行政院國家科學委員會專題研究計畫 成果報告
Acinetobacter baumannii 對 fluoroquinolones 抗藥性菌
株之分子流行病學分析及抗藥性機轉研究
計畫類別: 個別型計畫 計畫編號: NSC91-2314-B-002-167- 執行期間: 91 年 08 月 01 日至 92 年 07 月 31 日 執行單位: 國立臺灣大學醫學院內科 計畫主持人: 張上淳 計畫參與人員: 盛望徽 報告類型: 精簡報告 處理方式: 本計畫可公開查詢中 華 民 國 92 年 10 月 31 日
I
Fluoroquinolone 抗藥性 Acinetobacter baumannii 菌株之分子流行病學分析及抗藥
性機轉研究 計畫主持人: 張上淳 教授 台大醫院 內科 研究人員: 盛望徽 醫師 台大醫院 內科 研究計畫起迄時間: 2002.8-2003.7 中文計畫摘要 Acinetobacter baumannii 屬於非葡萄糖發酵性革蘭氏陰性桿菌,常存在於自然 界以及醫院內的潮濕環境中,因為其致病力低,故而以往在醫院病患分離時會被 認為是不具臨床意義的移生菌,但是由於近年來侵襲性的檢查治療及免疫抑制劑 的廣泛使用導致高危險群宿主的迅速增加,因而發生 A. baumannii 感染的病例報 告也越來越多,加上 A. baumannii 本身即具有產生多抗藥性之能力,在抗生素的 選擇上尤其困難,導致臨床上得到抗藥性 A. baumannii 感染的病患會伴隨較高的 死亡率且不易治療,甚至常在住院病患間相互傳染而造成群突發(outbreak)。 Fluoroquinolones 是一類以抑制細菌核酸形成的廣效殺菌性抗生素,被認為是對抗 A. baumannii 之有效藥物,但是由於其在人類疾病以及畜牧業的過度使用,致使各 類細菌對於fluoroquinolones 逐漸產生抗藥性,fluoroquinolones 抗藥性的機轉主要 是透過細菌染色體上在複製時之重要target enzymes-DNA gyrase (由基因次單位元 gyr A, gyr B 控制)及 topoisomerase IV (次單位元 par C, par E 控制)的突變而形成, 此一重要的基因序列位於 Escherichia coli 及 Pseudomonas aeruginosa 第 81 至 103 核甘酸位置,稱為quinolone-resistance determining region (QRDR),此段基因突變 會造成fluoroquinolones 之最低抑菌濃度(minimal inhibitory concentrations, MICs)數 倍上昇。雖然學者認為 A. baumannii 之 fluoroquinolones 抗藥性的產生亦與其引發 之QRDR 突變有關,然而相關的証據卻很少。 本計畫以台大醫院1992 年至 2002 年所分離出的 A. baumannii 根據各個時期 作不同FQs 之最低抑菌濃度(MICs)分析,比較 A. baumannii 是否隨著不同年代而 有FQs 抗藥性逐年上升的趨勢,並與 FQs 使用之量是否有關。另外我們亦收集國 家衛生研究院1998 年至 2000 年自臺灣地區 22 家不同醫院所分離之 A. baumannii 菌株,對於具有高抑菌濃度之分離菌株以脈衝式電泳凝膠分子分型(molecular typing by pulsed-field electrophoresis)分析是否存在於某些特定具有高抗藥性菌株 之在醫院內或地區性流行。並且藉由聚合脢連鎖反應(PCR)分析不同醫院內或地區
內的高抑菌濃度之抗藥性菌株,其QRDR 抗藥性突變區之差異,以了解其發生
fluoroquinolones 抗藥性的機轉。
研究結果顯示,在臺大醫院的 A. baumannii 分離菌株,fluoroquinolones 抗藥 性有逐年增加之趨勢,以ciprofloxacin 為例,在 1997 年以前分離之 A. baumannii 對ciprofloxacin 之 MIC50及MIC90分別為0.5µg/mL 及 8µg/mL(MIC 範圍
II
0.06-≥128µg/mL),有 22%為 ciprofloxacin 抗藥性菌株,但至 2002 年 A. baumannii 分離菌株之對ciprofloxacin 之 MIC50及MIC90已上升至2µg/mL 及 64µg/mL,且抗
藥性菌株增加至47%,同時其他之 FQs 類之情況亦同,A. baumannii 一旦對某一 fluoroquinolone 產生抗藥性,也會同時對其他的 fluoroquinolones 產生抗藥性。在 本研究中ciprofloxacin 抗藥性 A. baumannii 同時亦有 100%對 norfloxacin 抗藥,98% 對ofloxacin 抗藥,94%對 levofloxacin 抗藥,93%對 gatifloxacin 抗藥,88%對 moxifloxacin 抗藥,我們亦將臺大醫院過去 6 年來 fluoroquinolones 使用之量作一 調查(1997-2002),發現 fluoroquinolones 的使用量與 fluoroquinolone 抗藥性 A.
