行政院國家科學委員會專題研究計畫 成果報告
愛滋病毒感染病患肺炎雙球菌疫苗接種後抗體效價反應之
前瞻性研究: 著重在使用抗愛滋病毒藥物使用後之影響
計畫類別: 個別型計畫 計畫編號: NSC93-2314-B-002-086- 執行期間: 93 年 08 月 01 日至 94 年 07 月 31 日 執行單位: 國立臺灣大學醫學院內科 計畫主持人: 洪健清 共同主持人: 張淑媛 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 94 年 8 月 23 日
A Prospective Study of Anti-capsular Antibody Responses to Pneumococcal Vaccination among HIV-infected Persons: Emphasis on Impact of Highly Active Anti-Retroviral Therapy
Chien-Ching Hung1, Sui-Yuan Chang21Department of Internal Medicine, National
Taiwan University Hospital and 2Department of Medical Technology, National
Abstract
A 4-year prospective longitudinal follow-up study was conducted to assess the immunogenicity of 23-valent pneumococcal polysaccharide vaccine (PPV) among 305 HIV-1 infected individuals who received PPV and HAART between June 2000 and June 2004. 89 randomly selected vaccinees were stratified into 3 groups based on their CD4 counts at baseline: Group 1 with CD4 counts less than 100 cells/µl; Group 2 with CD4 counts between 100 and 350 cells/µl; and Group 3 with CD4 counts higher than 350 cells/µl. Antibody responses to 3 capsular antigens (14, 19F, and 23F) were performed using home-made ELISA. Changes in these parameters from baseline to week 4, and every 6 months thereafter were determined and compared using Student’s t-test. Rates of response to vaccination in different groups of vaccinees were compared using χ2 test. Vaccinees with more than 2-fold increase in antibody
responses were defined as vaccine responders. We found that immunization did not cause any significant changes in CD4 counts or plasma HIV-RNA load between vaccinees of different groups. For type 14, both the percentage of responders (27% v.s. 57% v.s. 54%) and the geometric mean fold-rise of reactive antibody (1.38 v.s. 2.89 v.s. 3.05) were significantly lower for Group 1 compared with Groups 2 and 3. The duration of antibody responses in responders was similar between groups. Among the 24 responders who had been followed for more than 3 years, more than 75% of them remained seropositive for type 14 irrespective of their baseline CD4 counts. Our findings suggest that antibody responses to 23-valent PPV are poorer in HIV-infected vaccinees with baseline CD4 counts less than 100 cells/µl compared with those with baseline CD4 counts higher than 100 cells/µl. The responses may last for more than 3 years in 75% of the vaccine responders who continue to receive HAART regardless of baseline CD4+ counts.
Patients with HIV infection are at higher risk for invasive infections due to
Streptococcus pneumoniae as compared with those without HIV infection. Rates of
invasive pneumococcal infections among AIDS patients may be as high as 100-fold greater than in HIV-seronegative controls [1]. In the era of increasing rates of antimicrobial resistance among isolates of S. pneumoniae worldwide as well as an increased risk for developing invasive pneumococcal infections among HIV-infected patients, the US Centers for Disease Control and Prevention (CDC) has strongly recommended that pneumococcal vaccination be given to patients with HIV infection who are aged ≥2 years [2] and a CD4+ count ≥200 cells/µl [3]. In addition, revaccination is recommended when the CD4+ count increases to ≥200 cells/µl in patients with an initial CD4+ count of <200 cells/µl [3].
However, these recommendations are notwithstanding, clinical studies evaluating the benefits of pneumococcal vaccination among HIV-infected patients have yielded
inconsistent results [4-7]. This inconsistency may be due to variations in the study populations or designs, the receipt of antimicrobial prophylaxis or antiretroviral therapy, or study end points and outcome measures. In retrospective studies conducted in the US [4,6,7], vaccination reduced the risk for invasive pneumococcal infections among HIV-infected patients before or after the introduction of highly active antiretroviral therapy (HAART). In Uganda where HIV-infected patients had no access to antiretroviral therapy (ART) [5], immunization with the 23-valent capsular polysaccharide pneumococcal vaccine (PPV) paradoxically increased the incidence of invasive pneumococcal infections and all-cause pneumonia. The reasons for such failure of pneumococcal vaccination to confer protection against invasive pneumococcal infections in Ugandan subjects remains unclear, although destruction of B-cell function resulting from polysaccharide vaccination in those patients without HAART or an increase of plasma HIV load has been proposed [5]. There remain several potential explanations for the failure of the immunity of HIV-infected patients to protect them from pneumococcal infections, including decreased serum opsonic activity against S. pneumoniae in HIV-infected patients [8], and a local effect of altered pulmonary immunoglobulin responses to penumococcal infection [9].
