Title: Perinatal and childhood risk factors for early-onset type 1 diabetes: A population-based case-control study in Taiwan
Running Title:
Perinatal and childhood factors for T1DM
Authors’ names, academic degrees, and affiliations:
Hsin-Yu Lee1, Chin-Li Lu1,2, Hua-Fen Chen3, Hui-Fang Su4, Chung-Yi Li1,5
1 Department and Institute of Public Health, College of Medical, Cheng-Kung University, Tainan, Taiwan
2 Department of Medical Research, Ditmanson Medical Foundation, Chia-Yi Christian Hospital, Chia-Yi City, Taiwan
3 Department of Endocrinology, Far-Eastern Memorial Hospital, New Taipei City, Taiwan 4 Department of Health Care Management, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan
5 Department of Public Health, College of Public Health, China Medical University, Taichung, Taiwan
Hsin-Yu Lee and Chin-Li Lu contributed equally to the work. Address correspondence to:
Chung-Yi Li, PhD
Department and Graduate Institute of Public Health College of Medicine, National Cheng Kung University #1, University Rd., Tainan, Taiwan, 701
E-mail: cyli99@mail.ncku.edu.tw
TEL: 886-6-2353535 ext.5862, FAX: 886-6-2359033 Funding:
This study was supported by a grant from the National Scientific Council (NSC101-2314-B-006-076-MY3) located in Taipei, Taiwan
Conflicts of interest:
ABSTRACT:
Background: Certain factors originating from the perinatal and childhood periods are suspected of contributing to the recent increasing trend of childhood type 1 diabetes (T1D) incidence. This study sought to investigate the relationships between various perinatal and childhood risk factors and T1D incidence in young children (<10 years). Methods: We used a nested case-control design based on 1,478,573 live births born in 2000 to 2005 in Taiwan. Cases were 632 incident cases of T1D between 2000 and 2008. Ten matched controls for each case were randomly selected. Information on various perinatal risk factors was also identified from claim data. Multiple conditional logistic regression was employed to estimate odds ratio (OR) and 95 confidence interval (CI) of T1D. Results: Childhood infection was significantly associated with an increased risk of T1D (OR=1.46, 95% CI=1.23-1.73). Increased risk of T1D was also noted in children born to younger mothers (<25 years)
(OR=1.94, 95% CI=1.34-2.81), older fathers (>30 years) (OR=1.56 (95% CI=1.16-2.10)-1.57 (95% CI=1.19-2.05), mothers with Cesarean section (CS) (OR=2.35, 95% CI=1.52-3.64), and mothers with gestational diabetes mellitus (OR=4.36, 95% CI=2.76-7.77). Fathers with T1D (OR=7.36, 95% CI=1.02-57.21) or type 2 diabetes (OR=1.54, 95% CI=1.04-2.26) were observed to substantially increase the risk of offspring T1D. Conclusions: Certain modifiable perinatal factors such as infection and CS may predispose incidence of T1D in young
children.
Keywords: type 1 diabetes, nested case-control study, perinatal risk factors, childhood infection, Cesarean sections
Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by known genetic risk factors with T cell-mediated infiltration and destruction of the beta cells within pancreatic islets.1 T1D affects millions around the world, and the incidence of T1D in children is increasing worldwide at a rate that cannot be explained by genetics alone.2 Although extensive scientific research has yielded important insights into the immune mechanisms involved in pancreatic β-cell destruction, little is known about the events that trigger the autoimmune process.3 Despite that a number of environmental risk factors have been identified to be associated with the incidence of T1D, the results are inconclusive. 1
Taiwan showed a slightly increased secular trend in childhood (<19 years) T1D incidence over the past 10 years (2000-2009), with an annual figure increased from 4.21 to 5.79 per 100,000.4 Given the fact that the exact nature of causative environmental factors including perinatal factors is unknown and much debated,1 this study sought to investigate the relationships between various perinatal and childhood risk factors and T1D incidence in young children (<10 years) of Taiwan.
Methods
Source of Data
Data analyzed in this study were retrospectively retrieved from the medical claims of the National Health Insurance Research Database (NHIRD) provided by Taiwan National Health Insurance Administration (NHIA). The NHIRD provides all inpatient and ambulatory
medical claims for around 99% of Taiwanese people.5 Access to research data has been reviewed and approved by the Review Committee of the National Health Research Institutes.