baumannii 的增加呈現正相關。從國衛院(NHRI)共收集 1998 年至 2000 年的 A.
baumannii 174 株,其中有 80 株為 fluoroquinolone 抗藥(46%),其 ciprofloxacin MIC50
為2µg/mL,MIC90為及32 µg/mL,範圍為 0.25 至≥128µg/dL,與臺大醫院類似。 Fluoroquinolone 抗藥性 A. baumannii 的分布在各個不同的地區(北、中、南、東區) 似乎並無差別,顯示台灣地區的fluoroquinolone 抗藥性 A. baumannii 是十分嚴重 普遍性存在的問題。 我們將臺大醫院分離出的fluoroquinolone 抗藥性 A. baumanni 及自國衛院收集 的抗藥性菌株作分子電泳派衝分型分析,發現在1992 至 1997 年的抗藥性菌株並 未有群聚(clustering)之情況,但在 1998-1999 以及 2000-2002 年的抗藥性菌株已有 少數群聚之情形,尤其在較高抗藥性(ciproprofloxacin MIC≥64µg/mL)之菌株,其群 聚現象特別明顯,顯示抗藥性菌株的增加,不單只是抗生素使用造成的篩選壓力 導致(selective pressure),院內感染的群突發(outbreaks)造成抗藥性菌株醫院內散播 亦為主因(研究中選取菌株已避開相同時間及相同病房及病患),因此減少抗生素之 使用與同時做好院內感染管制均為控制fluoroquinolone 抗藥性 A. baumanni 菌株散 佈重要的方式。在國衛院以不同地區(北、中、南、東區)劃分的 fluoroquinolone 抗藥性 A. baumannii 菌株發現各個不同區域的醫院之間 fluoroquinolone 高抗藥性 A. baumannii 亦有類似的分型出現,顯示高抗藥性菌株的分布可能隨由病患在不同 的醫院轉診有關,在臺大醫院及國衛院的菌株均發現相同的PFGE 分型也可能有 不同的MIC(由敏感性菌株 0.25µg/mL,低濃度抗藥 2µg/mL 至高濃度抗藥 128µg/mL),表示 fluoroquinolones 的抗藥性可以是一步步經由 fluoroquinolone 藥 物篩選(selection)而產生基因(DNA gyrase)突變增加由低抗藥性而變為高抗藥性。 最後我們亦將33 株 fluoroquinolones 抗藥性 A. baumannii 菌株作 DNA gyrase (gyrA) 及topoisomerase IV (parC)之抗藥性基因(QRDR)分析,並未發現有 gyrA 或 parC 之 基因(gyr A: Gly 81, Ser 83, Ala 84; par C: Ser 80, Glu 84)變化,因此台灣地區
fluoroquinolones 抗藥性 A. baumannii 之抗藥性機轉仍未明,可能與膜蛋白有關, 有待進一步研究。
總而言之,fluoroquinolones 在台灣過度的使用已造成 A. baumannii 抗藥性菌 株逐年增加,在不同fluoroquinolones 之間其抗藥性可能有重疊之現象
(cross-resistance),甚至在新藥如 moxifloxacin(2002 年上市)及 gatifloxacin 還未上 市之前就已存在對此新抗生素之抗藥性 A. baumannii。Fluoroquinolones 抗藥性 A.
III
baumannii 的增加也不單只是抗生素的篩選壓力(selective pressure),未做好院內感
染管制而造成抗藥性細菌散佈亦為原因之一,因此減少不必要的抗生素使用,以 及持續的院內感染管制(如洗手或無菌操作)及抗藥性細菌監測亦為控制
fluoroquinolones 抗藥性 A. baumannii 散佈之重要方式。
關鍵詞:Fluoroquinolones,Acinetobacter baumannii,最低抑菌濃度,聚合脢連鎖 反應 (PCR),脈衝式電泳凝膠分型 (PFGE)
Molecular Epidemiology and Clinical Characteristics of Fluoroquinolones- Resistant Acinetobacter baumannii in Taiwan
Abstract (English)
Acinetobacter baumannii is a new emerging nosocomial pathogen since 1990s. It
is usually highly resistant to various antibiotics and difficult to treat. The yearly surveillance of nosocomial infection at National Taiwan University Hospital (NTUH) showed A. baumannii infections increased from 2% to 5% in last decade.
Fluoroquinolones have once shown good activities against A. baumannii in early 1990s, however, decreased susceptibilities of fluoroquinolones had been reported recently.
