimilation (A) until light saturation (Fig. 1).
No significant difference in A was found between the two species for each given PFD, except at PFD = 0. Consequently, no significant difference was found in Amax and apparent quantum yield between leaves of
greenhouse grown B. pilosa and B. bipinnata plants (Table 2). While at PFD = 0, leaves of B. pilosa var. radiata had significantly higher dark respiration rates than those of B. bipinnata (Table 2).
Similar to results of field investigation, greenhouse grown B. pilosa var. raidata had higher SLA than B. bipinnata (Table 2).
In the early growing stage, B. bipinnata grew higher hence had significantly higher RGR in plant height than B. pilosa var. radiata (Table 3). However, both species accumulated similar biomass during the 64 days of growing period. Further analysis reveals that two species differed significantly in the pattern of biomass allocation. B. pilosa var. radiata allocated proportionately more biomass to leaves and less to stems and roots (P < 0.05), while B. bipinnata more to stems than to leaves and roots. In comparison between species, B. pilosa var. radiata allocated significantly
Table 1. Photosaturated photosynthetic rate (Amax) and
specific leaf area (SLA) of field growing plants B. pilosa var.
radiata (n = 12) and B. bipinnata (n =10). Values (mean ± s.e.)
within the same row followed by different superscripts represent significant difference at P = 0.05.
B. pilosa var. radiata B. bipinnata
Amax
(μmol CO2 m-2 s-1) 24.0 ± 1.5
a 24.8 ± 1.4a
SLA
(cm2 g-1) 453.4 ± 19.3a 381.7 ± 27.4b
Table 2. Means ± s.e. (n = 6) of relative growth rate in plant height (RGR) and biomass allocation variables, i.e. total biomass, root mass ratio (RMR), stem mass ratio (SMR), leaf mass ratio (LMR), capitula mass ratio (CMR), root/shoot ratio and leaf area ratio (LAR), for potted B. pilosa var. radiata and
B. bipinnata plants after being grown in a greenhouse for 64
days (from July to Sept., 2005). Values within the same row followed by different superscripts represent significant difference at P = 0.05.
Variables B. pilosa var. radiata B. bipinnata
RGR (cm cm-1 day) 0.65 ± 0.16b 2.66 ± 0.22a Total Biomass (g) 4.15 ± 0.88a 4.32 ± 0.55a RMR (g g-1) 0.28 ± 0.02a 0.17 ± 0.01b SMR (g g-1) 0.30 ± 0.03b 0.49 ± 0.01a LMR (g g-1) 0.42 ± 0.01a 0.27 ± 0.01b CMR (g g-1) 0.00 ± 0.00b 0.07 ± 0.01a Root/Shoot 0.39 ± 0.04a 0.21 ± 0.01b LAR (cm2 g-1) 124.1 ± 7.2a 97.2 ± 7.0b
Table 3. Photosaturated photosynthetic rate (Amax), apparent
quantum yield (AQY), dark respiration rate (Rd) and specific leaf area (SLA) of the most recently, fully expanded leaves of potted B. pilosa var. radiata and B. bipinnata plants after being grown in a greenhouse for 83 days. Values (mean ± s. e., n = 5) within the same row followed by different superscripts represent significant difference at P = 0.05.
Variables B. pilosa var. radiata B. bipinnata
Amax (μmol CO2 m-2 s-1) 20.1 ± 0.7 a 20.3 ± 0.6a AQY (μmol CO2 μmol photons-1) 0.063 ± 0.003 a 0.068 ± 0.003a Rd (μmol CO2 m-2 s-1) 1.8 ± 0.1 a 1.3 ± 0.2b SLA (cm2 g-1) 467.1 ± 16.2a 425.2 ± 23.6a
more biomass to leaves and roots than B. bipinnata did. In B. pilosa var. radiata, leaves and roots account for 70% of total biomass. While plants of B. bipinnata allocated more biomass to stems than those of B. pilosa var. radiata did, their stems account for almost 50% of
Fig. 1. Net CO2 assimilation as a function of photosynthetic
photon flux density (PFD) for B. pilosa var. radiata (B. p. r) and B. bipinnata (B. b.) grown in a greenhouse. Standard errors are indicated by bars (n = 5), if larger than symbols.
total biomass. Leaf area ratio was found significantly higher in B. pilosa var. radiata than in B. bipinnata (Table 3).