Study Design and Identification of Study Subjects
This was a nested case-control study based on a cohort of all live births in 2000-2005, who were followed to the end of 2008. Overall, 1,478,573 newborns were registered in the registry for beneficiaries between 2000 and 2005. Among them, 30,214 (2.04%) were excluded because their health insurances were not covered by their parents as beneficiaries, with 1,448,359 newborns left in the study cohort. According to Taiwan’s National Health
Insurance (NHI) policy, children should be insured under the names of their parents or other family members as beneficiaries.6
Cases were children who received ambulatory or inpatient cares for T1D (International Classification of Disease 9th version, Clinical Modification (ICD-9-CM) codes: 250x1 or
250x3) between date of birth and the last day of 2008. In the NHI system, the NHIA issues catastrophic illness/injury certificates to all patients who suffer from T1D, and these patients are exempt from copayment to the NHI if they sought cares for T1D. This certification is only applied when detailed and specific clinical data are met, e.g., regular insulin use with a history of diabetic ketoacidosis, a positive glucagon test, or the presence of glutamic-acid-decarboxylase antibodies. Only those T1D patients with catastrophic illness/injury certificates were selected (n=632). The date of first ever T1D diagnosis was regarded as the index date.
In selecting controls, we first excluded all children in the study cohort who ever experienced ambulatory care visits or admissions for diabetes mellitus (ICD-9-CM codes: 250xx) during 2000-2008. For each case, we randomly selected 10 controls who were matched on age, sex, index date, and beneficiary (mother or father). Among the case/control sets, 259 cases and 2,590 controls were insured with their mothers; while the insurance plans of 373 cases and 3,730 controls stayed with their fathers (supplementary Figure 1).
Perinatal Risk Factors of Children
The information on perinatal and childhood factors was retrieved from the inpatient claims, which included codes for birth weight / gestational age , and events of certain conditions originating in the perinatal period (ICD-9-CM codes: 760-779), including conditions which have their origin in the perinatal period, before birth through the first 28 days after birth.
Inclusion of this group of diagnosis in the analysis was mainly due to the fact that
endogenous autoantibodies predictive of future T1D may be detectable by 6-12 months of age, suggesting that environmental factors may operate before this age in some cases.7 Additionally, studies also found that maternal or intrauterine conditions may also modulate genetic risk of T1D, and the disease process culminating in T1D typically begins in early life, but it is not clear whether the trail begins before or after birth.7 We also identified admissions for various infections (listed in Table 3) suspected of being associated with diabetes.8,9.
Based on the number of residents and area for each of the 359 townships in Taiwan, we calculated the population density of each township, and categorized into 4 groups according to quartiles (<=235, 236-636, 637-1738, and >1738 people per km2). The population density has frequently been used as a surrogate for the urbanization level in previous Taiwanese studies.10
Perinatal Risk Factors of Parents
The maternal perinatal factors analyzed in this study included age at delivery, mode of delivery (vaginal birth and CS), a discharge code of gestational diabetes mellitus (GDM) (ICD-9-CM codes: 6488), admission for preeclampsia (ICD-9-CM codes: 6424-6427), and history of admission for infection during pregnancy. The infections of interest were the same as those described above. Another maternal co-morbidity during pregnancy included
ambulatory care visits for diabetes (ICD-9-CM codes: 250). The perinatal factors for fathers included only age at delivery and ambulatory care visits for diabetes.
Statistical Analysis
We compared the distribution of perinatal risk factors between cases and controls. We then analyzed data using conditional logistic regression models and estimated odds ratios (ORs) and their 95% confidence intervals (CIs) from the models.
All children’s perinatal and childhood risk factors were included in the multiple
regression model. The analysis of maternal (or paternal) perinatal risk factors simultaneously also adjusted for the children’s characteristics. All statistical analyses were performed using the SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). A two-tailed p-value <0.05 was considered significant.
