增進台灣國家衛生研究院授權活動成效之研究 -以美國國家衛生研究院授權活動為例 - 政大學術集成
134
0
0
全文
(2) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Research to Increase Taiwan National Health Research Institutes Licensing Performance by Comparing The Licensing Practice of United States National Institutes of Health English Abstract Since the passage of U.S. Bayh-Dole Act in 1980, government-funded research inventions were no longer considered as government property. Invention could be patented and be licensed to industry through the process of commercialization for revenue return. Commercialization will create revenue in the form of royalty and taxes. 政 治 大. to the government, further driving scientific improvement and increase job. 立立. opportunities.. Technology commercialization is a very delicate process and there are a lot of. •‧ 國. ㈻㊫學. factors that might alter the success, including but not limited to i) the quality of. •‧. invention, ii) legislation restriction, iii) policy incentives, iv) industry interest, v) availability of information and etc. If managed properly, technology commercialization. sit. y. Nat. could bring high value to the academic institutes that developed an invention, to. er. io. government that financially support academic research and to general public that could. al. benefit from the invention itself.. n. v i n This study intends to C identify the factors ofU h e n g c h i the weak licensing performance in Taiwan government-funded national biomedical research organization, National Health Research Institute (NHRI). To evaluate the licensing performance of NHRI, this study will compare the licensing performance of NHRI with National Institute of Health (NIH) in the United States. To get accurate and formal data, this study will mainly retrieve data from official annual report and website. By comparing the practice of technology commercialization process of both institutes, this study could suggest possible flaws in NHRI’s licensing process in comparison to NIH. At the same time, this study will give suggestions to achieve a better licensing performance. This study concluded that both institute performed equally in FY 2010, but it has been noticed that NHRI were selectively in disclosing its licensing performance i.
(3) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. statistics and it is difficult to retrieve general information from NHRI, despite the availability of Freedom of Government Information Law (Taiwan). It’s the basic right for the general public to be able to supervise and surveillance a government agency’s performance as it utilizes the taxes contributed by a citizen in a country. The limitation of information disclosure by NHRI has made it difficult for general public supervise its licensing performance, of which might further contribute to even weaker licensing performance due to lack of supervision. This research also concluded that few options of licensing contracts, localization in licensing strategy, confusion in technology disclosure, possible misalignment of patenting & licensing strategy of the IP Management Committee contributes to weak licensing performance in the NHRI.. 政 治 大 Keywords: Licensing performance, Freedom of Information, FOIA, NIH, NHRI 立立 •‧. •‧ 國. ㈻㊫學. n. er. io. sit. y. Nat. al. Ch. engchi. ii. i n U. v.
(4) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 增進台灣國家衛生研究院授權活動成效之研究 -以美國國家衛生研究院授權活動為例 中文摘要 1980 年,在美國拜杜法案(Bayh-Dole Act)通過以後,政府補助研發成果 從原屬於國家財產,下放歸屬權於研發單位。因此研發單位可自行管理並將研發 成果授權至產業界,將研發成果商品化,此過程稱之為「技術移轉」。技術移轉 是一個非常細膩且複雜的過程,有許多因素會左右技術移轉的成敗,其中包括: 技術之品質、法律限制、政策因素、產業需求和資訊流通等因素。技術移轉將可. 治 政 和增加就業機會,為國家創造經濟收入。 大 立立 本研究旨探如何提升政府補助國立生醫研究單位-台灣國家衛生研究院. 以使研發成果商品化, 有助於提升政府稅收和權利金收入,並且推動科學發展. •‧ 國. ㈻㊫學. (NHRI)之授權績效。本研究將採用美國國家衛生研究院(NIH)之授權績效和 授權執行方式當參考指標。為了得到精準和可信的數據,本研究僅截取官方之年. •‧. 報和網站的資料。與此同時,本研究末將會提供如何提升授權績效之建議。 . y. Nat. 此研究發現就 2010 年而言,NIH 和 NHRI 之授權績效可謂旗鼓相當。儘管. io. sit. 台灣立法規定「政府資訊公開法」,此研究發現台灣國家衛生研究院有選擇性的. n. al. er. 發表其授權績效的跡象。由於政府所有開銷均從其預算而來,而政府預算部分自. i n U. v. 人民納稅所得,因此監督政府績效是普羅大眾的基本權利。人民有權監督,並且. Ch. engchi. 要求政府就其管理、執行成效做出解釋。然而,台灣國家衛生研究院之資訊不透 明舉動,限制了人民監督的權利,此舉將會因缺乏監督而惡性循環的造成授權績 效更為疲弱。此外,此研究亦發現較少的授權契約種類、授權策略在地化、研發 成果披露之不明確性、智慧財產委員會專利申請和授權策略失衡是造成 NHRI 授 權成果疲弱的因素。 關鍵字:授權績效、資訊公開法、FOIA、NIH、NHRI、國家衛生研究院. iii.
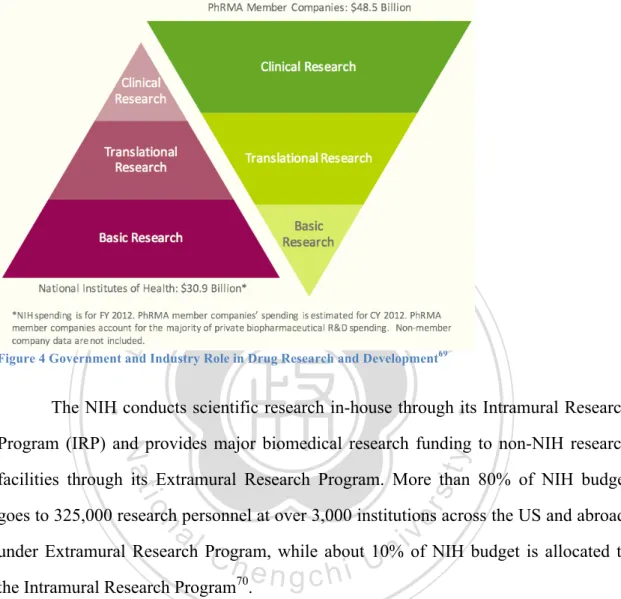
(5) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Table of Content English Abstract ....................................................................................................................... i 中文摘要 ................................................................................................................................. iii Figure Index ............................................................................................................................ vi Table Index ............................................................................................................................ vii Chapter 1: Introduction.......................................................................................................... 1 1.1 Background .................................................................................................................................. 1 1.2 Statement of Problem ................................................................................................................. 3 1.3 Research Objective ...................................................................................................................... 5 1.4 Research Methods ....................................................................................................................... 6 1.5 Research Structure...................................................................................................................... 7 1.6 Research Restriction ................................................................................................................... 8 1.7 Abbreviated Terminologies ........................................................................................................ 9 Chapter 2: Introduction to Intellectual Property Rights .................................................. 10 2.1 Types of Intellectual Property Rights ..................................................................................... 10 2.1.1 Patents ..................................................................................................................................... 11 2.2 Objective of Providing Patent Protection ............................................................................... 13 2.2.2 PCT- Patent Cooperation Treaty ......................................................................................... 15 . 立立. 政 治 大. •‧. •‧ 國. ㈻㊫學. Chapter 3: Introduction to Technology Licensing ............................................................. 18 3.1 Background ................................................................................................................................ 18 3.2 Due Diligence ............................................................................................................................. 19 3.3 Technology Transfer: Licensing .............................................................................................. 22 3.4 The Process of Technology Licensing ...................................................................................... 23 3.5 US Legislation: Bayh-Dole Act ................................................................................................ 26 3.6 Taiwan Legislation: Fundamental Science and Technology Act .......................................... 29 3.7 Locally Manufacture Clause .................................................................................................... 30 . sit. y. Nat. n. al. er. io. Chapter 4: Freedom to Information Law ........................................................................... 36 4.1 Background ................................................................................................................................ 36 4.2 United States Freedom of Information Act (FOIA) ............................................................... 37 4.3 Taiwan Freedom of Government Information Law, ............................................................. 40 4.4 The Importance of Information Transparency to Technology Transfer ............................. 42 4.4.1 Information Transparency: From the Point of View of TTO ............................................ 42 4.4.2 Information Transparency: From the Point of View of Technology Acquirer ................ 43 . Ch. engchi. i n U. v. Chapter 5: Government Funded Biotechnology Research ................................................ 45 5.1 Background ................................................................................................................................ 45 5.2 United States Government Funded Public Research Institute: National Institutes of Health (NIH) .................................................................................................................................... 45 5.2.1 Executive Orders and Source of Funding ............................................................................ 47 5.2.2 Organization and Structure .................................................................................................. 51 5.2.3 Intramural Research Program (IRP) ................................................................................... 51 5.2.4 Overview of Licensing Process at NIH ................................................................................. 53 5.2.4.1 Technology Protection (Patent Application) .................................................................... 54 5.2.4.2 Types of Licenses Available at NIH ................................................................................... 55 5.2.4.3 Licensing Process ................................................................................................................ 56 5.2.4.4 Post Licensing Performance Evaluation ........................................................................... 58 5.2.5 Reputation and Licensing Performance............................................................................... 59 5.2.5.1 Reputation ............................................................................................................................ 59 5.2.5.2 Licensing Reputation .......................................................................................................... 60 5.2.6 NIH OTT Effort to Increase Licensing Activity to Serve Global Public Health .............. 64 . iv.