In this study, clinical fluoroquinolone-resistant isolates of A. baumannii preserved between 1992 and 2002 at NTUH laboratory and isolates from various hospitals preserved at National Health Research Institute (NHRI) collected during 1998 to 2000 were analyzed. Minimum inhibitory concentrations (MICs) of fluoroquinolones by agar dilution method and pulsed-field gel electrophoresis (PFGE) analysis of FQs-resistant isolates were performed. The MIC50 and MIC90 of ciprofloxacin of A. baumannii
isolated before1997at NTUH were 0.5 µg/mL and 8 µg/mL (range, 0.06-≥128µg/mL) and 22% of the isolates were ciprofloxacin-resistant. However, the MIC50 and MIC90
had elevatedto 2µg/mL and 64µg/mL in 2002 with 47% of isolates were ciprofloxacin-resistant. The same circumstances were similar among other fluoroquinolones. Ciprofloxacin-resistant A. baumannii isolates were also
cross-resistant to other fluoroquinolones frequently; norfloxacin (100%), ofloxacin (98%), levofloxacin (94%), gatifloxacin (93%) and moxifloxacin (88%). Secular surveillance (1997 to 2002) revealed a trend of increasing high-level
fluoroquinolones-resistance (ciprofloxacin) for A. baumannii during recent years and correlated with increasing use of fluoroquinolones. Totally 80 of 174 isolates (46%) of
A. baumannii from NHRI were fluoroquinolones-resistant (ciprofloxacin MICs≧4
IV
gel electrophoresis (PFGE) of these isolates was performed and clonal spread within hospital or between hospitals (region), especially of A. baumannii with high-level FQs-resistance (ciprofloxacin MICs≧64 µg/mL) was noted. The same PFGE pattern of
A. baumannii isolates with different MICs suggested the resistance of fluoroquinolone
were developed step by step. We further investigated the mechanisms of
fluoroquinolone-resistance by analysis the sequence of DNA gyrase (topoisomerase II, gyr A) and topoisomerase IV (par C). No changes of aminoacid (gyr A: Gly 81, Ser 83, Ala 84; par C: Ser 80, Glu 84) of quinolone-resistance determination region (QRDR) of these isolates were detected. The actual mechanisms of fluoroquinolone-resistance in A.
baumannii in Taiwan needed further investigation.
In conclusion, the wide spread use of fluoroquinolones in Taiwan has resulted in the emergence and subsequent increase of fluoroquinolone-resistance, at rates greater than was anticipated. Spreading of high-level fluoroquinolone-resistant clones in recent years compatible with increasing use of fluoroquinolones. It is important that
continuous infection control and prudent use of these agents (selective pressure) be emphasized, not only treatment of human bacterial infections but also veterinary medicine to prevent the emergence and increase of resistant strains.
Key Words: fluoroquinolones, Acinetobacter baumannii, molecular epidemiology,
polymerase chain reaction (PCR), pulsed-field gel electrophoresis (PFGE),
1
Introduction
Non-fermenting gram-negative bacilli-Acinetobacter baumannii, were once regarded as low virulence bacteria [1]. They often colonize the environment and patients in hospital, and occur in mixed culture. As the introduction of
immunocompromised therapy and usage of broad-spectrum antimicrobials in hospitalized patients recently, A. baumannii infections have become much more frequent than before and have been associated with substantial morbidity and mortality [1-3]. The yearly surveillance of nosocomial infection at National Taiwan University Hospital (NTUH) showed A. baumannii infections increased from 2 % to 5 % at the last decade [4]. Choice of antibiotic therapy for the management of A. baumannii infections in the hospitalized patients continues to challenge the clinician because it is usually highly-resistant and difficult to treat. Fluoroquinolones, a family of newly developed quinolones including norfloxacin, ofloxacin, ciprofloxacin, levofloxacin, sparfloxacin, lomefloxacin, moxifloxacin, and gatifloxacin have shown good activities against a variety of gram-negative bacilli including A. baumannii [5]. Although isolates of A.
baumannii had once been demonstrated quite susceptible to fluoroquinolones in early
1990s, rapidly decreased of susceptibility had been reported recently [6, 7]. In our previous study, we represents that there is a high prevalence rate of
fluoroquinolones-resistance in this area [8]. In order to know the epidemiological status and trends of fluoroquinolones-resistant A. baumannii in Taiwan, isolates of A.
baumannii preserved at NTUH laboratory between 1992 and 2002 and the strains at
National Health Research Institute (NHRI) collected from other 22 hospitals during 1998 and 2002 were recruited for analysis.
The specific aims for this project are:
1. To determine the yearly secular changes of FQs-resistance for A. baumannii at a hospital and in Taiwan (NHRI) and the correlation of amount of FQs usage. 2. To determine whether or not there are some dominant epidemiological-related
FQs-resistant A. baumannii strains spread at a hospital (NTUH) and in Taiwan (NHRI).
3. To determine the resistance mechanism of FQs-resistant A. baumannii and if the infection control measures can reduce the opportunity for development of FQs-resistance.