Results
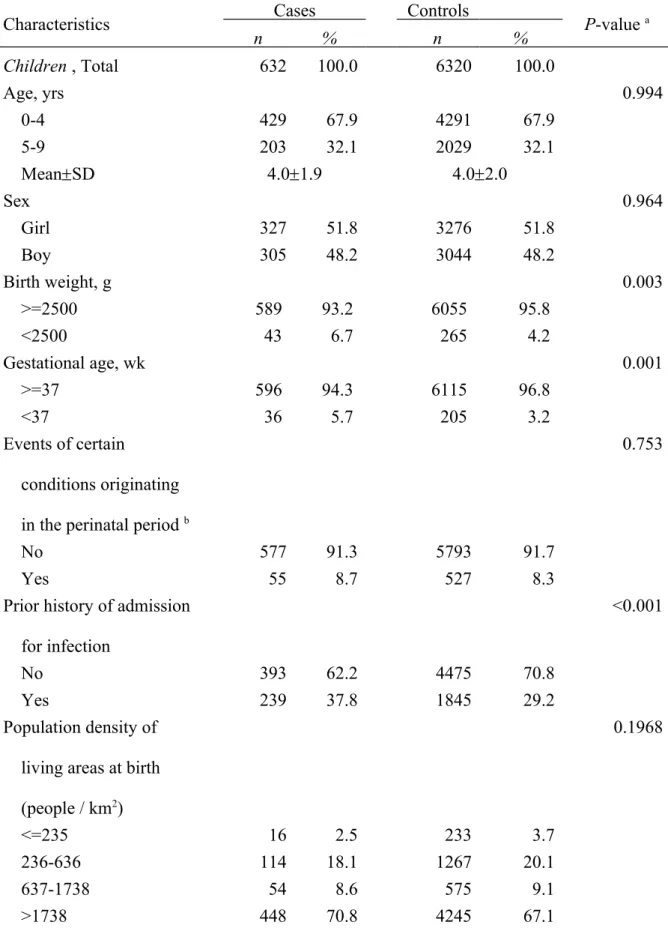
Cases and control were comparable on both age and sex. The prevalence of low birth weight (<2500 grams) and preterm (<37 weeks of gestation) was 6.7% and 5.7%, respectively for cases. The corresponding figures for controls were 4.2% and 3.2%. Additionally, 37.8% of cases had prior hospitalization for infectious disease, but only 29.2% of controls were ever admitted for infectious disease. Only 2.5% of cases and 3.7% of controls lived in townships with low population density (<235 people / km2) at birth. Compared to controls, cases tended to live in areas with higher population densities. (Table 1)
Table 1 also shows that mothers of cases were younger than mothers of controls
(29.35.2 vs 31.25.6 years). The prevalence rates of CS (51.2% vs 30.1%) and preeclampsia during pregnancy (0.8% vs 0.1%) were higher in cases’ mothers than in control mothers. The prevalence of GDM was much higher in case mothers (10.4%) than in control mothers (2.1%). The prevalence of diabetes in parents was also significantly different between case and controls. The prevalence of T1D was much higher in parents of cases. On the other hand, the prevalence rate of Type 2 diabetes in controls was almost double that of cases (5.7% versus 2.7%). Unlike the distributions of age at delivery and diabetes in mothers of cases and controls, fathers of cases were slightly older (32.35.1 vs 31.15.7 years) and had higher prevalence of diabetes. The prevalence rates of type 1 (0.5% vs 0.05%) and type 2 (9.2% vs
5.7%) diabetes were also higher in case fathers than in control fathers.