(6) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 5.3 Taiwan Government Funded Public Research Institute: National Health Research Institute (NHRI) .............................................................................................................................. 67 5.3.1 Background ............................................................................................................................. 67 5.3.2 Executive Orders and Source of Funding ............................................................................ 68 5.3.3 Organization and Structure .................................................................................................. 69 5.3.4 Overview of Licensing Process at NHRI .............................................................................. 72 5.3.4.1 Reputation and Licensing Performance ............................................................................ 74 . Chapter 6: Comparison of Licensing Performance ........................................................... 78 Chapter 7: Conclusion .......................................................................................................... 84 Chapter 8: Discussions .......................................................................................................... 86 8.1 Background ................................................................................................................................ 87 8.2 The Announcement of Licensing Opportunities and Licensee Selection ............................. 87 8.3 Scope and Types of Licenses .................................................................................................... 94 8.4 Notice for Available Technology and Time to License .......................................................... 94 8.5 Effort to Market of New Invention .......................................................................................... 95 8.6 Patenting & Licensing Manager (NIH) vs. IP Management Committee (NHRI) ............... 97 8.6.1 Decision Making of IP Management Committee ................................................................ 98 8.6.2 Evaluation Standard for Exclusive or Non-Exclusive Licensing ....................................... 99 8.7 Accessibility of Information ................................................................................................... 100 8.7.1 Communication Language .................................................................................................. 100 8.7.2 Freedom to Access Information .......................................................................................... 101 8.8 Future Suggestions .................................................................................................................. 105 . 立立. 政 治 大. •‧ 國. ㈻㊫學. Reference .............................................................................................................................. 108 . •‧. 參考資料 .............................................................................................................................. 113 Appendix .............................................................................................................................. 116 . n. er. io. sit. y. Nat. al. Ch. engchi. v. i n U. v.
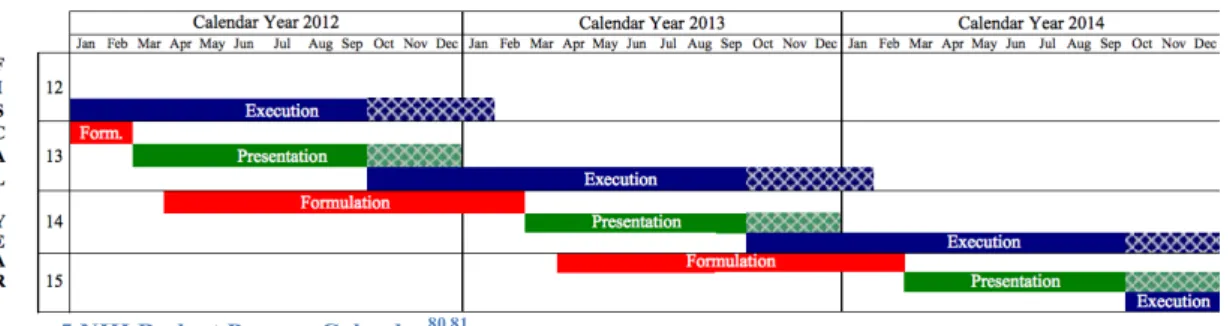
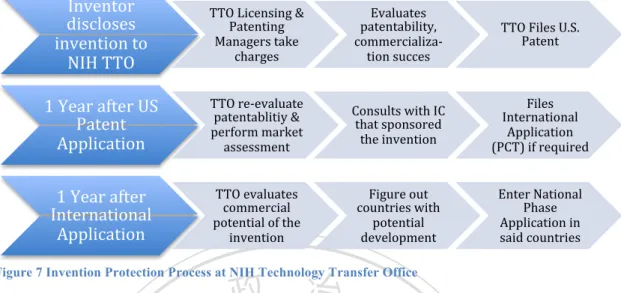
(7) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Figure Index Figure 1 PCT Application Timeline ........................................................................................ 16 Figure 2 Timeframe of Technology Commercialization and Factors Influencing Licensing Success .................................................................................................................................... 24 Figure 3 General Technology Licensing Process by a Technology Transfer Office .............. 25 Figure 4 Government and Industry Role in Drug Research and Development ...................... 46 Figure 5 NIH Budget Process Calendar, ................................................................................. 49 Figure 6 Organization Chart of NIH ...................................................................................... 51 Figure 7 Invention Protection Process at NIH Technology Transfer Office........................... 54 Figure 8 The Licensing Application Process Flow Chart ....................................................... 56 Figure 9 Increase Trend of NIH Royalty Income from FY1996 to FY2012 .......................... 63 Figure 10 NIH Licenses by Type of Agreement (FY2011-2013) .......................................... 64 Figure 11 NHRI Organization Structure , ............................................................................... 69 Figure 12 NHRI Technology Licensing Process..................................................................... 72 Figure 13 Technology Licensing Process (Discussion) .......................................................... 86 Figure 14 Snapshot of NIH's Available For-Licensing Technology Website Interface ......... 89 Figure 15 NHRI TTO's Announcement Seeking for Licensor Website Screenshot ............... 91 Figure 16 "Research Result" Webpage of NHRI .................................................................... 92 . 立立. 政 治 大. •‧. •‧ 國. ㈻㊫學. n. er. io. sit. y. Nat. al. Ch. engchi. vi. i n U. v.
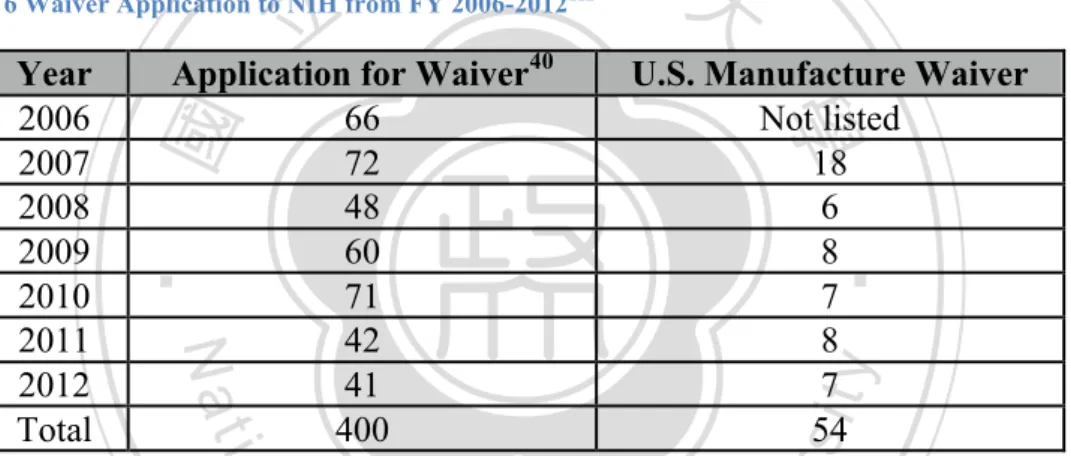
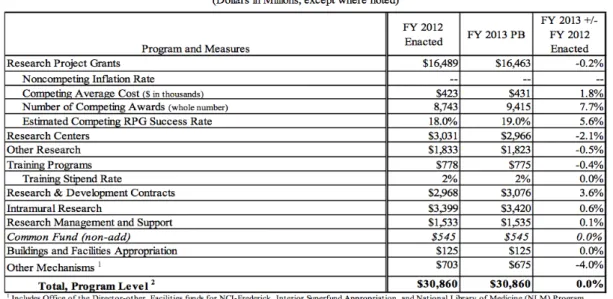
(8) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Table Index Table 1 Types of Patents in The United States ....................................................................... 13 Table 2 Type of Patents in Taiwan.......................................................................................... 13 Table 3 Number of Patents being Applied and Granted by AUTM Members from 1991-2012 ................................................................................................................................................. 27 Table 4 Cases of Licensing and Options by AUTM Members from 1991-2012 ................... 27 Table 5 Exception to U.S. Manufacture Requirement (35 USC § 204) ................................. 32 Table 6 Waiver Application to NIH from FY 2006-2012 ....................................................... 33 Table 7 Budget Funding for NIH in FY 2013 (in USD$) ....................................................... 50 Table 8 Types of Licenses Available at NIH .......................................................................... 55 Table 9 NIH Technology Transfer Reputation in FY 2011-2013 (in USD$) ........................ 59 Table 10 NIH Royalty Income since 2008 (in Million USD$) ............................................... 62 Table 11 NIH Executed Licenses since FY 2008 ................................................................... 64 Table 12 Patents Obtained by NHRI from 2004-2013 ............................................................ 76 Table 13 Research Funding from MOHW to NHRI and NHRI’s Licensing Performance from 2008-2013................................................................................................................................ 77 Table 14 Comparison between NIH IRP and NHRI and It's Licensing Performance on 2010 ................................................................................................................................................. 78 Table 15 Performance Index of Licensing Performance ......................................................... 79 Table 16 Comparison of Licensing Friendliness between NIH and NHRI............................. 93 . 立立. 政 治 大. •‧. •‧ 國. ㈻㊫學. n. er. io. sit. y. Nat. al. Ch. engchi. vii. i n U. v.