In an extended growth period (the 2nd experiment),
plants of B. pilosa var. radiata were significantly higher (Fig. 2) and accumulated more biomass (Table 4), about 100% more, than B. bipinnata at harvest. B. bipinnata was found to allocate proportionally more biomass to reproduction component than B. pilosa var. radiata did. More than half of the above ground biomass of B. bipinnata was allocated to sexual reproductive component. Lateral branch mass ratio was higher in B. pilosa var. radiata than in B. bipinnata.
Germination rate
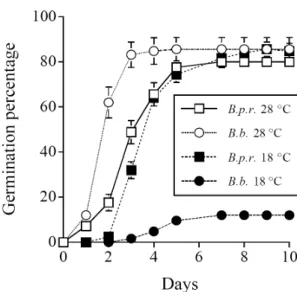
At 28°C, more seeds of B. bipinata germinated than those of B. pilosa var. radiata during the first 3 days of treatment, on the 7th days both species had similar
number of seeds geminated, and at the end of treatment no significant difference was found in the germination percentage (about 80%) between the two species (Fig. 2).
In the first 3 days of treatment, low temperature inhibited seed germination of both species, consequently, both species had significantly less seeds germinated at 18°C than at 28°C. But the degree of inhibition differed between the two species, the inhibition was more severe in B. bipinnata than in B. pilosa var. radiata. On the 4th
day, the effect of inhibition was not found in B. pilosa var. radiata. i.e., no significant difference was found in germination percentage between 28 and 18°C. In
Table 4. Means (± s.e., n =6) of relative growth rate of plant height (RGR), total biomass, root mass ratio (RMR), shoot mass ratio (SMR), lateral branch mass ratio (LBMR) and reproductive part (including capitula and seeds) mass ratio (RPMR) and root/shoot ratio for potted B. pilosa var. radiata and B. bipinnata plants after being grown in a greenhouse for 113 days (from Jan. to May, 2005). Values within the same row followed by different superscripts represent significant difference at P = 0.05.
Variable B. pilosa var. radiata B. bipinnata
RGR (cm cm-1 day) 1.21 ± 0.08a 0.35 ± 0.09b Total Biomass (g) 50.2 ± 1.8a 24.0 ± 0.7b RMR (g g-1) 0.08 ± 0.01a 0.05 ± 0.00b SMR (g g-1) 0.92 ± 0.01b 0.95 ± 0.00a LBMR (g g-1) 0.81 ± 0.01a 0.73 ± 0.02b RPMR (g g-1) 0.04 ± 0.04a 0.55 ± 0.01b Root/Shoot 0.09 ± 0.01a 0.05 ± 0.00b
contrast, the inhibition effect persisted in B. bipinnata, only 10% of its seeds were found germinated within 10 days. As a result, at 18°C B. pilosa var. radiata had significantly higher percentage of seed germination than B. bippinata.
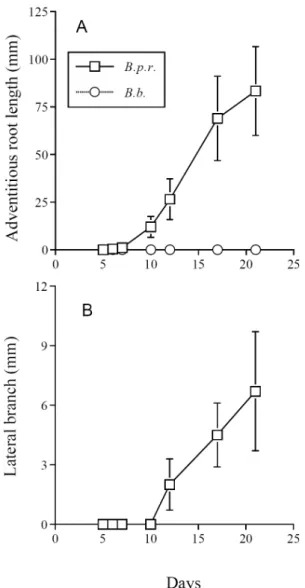
Growth of adventitious roots
Shoot segments of B. pilosa var. radiata were observed to grow adventitious roots one week after being immersed into water (Fig. 3a). Production and growth of lateral branches from the shoot segments was also found following the growing of adventitious roots in B. pilosa var. radiata (Fig. 3b). In contrast, neither adventitious root nor production of lateral branch was found in any of shoot segments cut from individual plants of B. bipinnata.
DISCUSSION
This is the first study comparing the physiological and morphological traits and life history between the two exotic Bidens weeds, one invasive and the other naturalized, in Taiwan. Results from the comparison suggest that some morphological and life history traits play important roles in outcompetition of B. bipinnata by B. pilosa var. radiata in the study farm, those traits might also contribute to the success of B. pilosa var. radiata in Taiwan.
One mechanism by which invasive plants may achieve success is through maximizing photosynthesis (Baruch and Goldsein, 1999; Durand and Goldstein, 2001). For example, Amax was identified as one of the
most useful variables to distinguish between invasive and noninvasive Rubus species (McDowell, 2002). In
Fig. 2. The percentage of seeds of B. pilosa var. radiata (B. p. r) and B. bipinnata (B. b.) germinated at 28 and 18°C for 10 days. Bars represent s.e.. (n = 5)
this study, neither field growing nor greenhouse grown plants of the invasive and non-invasive Bidens species showed significant differences in their Amax (Table 1 and
2), photosynthesis-irradiance response curve (Fig. 2), and apparent quantum yield (Table 2). B. pilosa var. radiata even had higher respiratory cost (per unit leaf area) than B. bipinnata (Table 2). Thus, it is unlikely that B. pilosa var. radiatata is superior to B. bipinnata in leaf-level photosynthetic traits.