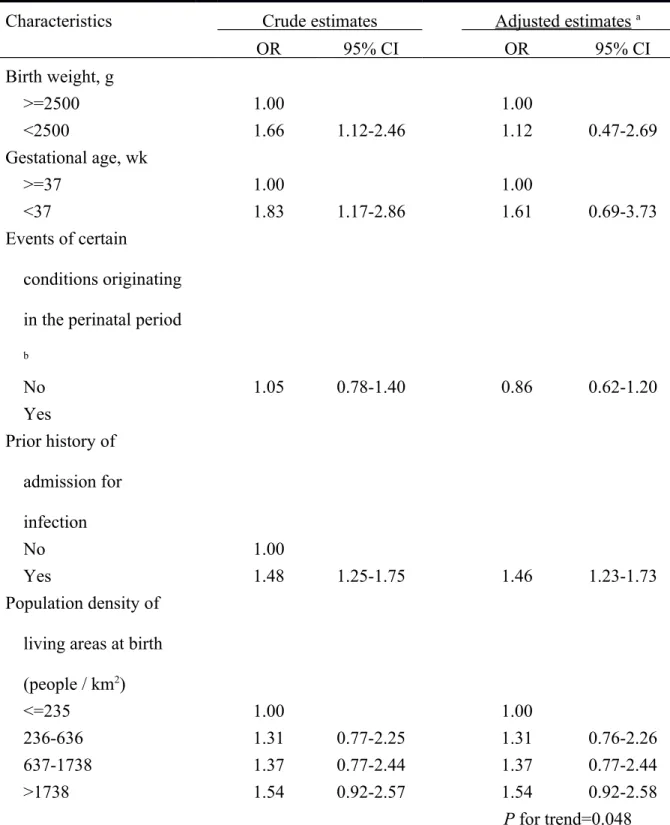
The multiple regression analysis indicated that preterm or low birth weight infants experienced an increased, but insignificantly, OR of T1D. When more detailed classifications of birth weight (<1500, 1500-2499, and >=2500 g) and gestational age (<33, 33-36, and >=37 wks) were used in the analysis. The study results were essentially the same. The adjusted ORs for birth weight of <1500 and 1500-2499 g were estimated at 1.05 (95% CI=0.46-2.42) and 1.14 (95% CI=0.76-1.71). The corresponding figures for gestational age of <33 and 33-36 wks were 1.50 (95% CI=0.64-3.50) and 1.62 (95% CI=0.70-3.77) (data not shown in Tables). Children with a prior history of admission for infection were at a significantly elevated OR of developing T1D (OR=1.46, 95% CI=1.23-1.73). We noted a significant linear trend (P for trend=0.048) in ORs of T1D in relation to increasing population density.
Compared to those living in areas with a population density <=235 people/km2 ), children residing in areas with a population density of 236-636 (OR=1.31, 95% CI=0.76-2.26), 637-1738 (OR=1.37, 95% CI=0.77-2.44), and >637-1738 (OR=1.54, 95% CI=0.92-2.58) people/km2 all had an increased, but insignificantly, OR of developing T1D. (Table 2)
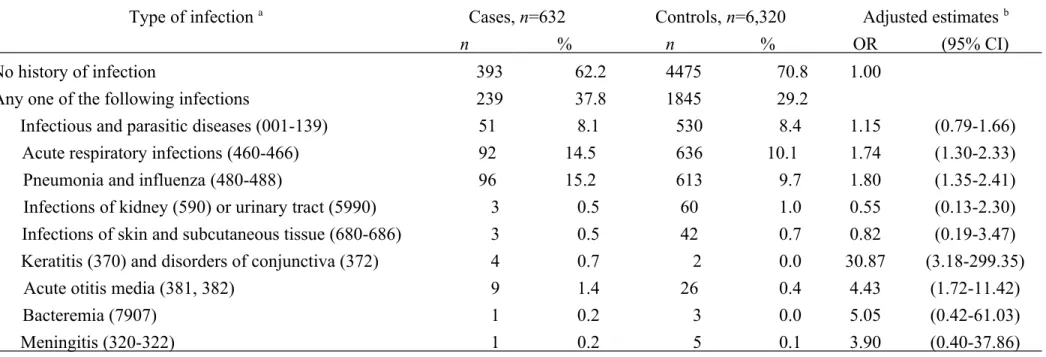
Table 3 shows that prior histories of admission for acute respiratory infections (OR=1.74, 95% CI=1.30-2.33), pneumonia and influenza (OR=1.80, 95% CI=1.35-2.41), keratitis and disorders of conjunctiva (OR=30.87, 95% CI=3.18-299.35), and acute otitis
media (OR=4.43, 95% CI=1.72-11.42) were all significantly associated an increased OR of T1D.