(9) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Chapter 1: Introduction 1.1 Background Science is a study of human pursues to understand the nature. By understanding the nature, human could utilize the nature to invent, in order to live more comfortably. This ritual runs from our ancient ancestors: starting fire with two stones or utilizing wheels to transport heavy stuffs; to sophisticated machine or computer calculation software that exists today. Nowadays, a lot of scientific discoveries and inventions happen in university. 政 治 大 Science today had branched into very small and professional niche, making new 立立 laboratories, in glasses, test tubes, solutions, machines or computers. Furthermore,. scientific discoveries seem “useless” if being inspect independently. Yet, scientific. •‧ 國. ㈻㊫學. discoveries, if being gathered in a pool, could make a change in our life, rather than sitting on the shelf or only being published on academic publications. For example, a. •‧. single component in an iPhone might be worthless, but when Apple put thousands of. sit. y. Nat. components together, an iPhone worth more than seven hundred U.S. Dollars.. io. er. Scientific discoveries that could be made into a unique device, method, composition, process, or make significant improvement to a current practice is. al. n. v i n considered a new invention.C“Intellectual Property Rights” are the rights given to hengchi U person over the creations of minds, for a given period of time. It is a kind of intangible. right that works like tangible property right. Invention that is novel and not obvious to person having ordinary skill in the art1 could be patented. Inventor applies patent from a nation’s Patent and Trademark Office. He or she then has the right to exclude others from practicing its invention by applying for patent protection. Once an inventor applied and granted a patent, they could choose to make use, sell, or license the patent to a business counterpart. A patented technology could be “transferred” from laboratories’ bench side to industry, transformed from basic science to business, evolved from spending money to 1. Often abbreviated as PHOSITA in the US. 1.
(10) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. making money through a process known as “technology transfer”. Technology transfer could involve licensing process or setting up joint ventures and partnership, with the ultimate goal to bring new technology to the market. Corporates, universities and governmental organizations, often have the “Office of Technology Transfer” (OTT, or TTO) to oversee and help with the licensing process. Technology licensing is a crucial “bridging” process between science and business. A successful technology licensing not only fuel the future scientific discovery through the royalties’ income, it also helps progress the technology advancement. In order for technology licensing to succeed, a lot of factors shall be taken into consideration, including but not limited to technology, talent, market, regulation,. 政 治 大. business operation, money, legal issue, intellectual property, employer, government,. 立立. competitor, and the list goes on.. •‧. •‧ 國. ㈻㊫學. n. er. io. sit. y. Nat. al. Ch. engchi. 2. i n U. v.
(11) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 1.2 Statement of Problem Every year, thousand of inventive finding have been developed by research organizations through money funded by government. Take drug discovery for an example, a potential drug candidate that could change the world could be licensed to a local biotech company for further research & development, or to an international pharmaceutical company for commercialization into products. Taiwan is one of the countries around the world that highly invest in research funding. Taiwan overall R&D spending as percentage of GDP was 2.57% (about NTD$ 331 Billion) in 2007 and was climbing to 3.02% (about NTD$ 413 Billion) in 20112. According to the R&D expenditure by Field of Science and Technology (See Appendix. 政 治 大. 1), Medical Science R&D expenditure was NTD$ 29.7, which made up 7.2% of total R&D expenditure in 2011.. 立立. Table 13 in this study showed that NHRI received NTD$ 2.26 Billion in 2011. •‧ 國. ㈻㊫學. from MOHW, which is about 7.6% of Taiwan overall medical science R&D budget. Since NHRI did receive other funds from other funding agencies in Taiwan, for. •‧. example National Science Council and etc., the ratio of NHRI funding over total medical science R&D budget should be higher than 7.6%.. Nat. sit. y. Research result of government-funded research could be patented and licensed. er. io. for commercialization, channeling the income to the inventor and back to the government. Research result should be managed properly and licensed to industry. n. al. Ch. i n U. v. locally and internationally to benefit the tax-payer, which indirectly supports the. engchi. research. According to Taiwan National Science Council’s Indicators of Science and Technology (FY 2012), the “ratio of technology payment balance”3 of the United States was 1.46 in 2010 (4 years average from 2007 to 2010 was 1.56), while Taiwan’s ratio of technology payment balance was 0.18 (4 years average from 2006-2010 was 0.24). It is apparent that Taiwan imports more technology than exporting it to other countries. The process of technology commercialization is very complicated and it involves a lot of professions. Many factors including legislation restriction, government 2. Indicators of Science and Technology (FY 2012) (n.d.), Taiwan National Science Council, Retrieved from https://nscnt12.nsc.gov.tw/WAS2/technology/AsTechnologyDataIndex.aspx 3 Ratio of receipts from other countries for technologies exported and payments made to other countries for technologies imported. High imports (ratio smaller than 1) may imply that a country is poor at developing technology, or the country is keen on using new technology. High exports (ratio higher than 1) may imply that the country is an important developer and originator of a particular technology, but it may indicate that the country is not able to put new technology into efficient use, e.g. Leaving to others to commercialize it.. 3.
(12) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. policy, research quality, market demand and etc., might contribute to the success or failure of technology licensing. The process of technology commercialization could be briefly divided into two phases: Supply End and Demand End. The Supply End means the ability of research institutes to provide quality invention, while the Demand End implies the ability of industry to accept the supply end’s invention. Since the passage of Fundamental Science and Technology Act, legislative restriction has loosened to be friendlier to licensing process. The act opens up a lot of licensing opportunities to the Supply End of the commercialization process. The responsibility connecting both Supply and Demand parties relies on the Technology Transfer Office (TTO). It is crucial for TTO to convey sufficient information between the Supply and Demand parties. The enactment of Taiwan Freedom of Government. 治 政 crucial for the marketing and sales of an invention. 大 立立 Referring to information published on the NHRI website, NHRI has licensed 22 Information Law opens up a channel for the free flow of information – which is very. •‧ 國. ㈻㊫學. of its invention to business corporates since the establishment of NHRI TTO (2001), among them 14 had been terminated and only 8 is under execution. NHRI on average. •‧. has 10 patents applied in Taiwan (invention numbers not disclosed) and 2 licenses executed annually. Whereas it’s counterpart, NIH on average has more than 300. Nat. sit. y. inventions disclosed annually and 180 licenses executed annually.. er. io. It seems that there are still rooms for improvement during the licensing process for the NHRI. This study intends to figure out what factors contributing to the weak. n. al. Ch. licensing performance at the NHRI.. engchi. 4. i n U. v.