Specific leaf area (SLA) is important in regulating and controlling carbon assimilation and allocation and is related to relative growth rates (Lambers and Poorter, 1992; Reich et al., 1997). Therefore, this trait is also considered important in determining success of the invasive plants (Grotkopp et al., 2007; Feng et al., 2008). Leaves of B. pilosa var. radiata showed consistently higher SLA than those of B. bipinnata either in field-growing (Table 1) or in greenhouse grown plants (Table 3). Thus, high SLA could be an important factor in determining the success of B. pilosa var. radiata.
Greenhouse experiments revealed that the pattern of biomass allocation differed between the two species in early growing stage (Table 3). B. bipinnata allocated more biomass to organs for supporting (stems), while B. pilosa more to those for resource acquisition (leaves and roots). Greater biomass allocation to leaves would allow plants to increase light interception. Though both species had similar photosynthetic rates (Fig. 2) when irradiated with same PFD, B. pilosa var. radiata with more leaf area to intercept light and more roots to explore nutrients is expected to have higher carbon assimilation rate and accumulate more biomass per plant than B. bipinnata in an extend growing period. This expectation is confirmed
Fig. 3. The growth of adventitious roots (A) and lateral branches (B) from axillary buds of shoot segments of B.
pilosa var. radiata (B. p. r) and B. bipinnata (B. b.). Standard
errors are indicated by bars, if larger than symbols.
by results of the second experiment that B. pilosa var. radiata accumulated more than one-fold of biomass than B. bipinnata during ca. 113 days of greenhouse-growing period (Table 4). Thus, a higher biomass accumulation of B. pilosa var. radiata in comparison to that of B. bipinnata results from greater photosynthetic area rather than greater leaf-level photosynthetic rate or less respiratory cost. After comparing the performance of co-occurring native and alien invasive plants, Daehler (2003) also found that invaders are more likely to have higher leaf area. Less biomass allocation to stems could cause less supportive to the plant body. This may explain the architecture differences between field growing B. bipinnata and B. pilosa var. radiata, the shoot of the former is usually erect while that of the later more or less
prostrate and easily being blown down by wind (person. obs.). The character might also facilitate the spreading of B. pilosa var. radaita due to its capability in producing adventitious roots (Fig. 3).
Both species are reported to have sexual reproduction and produce achenes (Peng et al., 1998) which can be dispersed by animals. In the study farm, we found that plants of B. bipinnata died off after seed maturation, a typical characteristic of an annual. Though primary shoots, where flowers were produced, of B. pilosa var. radiata also stop growing after seed maturation, its lateral branches continued growing. When lateral branches have opportunities to contact soil, for example as being blown down by wind, they produce adventitious roots and become rametes (field observation). A large biomass allocation to lateral branch coping with the ability to produce adventitious roots would allow B. pilosa var. radiata adding new modules to the plant body continuously even after the onset of sexual reproduction. Consequently, B. pilosa var. radiata behaves like a clonal herb and is able to disperse laterally. Laboratory experiment also confirmed that shoots of B. pilosa var. radiata were capable of producing adventitious roots while those of B. pipinnata did not have such ability (Fig. 3). This additional mode of reproduction may increase fecundity and help spreading of B. pilosa var. radiata. In contrast, B. bipinnata depends solely on seeds for dispersal and for generating new modules. Due to the inhibition of seed germination by winter temperature (Fig. 1), very few seedlings of B. bipinnata were found during the winter in the study farm (person. obs.). It is thus deducible that through vegetative reproduction and/or producing new seedlings B. pilosa var. radiata could expand and occupy the habitat evacuated by B. bipinnata at times, for example in winter, when current generation of B. bipinnata had died while new seedlings could not be established in the study farm. In general, vegetative reproduction in addition to help B. pilosa var. radiata increasing population size at low population densities, especially if the species is self incompatibility, could also make the species a better colonizer and faster reproducer than plants with no such capability. A common trait found in many introduced, invasive species is that they are capable of clonal growth (Pysek, 1997; Liu et al., 2006). Thus, the vegetative reproduction also represents an important life history trait in contributing to the invasiveness of the species.