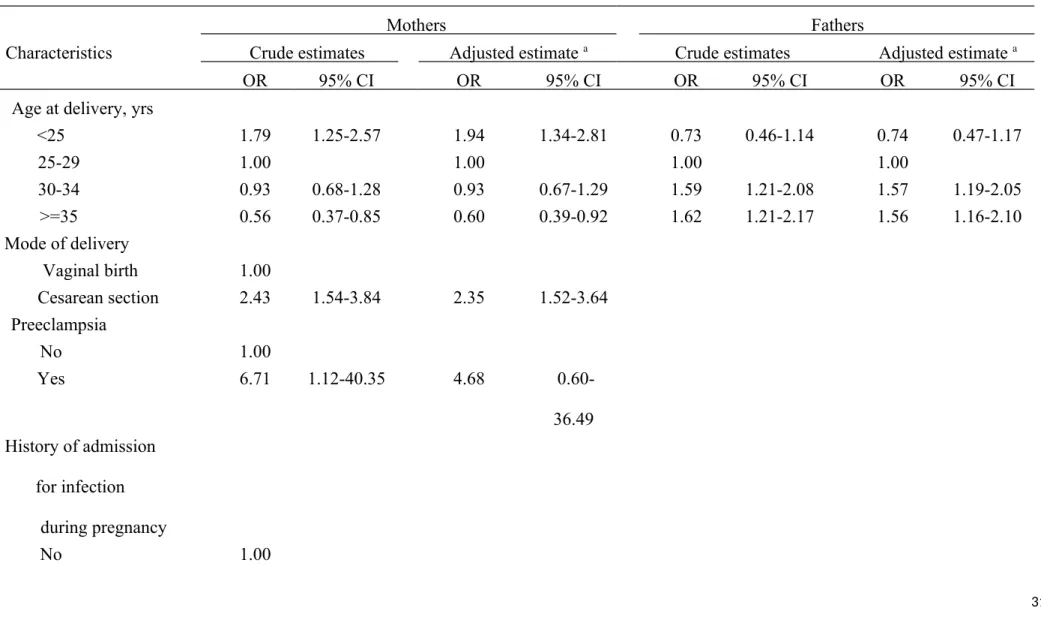
Compared to maternal age at delivery of 25-29 years, younger mothers (<25 years) were associated with an almost two fold OR (1.94, 95% CI=1.34-2.81). On the other hand,
children of older mothers (>=35 years) experienced significantly lower risk of T1D
(OR=0.60, 95% CI=0.39-0.92). Children born by CS or born to mothers with GDM were at a significantly increased risk of developing T1D, with an OR of 2.35 (95% CI=1.52-3.64) and 4.36 (95% CI=2.76-7.77), respectively. Mothers complicated with preeclampsia and those with a history of diabetes were associated with an increased risk of T1D in their children. (Table 4)
We noted that fathers aged 30 and older were more likely than those aged 25-29 years to have children with T1D. Additionally, a statistically increased risk of T1D was observed in children with fathers suffering from both type 1 (OR=7.36, 95% CI=1.02-57.21) and type 2 diabetes (OR=1.54, 95% CI=1.04-2.26). (Table 4)
Discussion
Main Findings
This study identified a number of perinatal risk factors for early onset T1D in Taiwan. Children ever admitted for infectious disease and those born by CS, born to mothers with GDM, and born to fathers with diabetes were also significantly associated with the onset of T1D. Our data also demonstrated a clear increasing trend of T1D incidence in areas with higher urbanization, which is consistent with the observation that the incidence of T1D has been reported to be higher in developed countries than in developing ones.11 As the incidence of T1D is relatively low, risk factors predicting the incidence of T1D have been rarely
investigated in Asia nations.