(13) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 1.3 Research Objective This study is intended to identify what critical factors contributes to successful technology licensing and what are the main reasons behind the relatively low licensing performance among Taiwanese research institutes, despite very high investment in research funding. One way to increase the low ratio of technology payment balance in Taiwan is to increase the licensing out performance of Taiwan technology. This study intends to figure out if it was due to licensing market demand not fulfilled, weak attractiveness of Taiwanese technologies, legislative policy that might slow down the licensing process, information asymmetry due to licensing information not disclosed, or other reasons.. 治 政 大 of Information Right gives right for citizens to surveillance it’s government. Freedom 立立individual, gaining individual access to information held by “the right to know” to an It is believed that freedom in accessing governmental information is a basic. •‧ 國. ㈻㊫學. the government.. The target of this investigation is limited to government-funded public research. •‧. organization in Taiwan and the United States, of which National Institute of Health (NIH, US) and National Health Research Institute (NHRI, Taiwan) were chosen in this. Nat. sit. y. study. No scholars have been researching on the licensing process and performance of. er. io. NHRI in Taiwan, except Chang C-P. in 20064 did a research on the technology transfer mechanism of research institutes in Taiwan. This research is considered a pioneer study. al. n. in the field.. Ch. engchi. i n U. v. After determining the reasons that contributed to weak success of technology licensing, this study will provide suggestions based on the success factor of NIH to Taiwanese government funded public research organization to improve the success rate of licensing out a new technology.. 4. 張正平(2006),我國國家生物技術研究機構技術移轉機制之探討,國立政治大學法律與科技整合研 究所,第四章, 政大機構典藏 2009-09-17T06:18:05Z. 5.
(14) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 1.4 Research Methods This research will compare the licensing practice between two governmentfunded public medical research organization in United States and Taiwan, namely NHRI and NIH. This study at the same time will compare the licensing performance of both research institutes to present a better perspective on the current status of licensing status in both institutes. To get accurate and formal data, this research will mainly retrieve data from official annual report and official websites. Using results computed from research statistics, it is possible to identify factors that bring synergy to licensing and point out factors that hinder Taiwanese public research organization from performing well in. 治 政 大 but not limited to website in licensing practice between two institutes, including 立立 scope of application, sales and marketing strategies, interface, licensing types,. technology licensing. This study would also compare and pin point the major difference. •‧ 國. ㈻㊫學. availability of technology friendly to licensee and etc.. This study will address the importance of freedom of information access during. •‧. technology licensing process. The author will practically talk to and request information that were absent on the public domain with both institute to evaluate to. Nat. sit. y. what extent does the government agency honor freedom of information access, since. er. io. responsible disclosure of sufficient information is potentially or often underrated. This study will also discuss and probe possible clues, which could further. n. al. Ch. i n U. v. improve the effectiveness of technology transfer in Taiwan.. engchi. 6.
(15) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 1.5 Research Structure Introduction Statement of Problem Research Objective Research Method Results . 立立. 政 治 大. Discussions and Suggestions . •‧. •‧ 國. ㈻㊫學. n. er. io. sit. y. Nat. al. Ch. engchi. 7. i n U. v.
(16) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 1.6 Research Restriction The research institute referred in this research focuses on government-funded public research institute that are governed by Bayh-Dole Act, of which the allocation of invention ownership of intellectual property rights is given to the inventor. The intellectual property rights being discussed in this research excludes trademark, copyright, trade secret and only limited to patent. To be more specific, this study only takes National Health Research Institute (NHRI) from Taiwan and National Institute of Health (NIH) from the United States into consideration. In Chapter 3, this study discussed about the supply (technology seller: research institute) and demand parties (technology buyer: industry) of technology licensing. 治 政 大research invention is abundant. demand party. This study assumes that the demand of 立立 could only apply for a patent in a country, to avoid Since an invention. value chain. This study only discusses the supply party and does not discuss about the. •‧ 國. ㈻㊫學. misinterpreting and exaggerating an institution’s licensing performance, this study only take local patent application into consideration, e.g. evaluation of NHRI’s performance. •‧. will be based on Taiwan Patent numbers, while evaluation of NIH’s performance will be based on U.S. Patent numbers.. Nat. sit. y. For NIH, while evaluating the performance of licensing activity, this research. er. io. only focuses on Intramural Research Program to allow fair and on par comparison with NHRI. Data on Extramural Research Program will be excluded in this study.. n. al. Ch. i n U. v. For NHRI, it is to be assumed that NHRI should be categorized as government. engchi. body as it receives research funding from Taiwanese government. Legislation of Ministry of Health and Welfare (MOHW) dictates that agencies receiving funding from MOHW should be viewed as government body. Thus, NHRI is bound to oblige with Taiwan Freedom of Government Information Act. Despite this study is able to retrieve all information about NIH licensing performance, the information available from NHRI was rather limited. The reporting practice in Taiwan made it impossible to retrieve a full set of data on licensing performance, including but not limited to invention disclosed, royalty information and etc. This study only able to retrieve full licensing data of NHRI in FY 2010, thus comparison on licensing performance will only be based on FY 2010.. 8.
(17) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 1.7 Abbreviated Terminologies Actual Terminology Academia Sinica (Taiwan) Association of University Technology Managers Biomedical Research and Development Price Index Board of Scientific Counselors Code of Federal Regulations Continued-in-part Freedom of Information Freedom of Information Act Fiscal Year Gross Domestic Product United States Department of Health and Human Science Institutes and Centers (of NIH) Intellectual Property Intramural Research Program Marine Hospital Service Ministry of Health and Welfare Main Science and Technology Indicators National Health Research Institute (Taiwan) National Institute of Health (United States) Office of Director (of NIH) Organization of Economic Co-operation and Development Office of Technology Transfer Patent Cooperation Treaty Pharmaceutical Research and Manufacturers of America Principal Investigator(s) Research and Development Small and Medium Enterprise(s) Start-up Exclusive Commercial License Agreement Start-Up Evaluation License Agreement Taiwan Intellectual Property Office Trade-Related Aspects of Intellectual Property Rights Technology Transfer Technology Transfer Office United States United States Code United States Patent and Trademark Office World Intellectual Property Orgnization World Trade Organization. 立立. 政 治 大. •‧. •‧ 國. ㈻㊫學. Abbreviation AS AUTM BRDPI BSCs C.F.R. CIP FOI FOIA FY GDP HHS ICs IP IRP MHS MOHW MSTI NHRI NIH OD OECD OTT PCT PhRMA PIs R&D SMEs Start-up ECLA Start-up EELA TIPO TRIPs TT TTO U.S. U.S.C. USPTO WIPO WTO. n. er. io. sit. y. Nat. al. Ch. engchi. 9. i n U. v.
(18) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Chapter 2: Introduction to Intellectual Property Rights 2.1 Types of Intellectual Property Rights The British Statute of Anne (1710) and the Statute of Monopolies (1624) are now seen as the origins of copyright and patent law respectively5, however it was not until the late 20th century that intellectual property rights have become a mainstream in the world. Intellectual property (IP) is an intangible right that could legally exclude people from using the owner’s creation of mind. Intellectual property covers everything that is created by human’s mind, including but not limited to musicals, literatures, art works, inventions, discoveries, inventions, business methods, new plant species, symbols, designs, words, phrases and etc. Patent, copyright, trademark, trade dress,. 治 政 to World Intellectual Property Organization (WIPO), 大 intellectual property should include rights relating to立立 : industrial design and trade secret are protected by intellectual property law. According 6. •‧ 國. ㈻㊫學. 1. Literary, artistic and scientific works;. 2. Performances of performing artists, phonograms and broadcasts;. •‧. 3. Inventions in all fields of human endeavor; 4. Scientific discoveries;. sit. y. Nat. 5. Industrial designs;. io. 7. Protection against unfair competition.. n. al. er. 6. Trademarks, service marks and commercial names and designations; and. i n U. v. The owner of an intellectual property does not own the absolute right to the. Ch. engchi. intellectual property, of which it means a patent owner could not practice his or her invention unless he or she obtains rights to all patents covering the basic construction of his or her invention. However he or she has the right to exclude others from practicing or using the invention without consent. This study focuses on the technology transfer in biotechnology industry, which further limits the scope of intellectual property rights to patents and trade secret. As mentioned on the Research Restriction, patent is the only intellectual property right being discussed in this research.. 5. Sherman, B., Bently, L. (1999). The making of modern intellectual property law: the British experience, 1760– 1911. Cambridge University Press. p. 207. ISBN 9780521563635 6 The Convention founding the World Intellectual Property Organization (WIPO), concluded in Stockholm on July 14, 1967 (Article 2(viii)).. 10.
