行政院國家科學委員會專題研究計畫 期中進度報告
以毒素及毒素/蛋白水解酵素複合物的結晶構造為基礎對蛇
毒毒素作結構─功能的分析(2/3)
計畫類別: 個別型計畫 計畫編號: NSC92-2311-B-002-107- 執行期間: 92 年 08 月 01 日至 93 年 07 月 31 日 執行單位: 國立臺灣大學生化科學研究所 計畫主持人: 邱式鴻 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 93 年 5 月 31 日
行政院國家科學委員會補助研究成果報告
計畫類別:■ 個別型計畫 □ 整合型計畫
計劃名稱:以毒素及毒素/蛋白水解酵素複合物的結晶構造為基礎對蛇
毒毒素作結構—功能的分析(2/3)。
計劃編號:NSC92-2311-B-002-107
執行期限:92 年 8 月 1 日至 93 年 7 月 31 日
主持人:邱式鴻 國立台灣大學生化科學研究所
一、中文摘要
蛇毒中的血小板醣蛋白Ib (GPIb) 之結合蛋白通常屬於C類植物凝血素,它們會與 GPIbα 結合於特定的位置且引發不同的血小板效應。在此我們提出一個從台灣 龜 殼 花 純 化 得 的 四 聚 合 血 小 板 凝 集 因 子( 分 子 量 為 121.1 千 道 耳 吞 )--- 稱 為 mucrocetin。雖然 mucrocetin 與 flavocetin 這兩個蛇毒凝血素有極高的序列相似 性 ( 94.6 % ),但卻與 GPIbα 結合於不同的位置。在此 mucrocetin 的晶體結 構已被解出至解析度2.8Å,它呈現一六層甜甜圈狀的有趣晶體堆積。這四個αβ-異質二聚體藉由內部αβ間頭尾相接的雙硫鍵穩定而排列成一獨特的四方環構 造。在進一步細部地比較 mucrocetin 和 flavocetin-A 的結構後,我們可推測在 結合的凹側表面不同的電荷分布可能是造成不同血小板效應的原因。Mucrocetin 結合面上獨特的正電區是主要造成血小板凝集活性的區域,此區由 α 次單元的 賴氨酸 102、108、109、精氨酸 123 以及 β 次單元的賴氨酸 22、102、116 精 氨酸 117 所組成。因此,可想而知地,此有趣之蛇毒因子與 GPIb-IX-V 複合物 結合將成為血小板凝集研究中一有效的工具。二、英文摘要
Platelet glycoprotein Ib-binding proteins (GPIb-BPs) from snake venoms are usually C-type lectins, which target on specific sites of GPIb α and elicit distinct effects on platelets. Here, we report a tetrameric platelet-agglutinating factor (Mr = 121.1 kDa), termed mucrocetin, purified from the venom of Taiwan habu (Trimeresurus
mucrosquamatus). Mucrocetin is a GPIb agonist with a distinct binding site from that
sequence identity (94.6%) between the two venom lectins. The crystal structure of mucrocetin was solved and refined to 2.8 Å resolution, which shows an interesting crystal packing of six-layer cylinders of doughnut-shaped molecules. The four αβ -heterodimers are arranged in an unusual square-shaped ring stabilized by four inter-dimer “head-to-tail” disulfide bridges. Detailed structural comparison between mucrocetin and flavocetin-A suggests that their disparate platelet effects are likely attributable to different charge distributions on the putative concave binding surface. A unique positively-charged patch on the binding surface of mucrocetin, formed by Lys 102 , Lys 108 , Lys 109 and Arg 123 in α-subunit coupled with Lys 22 , Lys 102 , Lys 116 and Arg 117 in β -subunit, appears to be the primary determinant of its platelet-agglutinating activity. Conceivably, this interesting venom factor may provide a useful tool to study platelet agglutination by binding to the GP Ib-IX-V complex. Key words: snake venom, lectin-like protein, platelet, glycoprotein Ib, mucrocetin, flavocetin-A.
三、Results and Discussion
Binding of plasma vWf to platelet membrane GP Ib-IX-V complex, with exposure to pathologic shear stress (e.g., stenosed arteries), is an important event in triggering thrombosis in embolic stroke and cardiovascular disease [1]. This binding process can be modified by several exogenous modulators, including the C-type lectin-like venom proteins. To date, a number of GPIb-BPs have been isolated from the venoms of crotalid and viperid snakes. These venom factors served as useful tools in probing the mechanism of platelet activation and developing a new class of anti-thrombotic agents.