2
Materials and Methods
Clinical Isolates and Data Collections
Clinical strains of A. baumannii preserved at National Taiwan University Hospital (NTUH) between 1992 and 2002 were recruited for testing. The isolates were from various clinical specimens of inpatients at various departments and wards distributed throughout the hospital. No duplicate isolate from the same patient and no strains from a single outbreak were included. Strains of A. baumannii at National Health Research Institute (NHRI) isolated from 22 other hospitals located elsewhere in Taiwan during 1998 to 2000 were also collected for antimicrobial susceptibilities determinations (minimal inhibitory concentrations) and epidemiological relatedness by pulsed field electrophoresis. The annual use of fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin) expressed as grams per discharged patient from 1997 to 2002 and ratio of fluoroquinolone resistance were also analyzed.
Antimicrobial Agents
The antimicrobial agents were supplied by individual pharmaceutical companies as standard reference powder for laboratory use. Ciprofloxacin and moxifloxacin were from Bayer AG (Germany), ofloxacin and levofloxacin from Daiichi Seiyaku (Japan), norfloxacin from Kyorin Pharmaceutical (Japan), and gatifloxacin from
Bristol-Squibb-Meyer (USA). Susceptibility Test
The minimum inhibitory concentrations (MICs) of each antimicrobial agent for the tested bacterial isolates were determined using the agar dilution method, as described by the National Committee for Clinical Laboratory Standards (NCCLS) of the USA [9]. Inocula of 104 colony-forming units (CFU) of A. baumannii were inoculated onto Mueller-Hinton agar plates containing a series of two-fold dilutions of tested
antimicrobial agents. Following inoculation, the agar plates were incubated at 35 ℃ in 5% CO2 for 18-20 h, with the MIC read as the lowest concentration of the antimicrobial
agents that completely inhibited the growth of bacteria. The concentration of
antimicrobial agents tested for all bacteria ranged from 0.03 µg/mL to 128µg/mL. P.
aeruginosa ATCC 27853 were used as internal controls for each test run.
Molecular Typing
Bacterial isolates resistant to fluoroquinolones in 1996-97 were checked for clonicity using pulsed-field gel electrophoresis (PFGE), as described in our previous report [10]. Electrophoresis on a 1% Pulsed Field Certified Agarose gel (Bio-Rad Laboratories) was performed for 22 h by using linearly rampedpulse times beginning
3
with 1 s and ending with 40 s at 6 V/cmat 14°C. The gel was stained with ethidium bromide for 1h and was destained for 1 h and then photographed by using Gel-Doc 2000 (Bio-Rad Laboratories). Isolates were initially alignedon the basis of band similarities and dendrograms generatedby using GelCompar II analysis software (Applied Maths).
Detection of Mutation and Resistance Mechanisms
Fuoroquinolone-resistant A. baumannii strains were randomized selected for further study of resistance mechanism. PCR and direct DNA sequencing was performed to identify mutations in the gyr A and parC genes of A. baumannii. The oligonucleotide primers for the PCR amplification will be as follows: for the gyr A gene the forward primer was 5’-CGGCGCGTACTGTACGCGTTGAC-3’ and the reverse primer was 5’-AATGTCTGCCAGCATTTCATGTGAGA-3’ and for the parC gene the forward primer was 5’-ATGCGCGATATGGGTTTGAC-3’ and the reverse primer was 5’-GGACAACAGCAATTCCGCAA-3’ [11, 12].
Results
The MICs of six fluoroquinolones for the tested A. baumannii from NTUH and NHRI during 1992 to 2002 are presented in Table 1. Totally 648 isolates of A.
baumannii from NTUH and 174 isolates from NHRI were analyzed for MIC checked.
Ciprofloxacin-resistant A. baumannii isolates were also cross-resistant to other
fluoroquinolones frequently; norfloxacin (100%), ofloxacin (98%), levofloxacin (94%), gatifloxacin (93%) and moxifloxacin (88%). Secular surveillance (1997 to 2002) revealed a trend of increasing high-level fluoroquinolone- resistance (ciprofloxacin) for
A. baumannii during recent years (MIC50:0.5 µg/mL in 1997 to 2 µg/mL in 2002;
MIC90:8 µg/mL in 1997 to 64µg/mL in 2002, NTUH) and correlated with increasing
use of fluoroquinolones (Figure 1). Totally 80 of 174 isolates (46%) of A. baumannii from NHRI were fluoroquinolones-resistant (ciprofloxacin MICs≧4 µg/mL); MIC50,
2µg/mL; MIC90, 32 µg/mL; ranged from 0.25-≥128µg/mL. For isolates collected from
different districts located in Taiwan (Northern, Middle, Southern and Eastern), there were no significances of susceptibilities among different districts (Table 2).