(19) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 2.1.1 Patents Patent right is an exclusive rights being granted to an inventor by government agency, often known as Patent and Trademark Office. An inventor must apply for a patent in order to get protection from the authority. In most countries, both natural persons and business entities could apply for a patent. A patent does not give the owner rights to make, use or sell an invention. The exclusive right granted to a patentee in most countries is the right to prevent others from making, using, selling, or distributing the patented invention without permission7. A typical patent application must include at least one or more claims that define the invention. A patent claim must meet with the requirement of patentability, which includes (i) Patentable subject matter; (ii) Novel; (iii) Non-obvious (or involve an. 政 治 大. inventive step in Europe patent system), and (iv) Useful (Susceptible of industrial application in Europe patent system). A patent must also fulfill “sufficient disclosure”. 立立. and “best-mode” requirement, of which it means during application, inventor has to. •‧ 國. ㈻㊫學. disclose sufficient information regarding the invention, allowing the public to receive knowledge of the preferred embodiments for practicing the claimed invention8.. •‧. Although patent gives the owner the exclusive right to make, use or sell the patent, that doesn’t mean the owner has the right to exploit the patent. A lot of patents. sit. y. Nat. are improvements of previous patents that might still valid; if the patent owner of an. io. the existing inventor before practicing his or her patent.. n. al. er. improved-patent based on an existing invention, he or she needs to get permission from. i n U. v. The grant and enforcement of patents are governed by national laws, and also. Ch. engchi. by international treaties, where those treaties have been given effect in national laws. Patents are granted by national or regional patent offices. In short, patent law is territorial in nature, a patent could only protect an invention in the country which that patent is granted in. However, when a patent application is published, the invention is thus disclosed into the public domain as prior art of every other country that the patent owner has not applied patent in. Patent in most countries fall into three categories, though it is classified differently in various countries:. 7. Patents: Frequently Asked Questions (n.d.), World Intellectual Property Organization, Retrieved from http://www.wipo.int/patentscope/en/patents_faq.html#protection 8 Eli Lilly & Co. v. Barr Laboratories Inc., 251 F.3d 955, 963, 58 USPQ2d 1865, 1874 (Fed. Cir. 2001).. 11.
(20) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. 1. Invention Patents (Patents, utility patents): Issued for the invention of a new and useful process, machine, manufacture, or composition of matter, or a new and useful improvement; 2. Utility Model Patents (Petty patents9): Issued for the creation of technical concepts that reflect the innovation of a form, construction, or installation of an article that possess a new purpose or improved efficacy. In most countries, utility model are cheaper to maintain (or free of maintenance fee), however the protection period are usually shorter; and 3. Design Patents (Industrial design patents): Issued for a new design in shape, pattern, color, or combination of an article to enhance its touch, view,. 政 治 大. quality, affinity or value through visual effects in order to increase market. 立立. competitiveness.. •‧ 國. ㈻㊫學. In the United States, the United States Patent and Trademark Office (USPTO) handles all patent applications10. Upon granting a patent, the owner of the patent must. •‧. pay a fee to maintain the patent for every few years. For research institutes that handle. y. Nat. the patent application on behalf of their inventors, a lot of considerations must be made. io. sit. before applying for a patent because the cost of maintaining the validity of a patent is. n. al. er. very expensive. In 2000, it costs a US patent owner to pay in between $10,000USD to. i n U. v. $30,000USD for obtaining and maintaining a patent depending on the claim numbers 11. listed on the patent .. Ch. engchi. 9. Utility model, also known as “petty patent” or “utility innovation”, registration of utility model are usually less strict because it required lower threshold of inventive steps compared to invention patent. Also, the registration is faster. Utility models are considered more suitable to SMEs to make “minor” modification on the current invention. Only a small but significant number of countries and regions provide the option of utility model protection. For more information, see http://www.wipo.int/sme/en/ip_business/utility_models/utility_models.htm 10 Types of Patents (n.d.), United States Patent and Trademark Office, Retrieved from http://www.uspto.gov/web/offices/ac/ido/oeip/taf/patdesc.htm 11 Lemley, M.A., Rational Ignorance at the Patent Office (February 2001). Northwestern University Law Review, Vol. 95, No. 4, 2001.. 12.
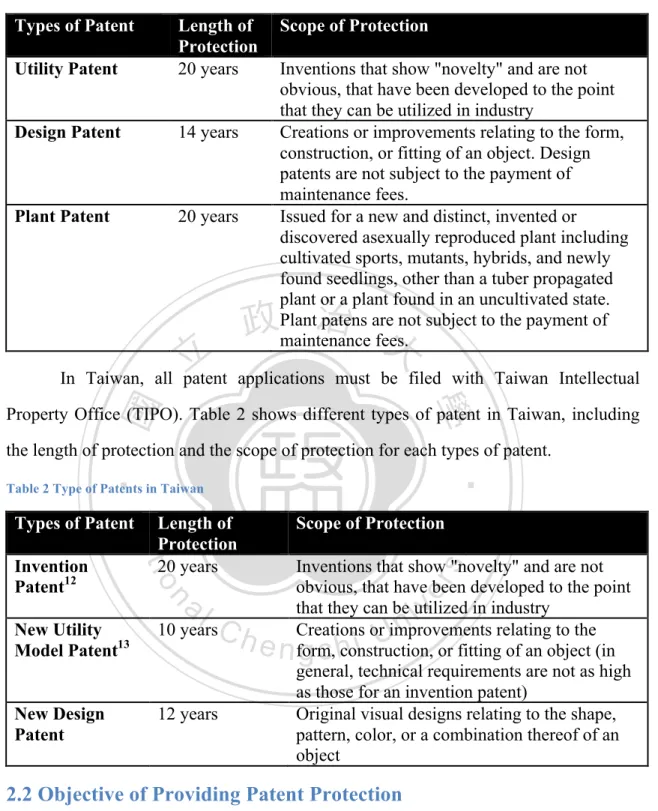
(21) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH Table 1 Types of Patents in The United States. Types of Patent Utility Patent. Length of Protection 20 years. Design Patent. 14 years. Plant Patent. 20 years. Scope of Protection Inventions that show "novelty" and are not obvious, that have been developed to the point that they can be utilized in industry Creations or improvements relating to the form, construction, or fitting of an object. Design patents are not subject to the payment of maintenance fees. Issued for a new and distinct, invented or discovered asexually reproduced plant including cultivated sports, mutants, hybrids, and newly found seedlings, other than a tuber propagated plant or a plant found in an uncultivated state. Plant patens are not subject to the payment of maintenance fees.. 政 治 大. 立立. In Taiwan, all patent applications must be filed with Taiwan Intellectual. •‧ 國. ㈻㊫學. Property Office (TIPO). Table 2 shows different types of patent in Taiwan, including the length of protection and the scope of protection for each types of patent.. n. al. sit. Scope of Protection. Inventions that show "novelty" and are not obvious, that have been developed to the point that they can be utilized in industry Creations or improvements relating to the form, construction, or fitting of an object (in general, technical requirements are not as high as those for an invention patent) Original visual designs relating to the shape, pattern, color, or a combination thereof of an object. er. io. Invention Patent12. Length of Protection 20 years. Nat. Types of Patent. y. •‧. Table 2 Type of Patents in Taiwan. Ch. New Utility Model Patent13. 10 years. New Design Patent. 12 years. engchi. i n U. v. 2.2 Objective of Providing Patent Protection Under the World Trade Organization’s (WTO) Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPs), every WTO member states should made patents available to its citizens for any invention, in all fields of technology, and 12 13. 發明專利 新型專利. 13.