In the previous study [10], we have evaluated the impact of vaccination with the 23-valent PPV on HIV-infected patients who were receiving HAART. Our data suggested that vaccination with 23-valent PPV reduced risks for pneumococcal diseases and was associated with a lower risk for death among HIV-1-infected patients receiving HAART. Vaccination did not increase the risks of all-cause community-acquired pneumonia and HIV progression, and CD4+ continued to increase and PVL decrease among HIV-infected patients receiving HAART. Despite the clinical benefits, the serologic responses to the 23-valent PPV has rarely been studied among HIV-infected patients receiving HAART; whether antibody responses increase with immune reconstitution and when the booster vaccination should be administered in HIV-infected patients receiving HAART remain to be studied. In this study, we aim to evaluate the serial changes of anti-capsular antibodies in HIV-infected patients who underwent vaccination with 23-valent PPV and received HAART with increase of CD4+ counts.
Materials and Methods Patients
Between June 2000 and June 2003, 350 HIV-infected patients who were followed at the National Taiwan University Hospital were immunized with the 23-valent PPV following the recommendations of the CDC of the US. Sequential blood specimens were collected while the patients returned for routine examinations for plasma viral load and CD4+ lymphocyte count every 4-6 months. The blood specimens were stored at -70℃ until determinations of anti-capsular antibody titers are performed. As of December 2004, it was estimated that the average number of blood specimens collected from each subject is 9. We will continue to collect blood specimens for routine PVL and CD4+ testing to determine anti-capsular antibody titers over the next one year. In addition, we will give booster vaccination to those whose CD4 count was 100 cells/µl or less at enrollment and to those who responded poorly to pneumococcal vaccination. The study is approved by the Institutional Review Board of the hospital, and every vaccinee gave written informed consent.
Based on the CD4+ lymphocyte count within 3 months of pneumococcal vaccination, three categories of patients are defined: vaccinees with CD4+<100 cells/µl; vaccinees with 100-350 cells/µl; and vaccinees with ≥350 cells/µl. The patients who have been followed for more than 5 years and respond poorly, booster vaccination will be administered. In each category of CD4+ lymphocyte count, serial blood specimens collected from each patient will be subjected to determinations of anti-capsular antibodies. Patients will continue their routine follow-up at the outpatient clinics for antiretroviral therapy. Respiratory or other relevant clinical specimens will be collected for bacteriologic studies if patients present with symptoms suggestive of infections. Radiographic studies and prescription of antibacterials are at the discretion of treating physicians.
Sera
Sera obtained before and after vaccination of HIV infected individuals with 23-valent pneumococcal vaccine will be separated from clotted blood by centrifugation and stored at –70℃. Before ELISA assay, sera will be adsorbed with cell wall polysaccharide (CWPS). Briefly, 1 ml of serum is mixed with 10 µg of CWPS and incubated at room temperature on a rocking platform for 30 min. Sera from patients with AIDS who had no antibody to S. pneumoniae serotypes 14, 19F, 23F, or 6B capsules and <1μg of antibody to CWPS per ml were used as negative controls.
Determinations of anti-capsular antibody titers
The determination of anti-capsular antibody levels among serially collected blood specimens from each enrolled vaccinee will be carried out with ELISA by following the methods described previously with minor modifications [11-13]. Capsular polysaccharide from S. pneumoniae serotypes 14, 19F, 23F, or 6B will be obtained from the American Type Culture Collection (ATCC). These capsular polysaccharide or CWPS will be suspended in phosphate-buffered saline (PBS, pH7.4) at concentration of 12.5μg/ml and used directly to coat wells by incubation at 4℃ overnight. After washing, blocking will be done with PBS containing 1% of bovine serum albumin at 4℃ overnight. Duplicate samples of sera will be studied in 1:100,
1:300, and 1:900 dilutions, a laboratory reference standard for each serotype that contains known amount of IgG reactive with specific capsular polysaccharide will be included in each plate as a positive control. Following washing, this first antibody incubation will be performed at 37℃ for 2h. After thorough washing of unbounded antibodies to wells, Horseradish Peroxidase (HRP)-conjugated goat antibody to human IgG at 1:2000 dilution will be used to detect IgG, and the reaction will be developed 10 min. at dark by addition of K-blue substrate, followed by adding 1N sulfuric acid to stop the reaction. All washings between each incubation will be done with PBS buffer containing 0.05% Tween20. Optical density will be read in an ELISA reader at 450 nm, with subtraction of optical density of the appropriate blank.