One hundred and ninety isolates of ciprofloxacin-resistant A. baumannii of NTUH randomly selected and all 174 isolates of NHRI were recruited for pulsed field gel electrophoresis (PFGE). Of these isolates performed, clonal spread within hospital or between hospitals (region), especially of A. baumannii with high-level FQs-resistance (ciprofloxacin MICs≧64 µg/mL) was noted (Figure 2, Figure 3). The same PFGE pattern of A. baumannii isolates with different MICs suggested the resistance of
4
fluoroquinolone were developed step by step. We further investigated the mechanisms of fluoroquinolone-resistance by analysis the sequence of DNA gyrase (topoisomerase II, gyr A) and topoisomerase IV (par C) of 33 ciprofloxacin-resistant A. baumannii. No changes of aminoacid (gyr A: Gly 81, Ser 83, Ala 84; par C: Ser 80, Glu 84) of
quinolone-resistance determination region (QRDR) of these isolates were detected (Table 3). The actual mechanisms of fluoroquinolone-resistance in A. baumannii in Taiwan needed further investigation.
Discussion
Acinetobacter baumannii is a new emerging nosocomial pathogen since 1990s. It
is usually highly resistant to various antibiotics and difficult to treat. Fluoroquinolones, such as norfloxacin, ofloxacin, ciprofloxacin, levofloxacin and newly developed fluoroquinolones (moxifloxacin and gatifloxacin) are new derivatives of quinolones since late 1990s. The fluorinated quinolones are more potent and have broader spectra of activity than the previous one (e.g. nalidixic acid) and had demonstrated to be good agents for the treatment of a variety of severe community-acquired or nosocomial bacterial infections, such as infections of bone and joint, respiratory, gastrointestinal, urogenital tracts, and systemic infections. However, as the clinical applications of the fluoroquinolone class increased, new acquisition of bacterial fluoroquinolone resistance becomes a challenge. In our study, we showed that A. baumannii isolates demonstrates decreased quinolone-susceptibility with the susceptible percentage to ciprofloxacin decreased from 78% in 1992 to 53% in 2000 at NTUH and similar circumstances in other districts (NHRI, 1998-2000 data) were also noted. A. baumannii have emerged as important causes of morbidity and mortality amongst hospitalized patients in the Taiwan area. Moreover, the progressively- increasing antibiotic resistance of these bacterial species has hindered desirable therapeutic management of patients infected by such bacterial agents. Since the fluoroquinolones were demonstrated to be less active against A. baumannii, they should only be used as an alternative regimen when the microbiological susceptibilities were clearly documented.
Pulsed field gel electrophoresis (PFGE) of these isolates was performed and clonal spread within hospital or between hospitals (region), especially of A. baumannii with high-level FQs-resistance (ciprofloxacin MICs≧64 µg/mL) was noted. The same PFGE pattern of A. baumannii isolates with different MICs suggested the resistance of fluoroquinolone were developed step by step.
5
the emergence and subsequent increase of fluoroquinolone-resistance, at rates greater than was anticipated. Spreading of high-level fluoroquinolone-resistant clones in recent years compatible with increasing use of fluoroquinolones. It is important that
continuous infection control and prudent use of these agents (selective pressure) be emphasized, not only treatment of human bacterial infections but also veterinary medicine to prevent the emergence and increase of resistant strains. The appropriate antimicrobial use of the new fluoroquinolones for future patient infection control should be encouraged in order to prevent the emergence of antimicrobial-resistant bacterial strains.
References (僅列重要參考文獻)
1. Bergogne-Berezin E. The increasing significance of outbreaks of Acinetobacter baumannii-the need for control and new agents. J Hosp Infect 1995;30:441-52. 2. Pascual A, Lopez-Hernandez I, Martinez-Martinez L, Perea EJ. In-vitro
susceptibilities of multiresistant strains of Acinetobacter baumannii to eight quinolones. Journal of Antimicrobial Chemotherapy. 1997;40:140-2. 3. Centers for Disease Control and Prevention. National Nosocomial Infection
Surveillance (NNIS) Report. October 1986-April 1996. Am J Infect Control 1996;24:380-8.
4. National Taiwan University Hospital. Nosocomial Infection Surveillance Data. 5. Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med
1991;324:384-94.
6. Choi KH, Baek MC, Kim BK, Choi EC. Resistance mechanism of Acinetobacter spp. Strains resistant to DW-116, a new quinolone. Arch Pharm Res
1998;21:310-4.
7. Chang SC, Hsieh WC, Liu CY. High prevalence of antibiotic resistance of common pathogenic bacteria in Taiwan. The Antibiotic Resistance Study Group of the Infectious Disease Society of the Republic of China. Diagn Microbiol Infect Dis 2000;36:107-112.
8. Sheng WH, Chen YC, Wang JT, Chang SC, Luh KT, Hsieh WC. Emergining fluoroquinolone-resistance for common clinically important gram-negative bacteria in Taiwan. Diagn Microbiol Infect Dis 2002;43:141-7.
9. National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. 2000;NCCLS documents, Wayne, Pennsylvania.
10. Chen YC, Chang SC, Hsu LY, Hsieh WC, Luh KT. In vitro antimicrobial activity of fluoroquinolones against clinical isolates obtained in 1989 and 1990. Journal of the Formosan Medical Association. 1993;92:1040-8.