(22) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. the term of protection available should be a minimum of twenty years14. Invention is given protection for a period of time, offering market exclusivity and economy monopoly to the inventor, in exchange inventor has to disclose and share the invention to the public. The protection offered by a patent exclusivity allows patent owner to pay off the associated research and development cost incurred during invention. An inventor has to disclose the “best-mode” of their invention to the public in exchange of getting an exclusivity protection. Pubic disclosure of an invention could help improving the world’s technology because everyone in the world will have the access to the disclosed invention and scientist could perform scientific research based. 政 治 大 to the public allows the probability of repeated R&D, thus could avoid spending 立立. on the findings and foundation laid by the people before them. Sharing new invention. unnecessary research funding.. •‧ 國. ㈻㊫學. Besides that, WIPO treaty is premised on the notion that protecting intellectual property right could effectively maintain economic growth. The WIPO Intellectual. •‧. Property Handbook gives two reasons for intellectual property laws:. y. Nat. sit. One is to give statutory expression to the moral and economic rights of creators. n. al. er. io. in their creations and the rights of the public in access to those creations. The. i n U. v. second is to promote, as a deliberate act of Government policy, creativity and. Ch. engchi. the dissemination and application of its results and to encourage fair trading which would contribute to economic and social development15. In 1996, being aware of the heavy reliance of OECD economies on knowledge and information, the OECD founded by 34 economic bodies has published a report on the “Knowledge Based Economy”. The truth that the export of knowledge-intense hightechnology products have doubled in late 1990s compared to 1970s indicated the arrival of knowledge-based economy. It is estimated that more than 50% of Gross Domestic. 14. Article 27.1, TRIPs Agreement. WIPO Intellectual Property Handbook (n.d.), p.3, World Intellectual Property Organization, Retrieved from http://www.wipo.int/export/sites/www/about-ip/en/iprm/pdf/ch1.pdf 15. 14.
(23) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Product (GDP) in the major OECD economies is now knowledge-based16. Besides that, research from WIPO and United Nations University measuring the impact of IP on six Asian countries found "a positive correlation between the strengthening of the IP system and subsequent economic growth17". A patent is just a worthless paper without protection given by a government. Shall a government strictly enforce intellectual property rights protection, it would give the inventor faith and security to safely invent and disclose their invention because they knew they could seek for legal remedy shall their patent been infringed. Thus, patent system not only could promote scientific improvement, but also contribute to economic. 政 治 大 2.2.2 PCT- Patent Cooperation Treaty 立立 and social development.. •‧ 國. ㈻㊫學. Patent Cooperation Treaty, frequently known as PCT is a treaty that allows applicant to seek for patent protection internationally and helps patent offices in 148. •‧. countries around the world with their patent granting decision. Besides that, PCT aims. sit. y. Nat. to facilitate public access to information relating to those inventions18.. io. er. PCT is created under an international patent law treaty in The Washington Diplomatic Conference and was signed on 19 June 1970. Any contracting state of the. n. al. Ch. i n U. v. Paris Convention for the Protection of Industrial Property could be a member of the. engchi. PCT. It provides a unified procedure for filing patent application among member states. Almost every major country in the world is a member of the PCT, with a few exceptions including Taiwan, Argentina and Macau19. Patent filed under the PCT is known as International Application, or PCT application, because it is not equivalent to a grant of a patent. There is no such thing known as “international patent” because the power of granting a patent remains under 16. The Knowledge Based Economy (1996), Organization of Economic Co-operation and Development, Retrieved from http://www.oecd.org/sti/sci-tech/1913021.pdf 17 Measuring the Economic Impact of IP Systems (2007), World Intellectual Property Organization, Retrieved from http://www.wipo.int/portal/en/news/2007/article_0032.html 18 PCT – The International Patent System (n.d.), WIPO, Retrieved from http://www.wipo.int/pct/en/ 19 A list of countries not the member of Patent Cooperation Treaty could be found here: http://www.patentideas.com/international-patents/countries-not-members-of-pct.aspx. 15.
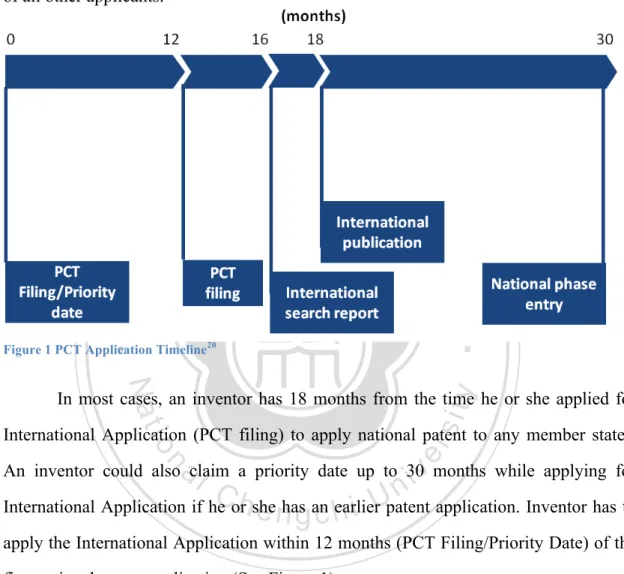
(24) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. control of a nation’s patent office, e.g. USPTO. What PCT offers is the convenience to an inventor for getting priority in a line internationally: It establishes a filing date in all contracting states when an inventor applies to PCT; upon granting a PCT application, the inventor could then apply for patent in each jurisdiction with an earlier date ahead of all other applicants.. 政 治 大. 立立. •‧. •‧ 國. ㈻㊫學. Figure 1 PCT Application Timeline20. y. Nat. sit. In most cases, an inventor has 18 months from the time he or she applied for. n. al. er. io. International Application (PCT filing) to apply national patent to any member states.. i n U. v. An inventor could also claim a priority date up to 30 months while applying for. Ch. engchi. International Application if he or she has an earlier patent application. Inventor has to apply the International Application within 12 months (PCT Filing/Priority Date) of the first national patent application (See Figure 1). International Application should be made in one language with a Receiving Office; Receiving Office could be WIPO Office, or any PCT contracting state’s national patent office. An International Searching Authority would then perform a search and issue a written opinion regarding the patentability of the invention before publishing the International Application. The inventor has an 18 months window to apply to any member states before PCT priority date expires. The granting of patents 20. What is the PCT? (Jun 8, 2011), Hawk IP Dashboard, Retrieved from http://www.hawkip.com/advice/whatis-the-pct. 16.
(25) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. remains under the control of the national or regional patent Offices in what is called the “national phase”21. With the priority date benefits provided by PCT, the 30 months time period is crucial for a technology to develop more maturely and allowing the inventor or patent holder to raise more capital to market their product. Since PCT Application is accompanied with an international preliminary search, it appeared that filing PCT Application would facilitate the national phase patent application process22.. 立立. 政 治 大. •‧. •‧ 國. ㈻㊫學. n. er. io. sit. y. Nat. al. Ch. engchi. 21. i n U. v. PCT – The International Patent System, WIPO, Retrieved from http://www.wipo.int/pct/en/ Oppendahl, C. (June 1999), Filing A PCT Application May Lead To A Faster, Cheaper US Patent, Oppedahl & Olson LLP, Retrieved from http://www.oppedahl.com/pubs/pctfaster.htm 22. 17.
(26) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Chapter 3: Introduction to Technology Licensing 3.1 Background Technology transfer (TT), is a process of transferring a knowledge, skills, manufacturing methods, or a product of intense knowledge investment between research institutes and business corporates. Technology transfer happened for one main reason: research institute does not have the ability to manufacture a product, or they are unable to commercialize a product by itself. By transferring a technology to a partner that could add synergy to the technology would bring benefits to the society by speeding up the progress technology improvement.. 政 治 大. Nowadays, as an effort to help with the transfer process, most research institutes. 立立. have Technology Transfer Office (TTO), dedicated to identify, examine and promote a. •‧ 國. ㈻㊫學. potentially successful to be sold invention to the business industry. TTO acts as a bridge between inventor and industry by (i) helping inventors to sell the invention; (ii). •‧. helping the industry to look for desired invention; and (iii) teaching the inventor what. y. Nat. the industry needs in the foreseeable future.. sit. There are four main players in a technology transfer: research institutes, small. n. al. er. io. companies23, big product companies24, and big product companies’ research center. In. i n U. v. nature, these players play different roles in technology transfer. Research institutes. Ch. engchi. follow an ideal-thinking tradition, which follow a pattern that is fundamentally different from industry thinking. They get funding from the government and the board, which usually focuses on more fundamental (basic science) research. Basic science research focuses on explaining science to the world, or discovers not-yet existing things, rather than utilize it. A big company research unit would sometimes leverage on research institutes’ resources as much as possible, besides its own research project to optimize and streamline the production of an invention by capturing interesting technology from the 23. Small companies in this study include small and medium enterprises (SMEs). Big product companies in this study generally referring to multinational companies that has a lot of chapters around the world. 24. 18.