In this paper, we present a GPIb-BP, mucrocetin, isolated from the venom of Taiwan habu (Trimeresurus mucrosquamatus). Mucrocetin has a structural organization almost identical to those of FL-A and convulxin [1,2]. The molecular weight of mucrocetin calculated from its amino-acid sequence is 121.1 kDa, smaller than those observed on SDS-PAGE (~135 kDa) and gel-permeation chromatography (150-200 kDa), indicating a low compactness in mucrocetin molecules. This is in agreement with the crystal structure of mucrocetin, i.e., a square flat ring with a large central pore. Mucrocetin eluted from the anion-exchanger column in the last fractions during the initial isolation step may reflect its acidic property. It is consistent with the observed negative charges on the surface of mucrocetin moloecules.
The predicted amino-acid sequence of mucrocetin was confirmed by protein N-terminal sequencing, and the complete sequence is also consistent with its crystal structure, including the distinct residues between mucrocetin and FL-A and those on
the concave binding surface. Sequence alignment and comparison of mucrocetin subunits with those of other snake venom GPIb-BPs show various degrees of identity from 38.5% to 60.7% in α-subunit and 52.0% to 71.2% in β-subunit with 24 and 30 identical residues, respectively, suggesting that the β -subunits are more conservedamong these lectin-like venom proteins. Kawasaki et al. proposed that the GPIb-binding site of venom GPIb-BPs resides on the β -subunit but not the α–subunit. As shown in the structures of mucrocetin and FL-A, the additional Cys 135 and Cys 3 in the α- and β-subunit, respectively, form an inter-dimer “head-to-tail” disulfide linkage. Thus, according to the sequence alignment, agglucetin probably possesses a similar interdimer disulfide bridge as well. Nevertheless, these two Cys residues are not found in the sequence of alboaggregin A, in spite of it oligomeric feature.
Mucrocetin dose-dependently induced platelet agglutinations on both PRP and washed platelets with similar turbidmetric profiles, indicating that vWf is not needed on the action of mucrocetin, distinguishable from what was observed on botrocetin and bitiscetin [3,4]. The inhibition studies, using FL-A and an anti-GPIb mAb on mucrocetin-induced platelet agglutination, suggest that mucrocetin is a GPIb agonist with a distinct binding site from that of FL-A on GPIb α.In addition, pretreatment of PRP with mucrocetin showed no effect on the platelet aggregation when stimulated with ristocetin; whereas, agglucetin, in a similar experiment performed by Wang and Huang [5], appeared to markedly prevent such a ristocetin-induced aggregation.
The crystal structure of mucrocetin shows a unique crystal packing of six-layer cylinders of doughnut-shaped molecules. The overall structure of mucrocetin is almost identical to the structures of FL-A and convulxin, with the αβ -heterodimer exhibiting the typical backbone fold of carbohydrate-recognition domain (CRD) of C-type lectins. The swapped loops in the α β -heterodimer, which are well conserved among the venom GPIb-BPs, are associated with the globular body of the adjacent subunit. The resulting contact interface, corresponding to the C-interface defined in 3D domain swapping, buries ~22.4% of the total surface area of one αβ -heterodimer, thus crucial in connecting the two subunits of mucrocetin. The interface between the αβ-heterodimers is mainly stabilized by interchain disulfide bridges, only with a few non-covalent interactions.
The distinct behaviors of lectin-like venom proteins to recognize different target molecules, such as coagulation factors, vWf and platelet GPIb, are thought to be in part due to the large hingelike motions at the bases of each swapped loop. Only small movements occur when the venom proteins target on similar molecules. Consistently, the backbone chain of mucrocetin superimposes well with that of FL-A, and the relative orientations of the α- and β-subunits of these two proteins are almost
identical. The structure of bitiscetin-vWf A1 complex reported by Maita et al. suggested that a positively-charged patch on the α-subunit of bitiscetin may interact with the C-terminal anionic region of GPIb α. However, this basic patch of bitiscetin was not observed in the structures of mucrocetin and flavocetin-A. In addition, two hydrophilic patches were observed in the structure of FL-A β–subunit, which are considered as possible candidates for the platelet GPIb-binding sites. In the structure of mucrocetin, these two peptide fragments were shown to exist as well. The central part of the concave surface in mucrocetin appears to bear more concentrated positive charges than that of FL-A, though the overall negative charges of mucrocetin structure are slightly greater. Thus, based on these observations, the disparate platelet effects between mucrocetin and FL-A are likely the consequence of different charge distributions on the putative binding surface. Namely, the unique positively-charged patch formed by several Lys and Arg residues on mucrocetin appears to be the primary determinant of the platelet-agglutinating activity of mucrocetin.