In conclusion, results of this study suggest that fast growth (due to a higher SLA and more biomass allocating to leaves and roots) and superior spreading capacity (owing to two modes of reproduction) of B. pilosa var. radiata play important roles in outcompeting its congener B. bipinnata. These traits may also contribute to the success of B. pilosa var. radiata as an invader in Taiwan.
ACKNOWLEDGEMENTS
We thank Mr. Pao-Yuan Chen and his family for allowing us to get access to the orchard farm and for their hospitality. This study was supported by a grant from the National Science Council (NSC 94-2621-B-002-010) of Republic of China.
LITERATURE CITED
Baruch, Z. and G. Goldstein. 1999. Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121: 183‒192. doi: 10.1007/s004420050920
Bryson, C. T., K. N. Reddy and J. D. Byrd. 2012. Growth, development, and morphological differences among native and nonnative prickly nightshades (Solanum spp.) of the southern United States. Invasive Pl. Sci. Managem. 5: 341‒352. doi: 10.1614/IPSM-D-11-00062.1
Chang, M., L. M. Hsu, C. I. Yuan, F. Y. Chen and Y. C. Chang. 2002. The harmful effect and ecology of invasive plants in Taiwan. In: The Harmful Effect and Field Management of Mikania micrantha. Hualien District Agricultural Research and Externsion, Agricultural Council, Taiwan. pp. 97‒109.
Daehler, C. C. 2003. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Ann. Rev. Ecol. Evol. Syst. 34: 183‒211.
D’Antonio, C. M. and S. Kark. 2002. Impacts and extents of biotic invasions in terrestrial ecosystems. Trends Ecol. Evol. 17: 202‒204.
Deng, X., W.-H. Ye, H.-L. Feng, Q.-H. Yang, H.-L. Cao, K.-Y. Xu and Y. Zhang. 2004. Gas exchange characteristics of the invasive species Mikania micrantha and its indigenous congener M. cordata (Asteraceae) in South China. Bot. Bull. Acad. Sin. 45: 213‒220.
Durand, L. Z. and G. Goldstein. 2001. Photosynthesis, photoinhibition, and nitrogen use efficiency in native and invasive tree ferns in Hawaii. Oecologia 126: 345‒354. doi: 10.1007/s004420000535
Feng, Y.-L., G.-L. Fu and Y.-L. Zheng. 2008. Specific leaf area relates to the differences in leaf construction cost, photosynthesis, nitrogen allocation, and use efficiencies between invasive and noninvasive alien congeners. Planta 228: 383‒390. doi: 10.1007/s00425-008-0732-2
Grotkopp, E. and M. Rejmanek. 2007. High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperm. Am. J. Bot. 94: 526‒532. doi: 10.3732/ajb.94.4.526
Grotkopp, E., M. Rejmanek and T. L. Rost. 2002. Toward a causal explanation of plant invasiveness: seedling growth and life history strategies of 29 pine (Pinus) species. Am Nat. 159: 396‒419. doi: 10.1086/338995
Hoffmann, W. A. and H. Poorter. 2002. Avoiding bias in calculation of relative growth rate. Ann. Bot. 90: 37‒42. doi: 10.1093/aob/mcf140
Hsu, H.-M. and W.-Y. Kao. 2009. Contrasting effects of aqueous tissue extracts from an invasive plant, Bidens pilosa L. var. radiata, on the performance of its sympatric
plant species. Taiwania 54: 255‒260. doi: 10.6165/tai.2009.54(3).255
Kao, W.-Y., T.-T. Tsai and C.-N. Shih. 2003. Photosynthetic gas exchange and chlorophyll a fluorescence of three wild soybean species in response to NaCl treatment. Photosynthetica 41: 415‒419.
van Kleunen, M., E. Weber and M. Fischer. 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 13: 235‒245. Lambers, H. and H. Poorter. 1992. Inherent variation in
growth rate between higher plants: a search for physiological causes and ecological consequences. Adv. Ecol. Res. 23: 188‒261. doi: 10.1016/S0065-2504(08)60148-8
LI-COR. 2004. Using the LI-6400 Portable Photosznthesis System. Version 5 (2004) LI-COR Biosciences, Inc. Lincoln, NE, USA. http://www.licor.com/env/products/ photosynthesis/measurements.html
Liu, J., M. Dong, S.-L. Miao, Z.-Y. Li., M. Song and R. Wang. 2006. Invasive alien plants in China: Role of clonality and geographical origin. Biological Invasions 8: 1461‒1470. doi: 10.1007/s10530-005-5838-x
Mack, R. N. 1996. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 78: 107‒121. doi: 10.1016/0006-3207(96)00021-3 McDowell, S. C. L. 2002. Photosynthetic characteristics of
invasive and non-invasive species of Rubus (Rosaceae). Am J. Bot. 89: 1431‒1438. doi: 10.3732/ajb.89.9.1431 Müller-Shärer, H., U. Shaffner and T. Steinger. 2004.