(27) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. institutes into the company’s business units. For smaller companies that could not afford a research unit, technology usually comes from research institutes. Small companies sometimes collaborate with research institutes to perform joint-research by providing funding or manpower. A research is preferred to be in a mature state and be able to fit into the receiver’s culture and business plan in order to be considered as a potential transfer target at very first place. Shall a technology could replace obsolete technology, increase efficiency, attract customers and engineers, and then it is more likely to be reviewed by technology receiver. When it comes to commercialization, commercial value is the only. 政 治 大 Technology transfer happens in a few ways, but all the aims are the same – to 立立. thing to be considered.. exploit the commercial value behind the invention. It could involve signing licensing. •‧ 國. ㈻㊫學. agreement, transferring the right of invention, setting up joint ventures, establishing partnership, or spinning off a new company to commercialize. In this study, the author. •‧. focuses mainly on technology licensing. However, prior to technology licensing,. Nat. sit. y. business corporates shall perform very detailed research and analysis on the to-be-. al. er. io. acquired technology to avoid ended with scam technology or technology that does not. v. n. fit with the company’s expectation through a process known as “Due Diligence”.. 3.2 Due Diligence. Ch. engchi. i n U. Due diligence is a term used to describe a number of concepts, including investigation of a business, or natural person prior to signing a contract. It could be a legal obligation under certain legislation, but more often it applies to voluntary investigations. A common example of due diligence is the process of which a potential acquirer evaluates a target company’s assets during a merger and acquisition transaction. Nowadays, more and more companies are highly aware about the value of IP, as it could act as an asset to expand their business, raise capital or to increase the 19.
(28) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. company’s gain, because IP has begun to be included into a company’s balance sheet to give their investor an idea of what the company owns. According to a report dated on Feb-12, 2013 in Intellectual Property Watch written by Kelly Burke, the median price paid for US patents sales was US$ 221,000, while the average price paid was US$ 374,000 in 201225. Before proceeding to acquire, or licensing a technology from a research organization, companies need to make sure that the patent is worth the price being paid. The cost to conduct a proper due diligence is not too high, however the process of gathering sufficient information is often painstakingly time consuming, and thus. 政 治 大 prefer not to disclose too much information on the subject invention, as a measurement 立立. might incur more cost to the due diligence process. Technology seller (TTO) often. to protect its own benefit and increase its leverage on technology buyer during. •‧ 國. ㈻㊫學. transaction. Disclosing too much information might expose the weakness of the subject invention, or narrows the Zone of Possible Agreement (ZOPA) during licensing. •‧. contract negotiation. Disclosing sufficient information to the public, enough to attract. Nat. sit. y. potential candidates, but not too much to weaken the position of TTO is an art, of. er. io. which is not easy to master. Thus, the cost of information gathering might be very high. al. v i n Ch “transparent government” administration, government agencies are required to e n gofcwhich hi U n. for technology buyer. However, more and more governments are moving into a. disclose government-held information with some exceptions. Government agencies are bound by law (Freedom of Information Act in the U.S. and Freedom of Government Information Law in Taiwan) to publish information publicly and promote openness. This initiative move the burden of proof of the party asking for information (technology buyer) to the government body (TTO), reducing cost and time of acquiring most technology-related information that is crucial for due diligence process.. 25. Kelly Burke (12 Feb, 2013), Report Finds Average US Patent Cost US$ 374,000 In 2012, Intellectual Property Watch, Retrieved from http://www.ip-watch.org/2013/02/12/first-patent-value-quotient-report-showsaverage-price-paid-for-issued-us-patents/?utm_source=post&utm_medium=email&utm_campaign=alerts. 20.
(29) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. There are a couple of reasons for a business corporates to spend huge amount of money to obtain a technology from others. The technology acquirer might be looking at i) a technology that complements with its current business; ii) a technology that could brings synergy to its current business; iii) a technology that could stop its competitor from competing with its current business; iv) a technology which the acquirer needs in order to start a new business; or v) a technology which could help the company out of an ongoing or potential litigation. That is why it is very important for a technology acquirer to understand the to-be-acquired patent thoroughly. Following shows the importance of patent due diligence:. 治 政 大details allow acquirer to judge if Understanding the potential technology in 立立 the technology fits the aim of the transfer. Due diligence could reveal more. 1. It allows the acquirer to understand the technology in details. •‧ 國. ㈻㊫學. information, restriction, possible application and limitation of the technology. A mature and ready to market technology usually value more. •‧. than an immature invention.. sit. y. Nat. 2. It allows the acquirer to understand the scope and limitation of the patent By understanding the claim, scope, length and the strength of the patent, it. io. n. al. er. gives the acquirer an overview of the protection a patent could provide. A. Ch. i n U. v. broader claim could provide more protection while at the same time risk. engchi. being invalidated by opponent during litigation. Technology or invention, which was applied as patents in more than a country, could gain multiple monopoly market access to acquirer. A patent with multiple claims, of which some of the claim might not be valuable to the acquirer might cost the acquirer excess money in maintenance. Since obtaining a patent does not means one could practice the invention, it is also very important to understand the freedom to operate an invention. 3. It gives the acquirer the fair market value of the patent Due diligence reveals the fair market value to the acquirer, allowing the acquirer to decide how much to offer during licensing transaction.. 21.
(30) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. A proper due diligence could reduce the cost of transaction for the technology acquirer due to the asymmetric information both parties pertained. The discovery of some gaps or defects of a patent allows the acquirer to be aware of its risk before investing in the technology licensing process.. 3.3 Technology Transfer: Licensing After making a proper due diligence, a technology will be ready to be transferred from a research state to a commercial stage. Licensing is one of the most common ways to transfer technology from research institutes to business organizations. According to an annual research done by Association of University Technology. 政 治 大. Managers (AUTM), there are 5,130 licenses being executed in FY 2012 among its. 立立. survey targets in the U.S. with a 9.5% increase compared to FY 2011. While the. •‧ 國. ㈻㊫學. income received in royalty marked a 6.8% increase, reaching USD$ 2.6 billion26. Usually business corporates enter into licensing agreement with research. •‧. organization to utilize its intellectual property right (usually patent). There are two. sit. y. Nat. types of licensing based on the scope of the license, namely Exclusive Licensing and. io. er. Non-Exclusive Licensing. Exclusive licensing limits licensor to license its patent to a licensee at a specific period of time and specific location. The word “exclusive” does. n. al. Ch. i n U. v. not mean “one and only” license granted, but that the licensor agrees not to grant other. engchi. licensees the same rights within the scope or field covered by the first exclusive license. Once a patent is entered into an exclusive license contract, patent owner can no longer utilize the patent or license it to a third party within the said period of time and location. Depending on the licensing contract, the licensee could utilize, manufacture, sell, import, sublicense or exclude others from practicing the patent during litigation. A licensor could issue any number of exclusive licenses with different rights in the same scope or licenses with the same rights in different scopes. 26. Association of University Technology Manager (2012). AUTM U.S. Licensing Activity Survey FY2012 Highlights. Retrieved from http://www.autm.net/AM/Template.cfm?Section=FY2012_Licensing_Activity_Survey&Template=/CM/Conten tDisplay.cfm&ContentID=11435. 22.
(31) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. In non-exclusive licensing, the licensor could enter into as many non-exclusive licenses as he or she wishes with different licensees. All licensees, including the patent owner, could practice the patent legally without infringing other licensee’s right. In summary, you get to practice the invention but the owner can let someone else utilize the invention too.. 3.4 The Process of Technology Licensing Licensing a novel technology from an inventor in academic institute to business sector for practice and selling is a very delicate and long process. A lot of factors will have to be taken into consideration and more than one parties have to take part in a technology licensing process.. 立立. 政 治 大. In a buy and sell market, there are always two parties in a transaction: Demand. •‧ 國. ㈻㊫學. Party (buyer) and Supply Party (seller). The supply party in a technology commercialization process in this study is public research institute (NIH or NHRI) that. •‧. could constantly provide quality inventions that were patented. While the demand party. sit. y. Nat. in this study would be the industries that are interested in licensing in quality and. io. er. protected inventions. Technology Transfer Office (TTO) is the party connecting both supply and demand party in the transaction. TTO acts like a broker in a real estate. n. al. Ch. i n U. v. transaction: matchmaking both buyer and seller, providing legal consultation or service,. engchi. acting as quality assurance referees and etc.. In reality, more than the abovementioned three parties are responsible for the success of technology commercialization process, for example government policies or legislative regulations are among the most important influence factors in technology licensing. Following is a timeframe of technology commercialization and factors influencing the success of licensing.. 23.