Results
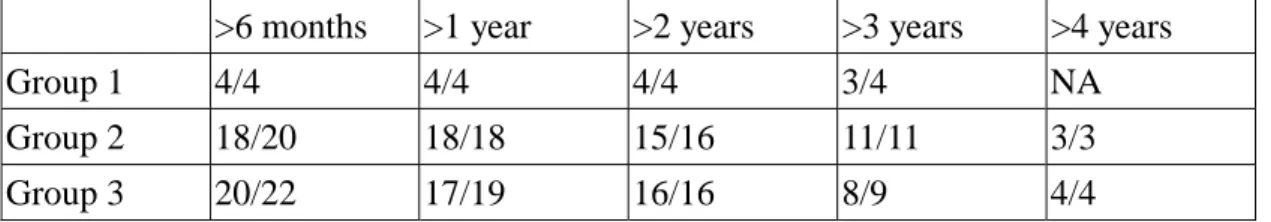
Baseline characteristics of the 89 subjects undergoing pneumococcal vaccination and serial determinations of anti-capsular antibody titers are shown in Table 1. Antibody responses to 23-valent PPV are poorer in HIV-infected vaccinees with baseline CD4+ counts of 100 cells/µl or less compared with those with baseline CD4+ counts higher than 100 cells/µl. The percentage of responders to PPS 14 was higher than those to PPS 19F or 23F (52% vs. 25% vs. 10%). (Table 2). For PPS 14, both the percentage of responders (27% vs. 57% vs. 54%) and the geometric mean fold-rise of reactive antibody (1.38 vs. 2.89 vs. 3.05) were significantly lower in Group 1 compared with Groups 2 and 3 (Table 3). The duration of antibody response was similar between groups (Table 4). Among the 24 responders who had been followed up for more than 3 years, more than 75% of them remained seropositive for PPS 14 irrespective of their baseline CD4+ counts. The responses may last for more than 3 years in 75% of the observed responders who continue to receive HAART regardless of baseline CD4+ counts (Table 4).
References
1. Redd SC, Rutherford III GW, Sande MA, et al. The role of human immunodeficiency virus infection in pneumococcal bacteremia in San Francisco residents. J Infect Dis 1990;162:1012-1017.
2. Centers for Disease control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practice (ACIP).
MMWR Morb Mortal Wkly Rep 1997;46 (RR-8):1-25.
3. 2002 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. USPHS/IDSA Prevention of Opportunistic Infections Working Group. MMWR Morb Mortal Wkly Rep 2002;51:1-64.
4. Gebo KA, Moore RD, Keruly JC, Chaisson RE. Risk factors for pneumococcal disease in human immunodeficiency virus-infected patients. J Infect Dis 1996;173:857-862.
5. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infceted Ugandan adults: double-blind, randomized and placebo controlled trial. Lancet 2000;355:2106-2111.
6. Breiman RF, Keller DW, Phelan MA, et al. Evaluation of effectiveness of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected patients. Arch Intern Med 2000;160:2633-2638.
7. Dworkin MS, Ward JW, Hanson DL, et al. Pneumococcal disease among human immunodeficiency virus-infected persons: incidence, risk factors, and impact of vaccination. Clin Infect Dis 2001;32:794-800.
8. Hidehiko Takahashi, Kazunori Oishi, Hiroyuki Yoshimine, et al. Decreased serum opsonic activity against Streptococcus pneumoniae in human immunodeficiency virus–infected ugandan adults. Clin Infect Dis 2003;37:1534-1540.
9. Stephen B. Gordon, David E. Miller, Richard B. Day, et al. Pulmonary immunoglobulin responses to Streptococcus pneumoniae are altered but not reduced in human immunodeficiency virus–infected Malawian adults. J Infect Dis 2003;188:666-670.
10. Hung CC, Chen MY, Hsieh SM, Hsiao CF, Sheng WH, Chang SC: Clinical experience of the 23-valent capsular polysaccharide pneumococcal vaccination in HIV-1-infected patients receiving highly active antiretroviral therapy: a 2-year prospective observational study. Vaccine 2004;22:2006-12.
11. Musher, D.M., D.A. Watson, and R.E. Baughn. Does naturally acquired IgG antibody to cell wall polysaccharide protect human subjects against pneumococcal infection? J Infect Dis 1990;161:736-740.
Pneumococcal polysaccharide vaccine in young adults and older bronchitics: determination of IgG responses by ELISA and effect of adsorption of serum with nontype specific cell wall polysaccharide. J Infect Dis 1990;161:728-735.
13. Carson PJ, Schut RL, Simpson ML, O’brien J, Janoff EN. Antibody class and subclass responses to pneumococcal polysaccharides following immunization of human immunodeficiency virus-infected patients. J Infect Dis 1995;172:340-345.