6
11. Vila J, Ruiz J, Goni P, Jimenez de Anta T. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J Antimicrob
Chemother 1997;39:757-62.
12. Vila J, Ruiz J, Goni P, Marcos A, Jimenez de Anta T. Mutation in the gyrA gene of quinolone-resistant clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 1995;39:1201-3.
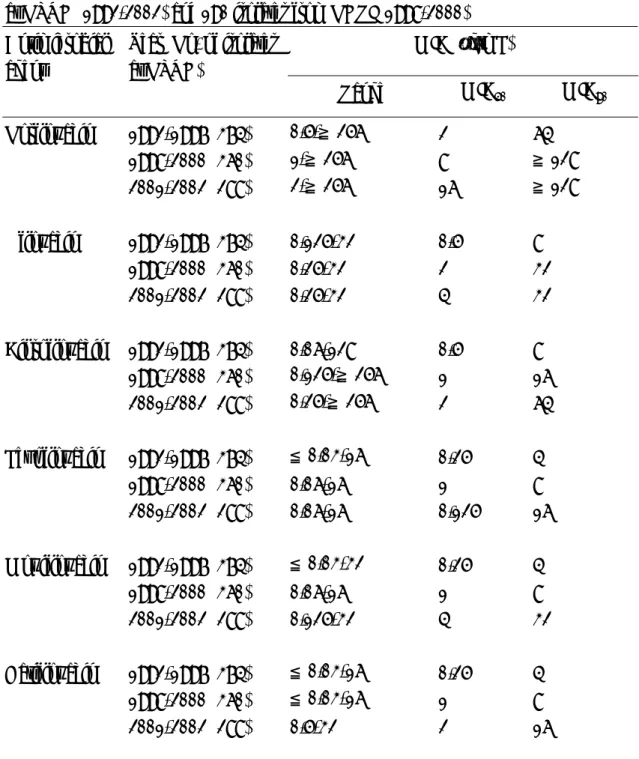
TABLE 1 Comparative in vitro activity of norfloxacin, ofloxacin, ciprofloxacin, levofloxacin and gatifloxacin against 648 Acinetobacter baumannii isolates preserved at NTUH (1992-2002) and 190 isolates from NHRI (1998-2000)
MIC (µg/mL) Antimicrobial
agent
Year (No. of isolates at NTUH)
Range MIC50 MIC90
Norfloxacin 1992-1997 (374) 0.5-≧256 2 64 1998-2000 (360) 1-≧256 8 ≧128 2001-2002 (288) 2-≧256 16 ≧128 Ofloxacin 1992-1997 (374) 0.125-32 0.5 8 1998-2000 (360) 0.25-32 2 32 2001-2002 (288) 0.25-32 4 32 Ciprofloxacin 1992-1997 (374) 0.06-128 0.5 8 1998-2000 (360) 0.125-≧256 1 16 2001-2002 (288) 0.25-≧256 2 64 Levofloxacin 1992-1997 (374) ≦0.03-16 0.25 4 1998-2000 (360) 0.06-16 1 8 2001-2002 (288) 0.06-16 0.125 16 Moxifloxacin 1992-1997 (374) ≦0.03-32 0.25 4 1998-2000 (360) 0.06-16 1 8 2001-2002 (288) 0.125-32 4 32 Gatifloxacin 1992-1997 (374) ≦0.03-16 0.25 4 1998-2000 (360) ≦0.03-16 1 8 2001-2002 (288) 0.5-32 2 16
7
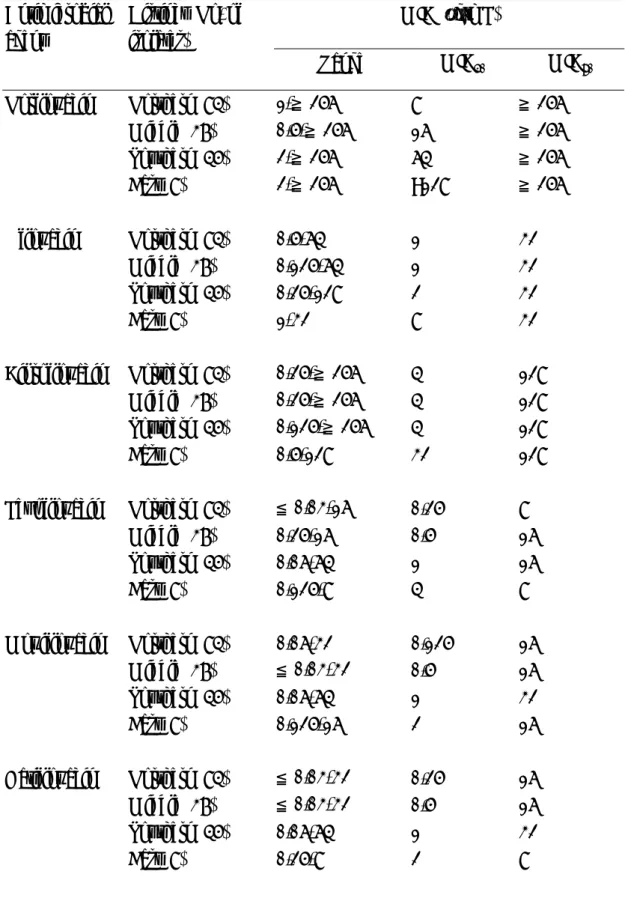
TABLE 2 Comparative in vitro activity of norfloxacin, ofloxacin, ciprofloxacin, levofloxacin and gatifloxacin against 174 Acinetobacter baumannii isolates collected from 22 other hospitals distributed at different districts in Taiwan (NHRI data)
MIC (µg/mL) Antimicrobial