(32) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Institute's Technology Transfer Of?ice (Matchmaker) Supply: Research Insistute 1. Freedom of Information Act allows Demand: Industry 1. Bayh-‐Dole Act allows ownership to be allocated to inventor. 2. The role of research institute set forth in Executive Order de?ines institute's position and strategy in licensing. . access to information freely by industry. 2. Sales and marketing technique is crucial in technology transfer. . 1. Locally Manufacture clause limits foreign market sales. 2. Market size or demand might in?luence the price and thus the success of licensing. . 政 治 大 As shown on Figure 2, there are main three parties, which are involved in 立立. Figure 2 Timeframe of Technology Commercialization and Factors Influencing Licensing Success. technology licensing process. The Bayh-Dole Act or Fundamental Science and. •‧ 國. ㈻㊫學. Technology Act (discussed later in this chapter) allows the university to obtain the. •‧. ownership of an invention, which then allows the university to license it to the industry according to the law. TTO, acting as the matchmaker, is the main player in the. Nat. sit. y. technology licensing process. The Freedom of Information Act (FOIA), which will be. er. io. discussed in Chapter 4, requires TTO to disclose all information related to the institute. al. v i n C h disclose licensing-related performance. The requirement to information is crucial for engchi U n. to the general public as an effort to allow public to supervise and inspect government. the licensing process, as technology buyer will need to acquire related information set forth in Bayh-Dole Act and Fundamental Science and Technology Act, could also tamper the success of technology transfer with a foreign entity.. 24.
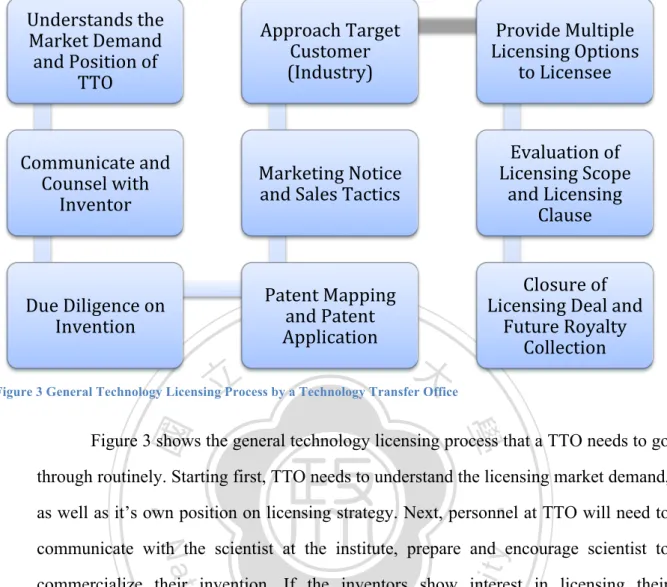
(33) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. Understands the Market Demand and Position of TTO . Approach Target Customer (Industry) . Provide Multiple Licensing Options to Licensee . Communicate and Counsel with Inventor . Marketing Notice and Sales Tactics . Evaluation of Licensing Scope and Licensing Clause . Due Diligence on Invention . Patent Mapping and Patent Application . Closure of Licensing Deal and Future Royalty Collection . 立立. 政 治 大. Figure 3 General Technology Licensing Process by a Technology Transfer Office. •‧ 國. ㈻㊫學. Figure 3 shows the general technology licensing process that a TTO needs to go through routinely. Starting first, TTO needs to understand the licensing market demand,. •‧. as well as it’s own position on licensing strategy. Next, personnel at TTO will need to. sit. y. Nat. communicate with the scientist at the institute, prepare and encourage scientist to. io. er. commercialize their invention. If the inventors show interest in licensing their invention, TTO shall perform due diligence investigation on the invention to avoid. al. n. v i n C hTTO will have toUgive advice and counseling to the “me-too!” product. If possible, engchi inventor about the need of the industry, allowing the inventor to make modification on its invention to better suit the industry demand. Proper patent mapping to figure out the potential of an invention and patent application are required next to give protection to the invention, TTO will decide if an invention should apply for patent in which countries. When an invention is ready to seek for potential licensee, TTO will draft a “notice for licensing” document, disclosing sufficient information of the invention to the public but not too much information to avoid negative impact on marketing. The notice to license will be placed online to be browsed by interested licensee or to be sent to targeted/potential licensee familiar to TTO. Before the closure of licensing deal,. 25.
(34) Research to Increase NHRI Licensing Performance by Comparing The Licensing Practice of NIH. TTO will have to have in-depth discussion with potential licensee, to negotiate the best licensing deal for both parties.. 3.5 US Legislation: Bayh-Dole Act In 1980, Birch Bayh and Bob Dole in the United States sponsored the Patent and Trademark Law Amendment Act, always known as the Bayh-Dole Act and was signed it into the law in December 12, 1980. The act was initially known as Patent and Trademark Law Amendment Act or The University and Small Business Patent Procedure Act 27 . The Bayh-Dole Act is codified in 35 U.S.C. §200-212 and is implemented by 37 C.F.R. 401.. 政 治 大. The key change of the law was the allocation of ownership of a federally funded. 立立. invention. Before the Bayh-Dole Act, federal research funding grants and contracts. •‧ 國. ㈻㊫學. require the inventor to assign the invention back to the federal government. Bayh-Dole Act allows a university, small business enterprise and non-profit organization the right. •‧. to retain ownership in preference to the government. If an organization does not elect to. sit. y. Nat. retain the title of the ownership, then the control of the invention goes back to the. io. er. government. However, the government could waive its right to take the invention, and the right goes back to the inventor.. n. al. Ch. i n U. v. The Bayh-Dole Act did two things in one stroke: By transferring the ownership. engchi. of an invention from the government that funded the research to the research institute that had actually carried out the research, it ensures the researcher involved to receive part of the revenue derived from the commercialized product. The feeling of being involved will give inventor more incentive to perform a better research in the future. Following are a few contributions Bayh-Dole brings to the society28: 1. Effectively promote the collaboration and interaction between business corporates and research institutes;. 27. 王偉霖、劉江彬(2010),p105,國際技術轉移制度理論與實務-兼論台灣立法與產學研因應之策略, 華泰文化。 28 王偉霖、劉江彬(2010),p106, 國際技術轉移制度理論與實務-兼論台灣立法與產學研因應之策略 , 華泰文化。. 26.
數據

+7
Outline
Introduction
Objective of Providing Patent Protection
Due Diligence
US Legislation: Bayh-Dole Act
Locally Manufacture Clause
Executive Orders and Source of Funding
NIH OTT Effort to Increase Licensing Activity to Serve Global Public Health
Overview of Licensing Process at NHRI
Comparison of Licensing Performance
Freedom to Access Information
相關文件
最新的權威性的美國市調公司─鮑爾市場研究公司 J.D.Power. 1)
巴斯德研究院(法語:Institut Pasteur)總部位於巴黎,是法國的一個私立的非營利研究 中心,致力於生物學、微生物學、疾病和疫苗的相關研究,其創建者巴斯德於
屏東科技大學森林系陳美惠教授帶領的社區林業研究團隊長期深 耕,陪伴墾丁國家公園(台 26 線)及屏北原鄉部落(台 24 線),建立森 林與部落的生態旅遊推動模式,研究成果於 2008
學博士,現為上海大學文學院教 授,兼任佛光山人間佛教研究院
認為它注重對四大師的研究而忽視支援這些大師布教活動的庶民之信仰的研 究。[13]
研究與發展(research and development, R&D) 係指進步的 科學知識或產品(與製程)之創新而言[23]。依據美國國家科學基 金會的報告,顯示在最近
而隨著道路之持續開發,隨之而來的大量環境破壞則成為促進道路生 態學發展的推手。歐美國家自 1920 年開始積極推動有關道路生態之 研究,藉以保護自然環境中之大型哺乳動物。表
中華大學應用數學研究所 研 究 生:黃仁性 敬啟 指導教授:楊錦章 教授 中華民國九十六年..