The crystal structures of platelet GPIb α and its complexes with vWf A1 domain or thrombin have been published. Three negatively-charged patches on the surface of GPIb α were localized to regions that interact with vWf A1 domain and the exosites I and II of thrombin, respectively. The interacting surfaces between GPIb α and its bound ligands show the heterogeneous charge distributions and reflect striking charge complementarities. These acidic patches on GPIb α may be the candidates of mucrocetin-targeting site. More structural studies of mucrocetin in complex with GPIb α are needed to elucidate the proposed interaction mechanism.
四、References
1. Kai-Fa Huang, Tzu-Ping Ko, Chin-Cjun Hung, John Chu, Andrew H.-J. Wang and Shyh-Horng Chiou (2004) Crystal structure of a platelet-agglutinating factor isolated from the venom of Taiwan habu (Trimeresurus mucrosquamayus). Biochem. J. 378, 399-407.
2. Murakami, M. T., Zela, S. P., Gava, L. M., Michelan-Duarte, S., Cintra, A. C. O. and Arni, R. K. (2003) Crystal structure of the platelet activator convulxin, a disulfide-linked α 4 β 4 cyclic tetramer from the venom of Crotalus durissus
terrificus. Biochem. Biophys. Res. Commun. 310, 478-482.
2. Fukuda, K., Mizuno, H., Atoda, H. and Morita, T. (2000) Crystal structure of flavocetin-A, a platelet glycoprotein Ib-binding protein, reveals a novel cyclic tetramer of C-type lectin-like heterodimers. Biochemistry 39, 1915-1923.
3. Fukuda, K., Doggett, T. A., Bankston, L. A., Cruz, M. A., Diacovo, T. G. and Liddington, R. C. (2002) Structural basis of von Willebrand factor activation bythe snake toxin botrocetin. Structure 10, 943-950.
4. Matsui, T., Hamako, J., Matsushita, T., Nakayama, T., Fujimura, Y. and Titani, K. (2002) Binding site on human von Willebrand factor of bitiscetin, a snake venom-derived platelet aggregation inducer. Biochemistry 41, 7939-7946.
5. Wang, W. J. and Huang, T. F. (2001) A novel tetrameric venom protein, agglucetin from Agkistrodon acutus, acts as a glycoprotein Ib agonist. Thromb. Haemost. 86, 1077-1086.
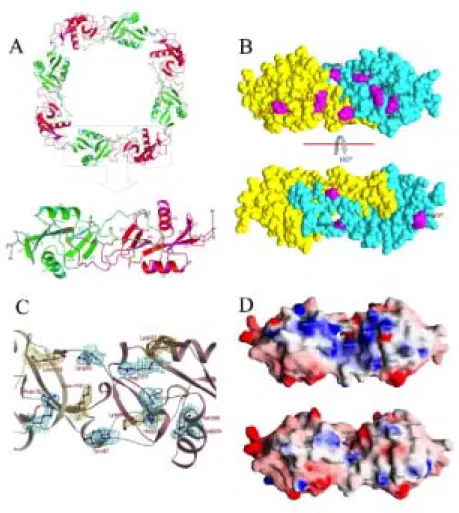
Figure 1. Overall structure of mucrocetin and the concave binding surface on the molecule (A) Four heterodimers of the mucrocetin α (red) and β (green)
subunits are arranged about a four-fold axis as shown with a ribbon diagram. Within a dimer (lower panel), the two subunits are associated by swapping of the loop-domains and a inter-subunit disulfide bridge, shown as ball-and-stick models. There are three other disulfide bonds within each subunit. The heterodimers are further connected by intermolecular, head-to-tail, disulfide bonds to form a circular tetramer. (B) The space-filling model of mucrocetin molecule denotes the substituted residues (magenta) on the surface of mucrocetin as compared with those in flavocetin-A. The mucrocetin α- and β-subunits are painted in yellow and cyan, respectively. (C) The 2Fo – Fc maps (contoured at the 1.0 σ level) of mucrocetin around some residues located at the concave binding surface are presented. The density maps for the substituted residues are drawn in cyan and those for the Lys residues that contribute to the positive charges of the binding surface are shown in yellow. (D) Diagrams of the surface charge potential on the mucrocetin (upper) and flavocetin-A (lower) molecules are shown. The surfaces are drawn in red, white and blue for negative, neutral and positive charges, respectively. The views are facing the concave binding surface on these two molecules.