Perinatal Risk Factors of Children
A Swedish study found that children who developed T1D at 1 to 15 years of age tended to have a larger for gestational age weight,12 and such findings were supported in a subsequent meta-analysis,13 but were not supported in others.14,15 Our data showed no significant
associations of low birth weight or preterm birth with risk of T1D. Interpretation of our study findings could be limited due to incomplete adjustment for certain predictors for T1D. For example, a lower birth weight might relate to a first born child, who is vulnerable to an increased risk of T1D.16 Also, birth order relates to family size,17 which is also related to risk
of T1D.18
Epidemiological and laboratory studies suggest a triggering role for enteroviruses, such as coxsackievirus B4 (CV-B4), in the development of T1D.19 In addition to enteroviruses, neonatal infections, eczema and rhinitis during infancy were also found to be significantly associated with development of T1D.20 Literature have accumulated a lot of evidence supporting that enteroviruses can contribute, at least in some patients, to the pathogenesis of T1D through various mechanisms.3 Additionally, space-time clustering in the presentation of T1D and clustering of births in children who subsequently develop diabetes also support a direct role for infections in the initiation and acceleration of the disease process.21 Although strong evidence showing that viruses can indeed infect pancreatic beta cells with consequent effects ranging from functional damage to cell death,22 it is still a challenge to date to prove a causal relationship between enteroviruses and T1D.23 Serious infections could be related to the ketoacidosis clinical onset, leading to the diagnosis of T1DM. The review by Afonso and Mallone argued that fulfilling the Koch's postulates, namely isolation of the infectious agents preferentially in T1D patients, including before disease onset would be required to
demonstrate the role of infection in T1D incidence.23 Our results are generally consistent with previous findings, suggesting that childhood infection may play as an important
Perinatal Risk Factors of Parents
We noted a substantially high association of CS with T1D. An earlier meta-analysis summarizing 20 studies has shown that children born by CS have a 23% higher risk of developing childhood-onset T1D compared to those vaginally born.24 Our study also
demonstrated a positive relationship between CS and risk of T1D, but with a much stronger OR (2.5-fold), which could be due to incomplete adjustment for all known risk factors of T1D such as birth order 25 and breast-feeding.12 Mode of delivery is thought to influence the development of the immune system in the offspring by several pathways including variation in bacterial colonization of the intestinal tract, different levels of adaptive stress of being born; and altered epigenetic regulation of gene expression, which were considered to pose long-term effect of autoimmune disorders including T1D.7 A recent review suggested some evidence supporting a direct causal role for exposure to maternal diabetes in utero in determining offspring long-term greater adiposity and adverse cardiometabolic health.27 A meta-analysis of 13 studies conducted by Aceti et al.28 reported that offspring of mothers with GDM had similar diastolic blood pressure (BP) to controls, but slightly higher systolic BP (1.39 mmHg, 95% CI=0.00-2.77 mmHg). Our study showed a possible link between GDM and subsequent risk of T1D, which needs to be replicated by other investigations.
for T1D, including eclampsia. However, the evidence for the link between maternal pre-eclampsia during pregnancy and T1D has been inconstant.14 A recent meta-analysis analyzed data of 16 studies including 8,315 children with T1D concluded that there was little evidence of an increase in the risk of T1D in children born to mothers who had pre-eclampsia during pregnancy (OR=1.10, 95% CI 0.96-1.27).29 Our study also provided no support for the significantly increased risk of T1D in relation to maternal pre-eclampsia. We noted that younger mothers (<25 years) were significantly associated with an increased risk of T1D, but older mothers had a significantly lower risk of having offspring who later developed T1D. A protective effect of being older mothers could be due to the fact that increased maternal age was usually associated with a longer duration of breast feeding, which was shown to reduce the risk of T1D.30
Probably due to limited number of mothers with a history of diabetes, we noted an increased, but insignificantly, risk of T1D in children born to mothers with T1D. On the other hand, children born to fathers with diabetes, especially T1D, were at a significantly increased risk of T1D. As indicated, family history of T1D among first-degree relatives and also including third-degree relatives add to the risk for T1D.12 Our study findings are also consistent with the observation that paternal diabetes confers higher risk than maternal diabetes,7 which might be due to a sexual dimorphism in transmission to offspring of expression of islet autoantibodies.31
Implications
Among the significant perinatal risk factors identified in this study, CS and childhood infectious disease are factors preventable through provision of prenatal cares and certain educational interventions. Taiwan has a high rate of CS, approximately 30 percent in the past decade.32A recent survey reported that of the women who had Cesarean deliveries in Taiwan, about 20% were without any medical indication.33 By using the prevalence rate of CS (30.1% in controls) and the OR associated with CS (2.35), we calculated the population attributable risk percentage (PAR%) to assess the potential public health impact of CS. The PAR% for CS was estimated at 28.9%. The corresponding figures for childhood infectious disease were 11.8%. The above estimates tended to highlight the potentially important role of CS in association with the incidence of T1D in Taiwan’s children. Because CS is a modifiable risk factor related to the development of T1D, it is suggested that counseling regarding mode of delivery should be offered early in pregnancy.33
Strengths and Limitations
Methodological strengths included the utilization of nested case-control design, which effectively reduces the chance of control selection bias. Second, the likelihood of potential recall bias is minimal as all the information was obtained from claim data rather than the parental recall. Several limitations should be mentioned here. First, due to limited
information available from claim data, we were unable to comprehensively take all potential genetic, environmental, and perinatal / neonatal risk factors into account. Second, we used only a half of study sample in the analysis of maternal / paternal risk factors in association with T1D, which may have raised concern about the potential selection bias. To address this concern, we compared between the children in the analysis of maternal perinatal and those used for the analysis of paternal factors, and found that these children did not differ with respect to all children’s characteristics list in Table 1. Third, the infections investigated included only those infections leading to hospitalization and excluded the majority of childhood infections. Fourth, due to unavailability of all medical claims for parents, we analyzed only the mothers’ inpatient claims on preeclampsia, GDM, and history of admission for infection during pregnancy, which may have under-reported the prevalence of these maternal conditions.