Evolution in invasive plants: implication for biological control. Trends Ecol. Evol. 19: 417‒422.
Pattison, R. R., G. Goldstein and A. Ares. 1998. Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species. Oecologia 117: 449‒459. doi: 10.1007/s004420050680
Peng, C.-I, K.-F. Chung and H.-L. Li. 1998. Compositae. In: Huang, T.C. et. al (eds), Flora of Taiwan. 2nd ed. IV: Editorial Committee, Dept. Botany, National Taiwan University, Taipei, Taiwan.
Pimentel, D., L. Lach, R. Zuniga and D. Morrison. 2000. Environmental and economic costs of nonindigenous species in the United States. BioSci. 50: 53‒65.
Pyšek, P. 1997. Clonality and plant invasion: Can a trait make a difference? In: Kroon, H. and J. van Groenendael (eds), The Ecology and Evolution of Clonal Plants. Backbuys, Leiden, Netherlands. pp. 405‒427.
Pyšek, P. and D. M. Richardson. 2007. Traits associated with invasiveness in alien plants: where do we stand for? In: Nentwig, W. (ed.), Biological Invasions. Springer, New York. pp. 97‒125. doi: 10.1007/978-3-540-36920-2_7 Reddy, K. N. and M. Singh. 1992. Germination and
emergence of hairy beggarticks (Bidens pilosa). Weed Sci. 40: 195‒199.
Reich, P. B., M. B. Walters and D. S. Ellsworth. 1997. From tropics to tundra: global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 94: 13730‒13734. doi: 10.1073/pnas.94.25.13730
Rejmanek, M. 2000. Invasive plants: approaches and predictions. Aust. Ecol. 25: 497‒506. doi: 10.1046/j.1442-9993.2000.01080.x
Ridenour, W. M. and R. M. Callaway. 2001. The relative importance of allelopathy in interference: The effects of an invasive weed on a native bunchgrass. Oecologia 126: 444‒450. doi: 10.1007/s004420000533
Schierenbeck, K. A., R. N. Mack and R. R. Sharitz. 1994. Effects of herbivory on growth and biomass allocation in native and introduced species of Lonicera. Ecology 75: 1661‒1672. doi: 10.2307/1939626
Schlepfer, D., M. Glaettli and M. van Kleunen. 2010. A multi-species experiment in their native range indicates pre-adaptation of invasive alien plant species. New Phytol. 185: 1087‒1099. doi: 10.1111/j.1469-8137.2009.03114.x
Schmidt, C. D., K. R. Hickman, R. Channel, K. Harmoney and W. Stark W. 2008. Competitive ability of native grasses and non-native (Bothriochloa spp.) grasses. Plant Ecol. 197: 69‒80. doi: 10.1007/s11258-007-9361-2 Smith, M. D. and A. K. Knapp. 2001. Physiological and
morphological traits of exotic, invasive, and native plant species in tallgrass prairie. Int. J. Plant Sci. 162: 785‒792. doi: 10.1086/320774
Sutherland, S. 2004. What makes a weed a weed: life history traits of native and exotic plants in USA. Oecologia 141: 24‒39. doi: 10.1007/s00442-004-1628-x
Vitousek, P. M. 1986. Biological invasions and ecosystem properties: can species make a difference. In: Mooney, H. A. and J. Drake (eds). Ecology of biological invasions of North America and Hawaii. Springer, Berlin Heidelberg, New York. doi: 10.1007/978-1-4612-4988-7_10
Walker, B. and W. Steffen. 1997. An overview of the implications of global change for natural and managed terrestrial ecosystems. Conservation Ecology 1: http://www.consecol.org/vol1/iss2/art2.
Wu, S.-H., C.-F. Hsieh and M. Rejmanek. 2004. Catalogue of the naturalized flora of Taiwan. Taiwania 49: 16‒31. doi: 10.6165/tai.2004.49(1).16
Xu, H.-G., H. Deng, M.-Y. Li, S. Qiang, J.-Y. Guo, Z.-M. Han, Z-G. Huang, H.-Y. Sun, S.-P. He, H.-R. Wu and F.-H. Wan. 2006. The distribution and economic losses of alien species invasion to China. Biol. Invasions 8: 1459‒1500. doi: 10.1007/s10530-005-5841-2