agent
Distinct (No. of isolates)
Range MIC50 MIC90 Norfloxacin Northern (84) 1-≧256 8 ≧256 Middle (37) 0.5-≧256 16 ≧256 Southern (45) 2-≧256 64 ≧256 East (8) 2-≧256 >128 ≧256 Ofloxacin Northern (84) 0.5-64 1 32 Middle (37) 0.125-64 1 32 Southern (45) 0.25-128 2 32 East (8) 1-32 8 32 Ciprofloxacin Northern (84) 0.25-≧256 4 128 Middle (37) 0.25-≧256 4 128 Southern (45) 0.125-≧256 4 128 East (8) 0.5-128 32 128 Levofloxacin Northern (84) ≦0.03-16 0.25 8 Middle (37) 0.25-16 0.5 16 Southern (45) 0.06-64 1 16 East (8) 0.125-8 4 8 Moxifloxacin Northern (84) 0.06-32 0.125 16 Middle (37) ≦0.03-32 0.5 16 Southern (45) 0.06-64 1 32 East (8) 0.125-16 2 16 Gatifloxacin Northern (84) ≦0.03-32 0.25 16 Middle (37) ≦0.03-32 0.5 16 Southern (45) 0.06-64 1 32 East (8) 0.25-8 2 8
8
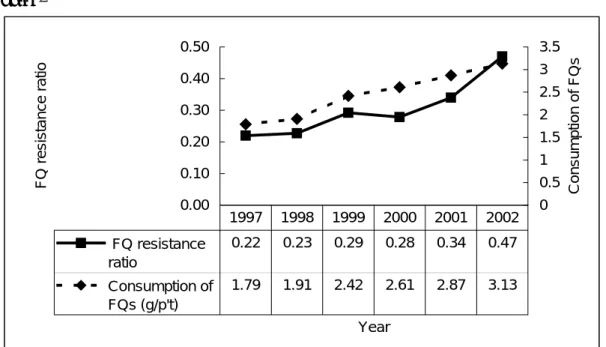
FIGURE 1 Relationship between use of fluoroquinolone (average, gram per hospitalized patient) and ratio of A. baumannii with fluoroquinolone (ciprofloxacin) -resistance. Dramatic increase in the prescription of the fluoroquinolones is compatible with the trends of increasing fluoroquinolone-resistant A. baumannii at NTUH were noted. 0.00 0.10 0.20 0.30 0.40 0.50 Year F Q r e s is tanc e r a tio 0 0.5 1 1.5 2 2.5 3 3.5 Cons umption of F Q s FQ resistance ratio 0.22 0.23 0.29 0.28 0.34 0.47 Consumption of FQs (g/p't) 1.79 1.91 2.42 2.61 2.87 3.13 1997 1998 1999 2000 2001 2002
TABLE 3 Mechanisms of fluoroquinolone-resistance by analysis the sequence of DNA gyrase (topoisomerase II, gyr A) and topoisomerase IV (par C) of 33
ciprofloxacin-resistant A. baumannii.