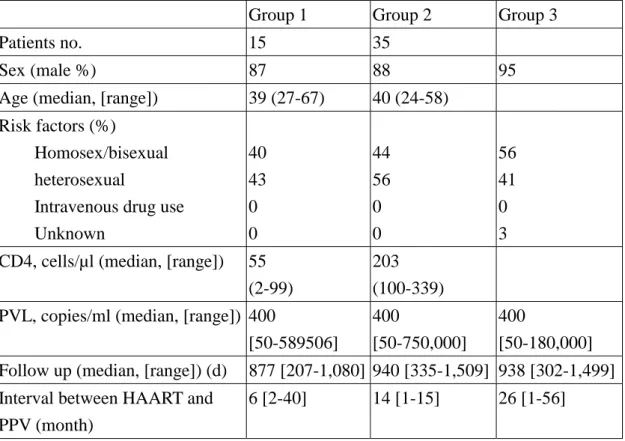
Table 1. Baseline characteristics of the randomly selected 89 HIV-infected patients from 350 HIV-infected vaccines between 2000 and 2003
Group 1 Group 2 Group 3
Patients no. 15 35
Sex (male %) 87 88 95
Age (median, [range]) 39 (27-67) 40 (24-58) Risk factors (%)
Homosex/bisexual heterosexual
Intravenous drug use Unknown 40 43 0 0 44 56 0 0 56 41 0 3 CD4, cells/µl (median, [range]) 55
(2-99)
203 (100-339) PVL, copies/ml (median, [range]) 400
[50-589506] 400
[50-750,000] 400
[50-180,000] Follow up (median, [range]) (d) 877 [207-1,080] 940 [335-1,509] 938 [302-1,499] Interval between HAART and
PPV (month)
6 [2-40] 14 [1-15] 26 [1-56]
Abbreviations: HAART: highly active antiretroviral therapy; PVL: plasma HIV-RNA load by RT-PCR; PPV: pneumococcal vaccination
Table 2. Percentage of responders to 23-valent polysaccharide pneumococcal vaccination Group 1 (CD4<100 cells/µl) Group 2 (CD4, 100-350 cells/µl) Group 3 (CD4≥350 cells/µl) Type 14 27 57* 54* Type 19 20 31 21 Type 23 0 17* 8* Any 47 54 50
NOTE: Responders are defined as persons who become seropositive for specific anti-capsular IgG or have more than 2-folds increase in antibody titer.
*: statistically significantly different (p<0.05) from Group 1 by chi-square test *: statistically significantly different (p<0.05) from Group 1 by chi-square test
Table 4. Duration of antibody responses in responders of different groups
>6 months >1 year >2 years >3 years >4 years
Group 1 4/4 4/4 4/4 3/4 NA
Group 2 18/20 18/18 15/16 11/11 3/3
Group 3 20/22 17/19 16/16 8/9 4/4
Numbers of observed responders with reactive antibody responses/numbers of responders observed
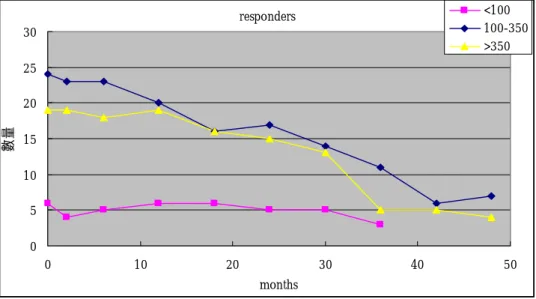
Figure 1. Number of subjects undergoing serial determinations of anti-capsular antibody responses following pneumocccal vaccination
responders 0 5 10 15 20 25 30 0 10 20 30 40 50 months 數量 <100 100-350 >350
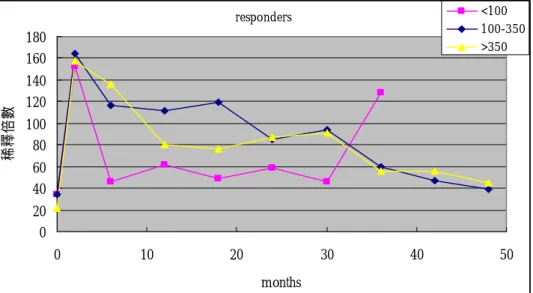
Figure 2. Sequential changes of fold-dilution of anti-capsular antibody following pneumococcal vaccination among vaccine responders with three different categories of CD4 lymphocyte counts. responders 0 20 40 60 80 100 120 140 160 180 0 10 20 30 40 50 months 稀釋 倍 數 <100 100-350 >350