A.baumannii gyrA parC
ATCC19606 Gly81 Ser83 Ala84 Ser80 Glu84
ATCC19606 GAC TCG GAA TCG -
Spain(susceptible) GAC TCG GAA TCG GAA GAC(28) TCG(25) GAA(28) TCG(26) GAA(25) NTUH (n=25)
TCA(3) TCA(2) GAG(3)
NHRI (n=8) GAC(5) TCG(5) GAA(5) TCG(5) GAA(5)
Mutant* Val81 Leu83 Pro84 Leu80 Lys84
9
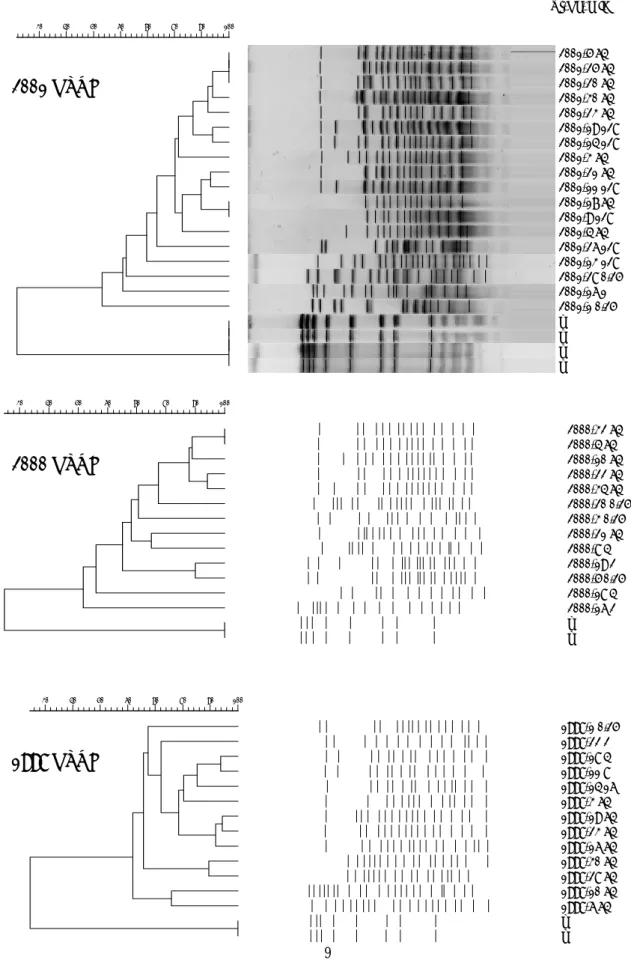
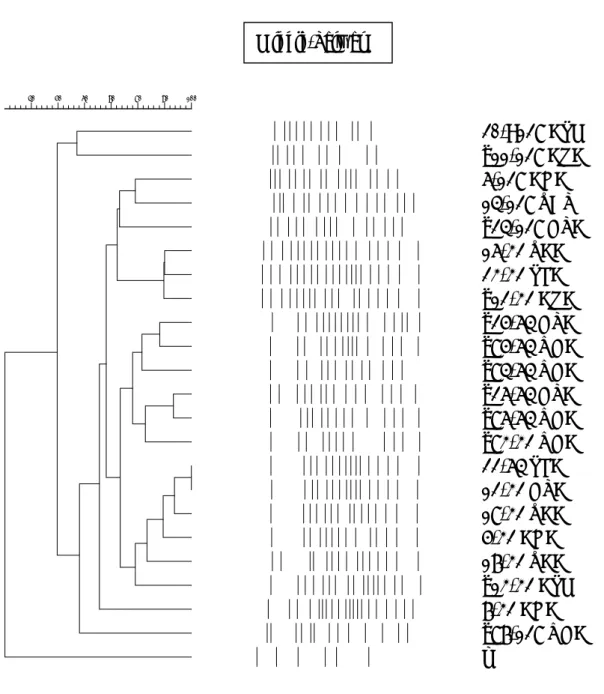
FIGURE 2 Pulsed field gel electrophoresis (PFGE) of A. baumannii isolates
recruited at NTUH. Highly diversity of fluoroquinolone-resistant A. baumannii isolates in 1998. Some clustering of fluoroquinolone-resistant A. baumannii isolates in 2000 and 2001 were noted.
Yr-No. MIC 2001 NTUH 2000 NTUH 1998 NTUH 2001-5 64 2001-25 64 2001-20 64 2001-30 64 2001-23 64 2001-19 128 2001-14 128 2001-3 64 2001-21 64 2001-11 128 2001-17 64 2001-9 128 2001-4 64 2001-26 128 2001-13 128 2001-28 0.25 2001-16 1 2001-1 0.25 m m m m 100 90 80 70 60 50 40 30 1998-1 0.25 1998-22 2 1998-18 4 1998-11 8 1998-14 16 1998-3 64 1998-19 64 1998-23 64 1998-16 64 1998-30 64 1998-28 64 1998-10 64 1998-6 64 m m 100 90 80 70 60 50 40 30 2000-32 64 2000-4 64 2000-10 64 2000-22 64 2000-34 64 2000-20 0.25 2000-3 0.25 2000-21 64 2000-8 4 2000-19 2 2000-5 0.25 2000-18 4 2000-16 2 m m 100 90 80 70 60 50 40 30
10
FIGURE 3 Isolates of NHRI recruited for pulsed field gel electrophoresis (PFGE). (Here showed part of analysis) Clonal spread within hospital and between hospitals (region) were shown, especially of A. baumannii with high-level fluoroquinolones- resistance (ciprofloxacin MICs≧64 µg/mL). (Part)
20->128 CSM b11-128 CRC 6-128 CHC 14-128 ZAT b24-128 GTC 16-32 ZCC 23-32 SLC b12-32 CRC b25-64 GTC b85-64 VGC b84-64 VGC b26-64 GTC b86-64 VGC b83-32 VGC 22-64 SLC 12-32 GTC 18-32 ZCC 5-32 CHC 17-32 ZCC b13-32 CSM 7-32 CHC b87-128 VGC m 100 90 80 70 60 50 40 Middle-Taiwan