Conclusions
Certain modifiable perinatal factors such as infection and CS may predispose incidence of T1D in young children. Prevention of childhood infections and avoidance of non-indicated CS are able to largely reduce the incidence of early onset T1D in Taiwan. Our study also suggests that children in urban areas should be the object of preventive strategies for T1D.
Acknowledgment:
This study was supported by a grant from the National Scientific Council (NSC101-2314-B-006 -076 -MY3) located in Taipei, Taiwan; and it has no role in both conducting the study and interpreting the results. The authors are grateful for the comments from Dr. Po-Lin Chen of Department of Internal Medicine, National Cheng Kung University Hospital.
Key points
Various perinatal factors have been found to be associated with increased risk of T1D in children, but the evidence is neither consistent nor adequate.
This population-based cohort study identified a number of perinatal risk factors for T1D including younger mothers, older fathers, paternal diabetes, maternal gestational
diabetes mellitus, Cesarean section, and childhood infection.
There is a positive dose gradient relationship between population density and risk of early onset T1D, also suggesting that urbanization might play a role of contributing the occurrence of early onset T1D.
Given a high Cesarean section prevalence rate (30.1%) in Taiwan, the population attributable risk percent for Cesarean section was estimated at 28.9%. Clinicians and public health policy should consider strategies that may effectively avoid the non-medically indicated Cesarean sections in order for further reduction of early onset T1D.
References
1. Thrower SL, Bingley PJ. Prevention of type 1 diabetes. Br Med Bull 2011;99:73-88. 2. Forlenza GP, Rewers M. The epidemic of type 1 diabetes: what is it telling us? Curr Opin
Endocrinol Diabetes Obes 2011;18:248-51.
3. Ghazarian L, Diana J, Simoni Y, Beaudoin L, Lehuen A. Prevention or acceleration of type 1 diabetes by viruses. Cell Mol Life Sci 2013;70:239-55.
4. Jiang YD, Chang CH, Tai TY, et al. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000-2009 Nationwide Health Insurance database. J Formos Med Assoc 2012;111:599-604.
5. Lu JFR, Hsiao WC. Does Universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff (Millwood) 2003;22:77-88.
6. Chiang TL. Taiwan’s 1995 health care reform. Health Policy 1997;39:225-39. 7. Stene LC, Gale EA. The prenatal environment and type 1 diabetes. Diabetologia
2013;56:1888-97.
8. Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care 2001;24:1044-9.
9. Shoshan A, Sella T, Shohat T, Goren I, Shalev V, Chodick G. A case-crossoverstudy of infectious diseases and new diagnosis of type 1 diabetes . Pediatr Diabetes 2012;13:583-6.
10.Li CY, Lin RS, Lin CH. Urbanization and childhood leukemia in Taiwan. Int J Epidemiol 1998;27:587-91.
11.Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000;23:1516-26.
12.Dahlquist G, Bennich SS, Kallen B. Intrauterine growth pattern and risk of childhood onset insulin dependent (type I) diabetes: population based case-control study. BMJ 1996(7066);313:1174-7.
13.Cardwell CR, Stene LC, Joner G, et al. Birthweight and the risk of childhood-onset type 1 diabetes: a meta-analysis of observational studies using individual patient data.
Diabetologia 2010;53:641-51.
14.Robertson L, Harrild K. Maternal and neonatal risk factors for childhood type 1 diabetes: a matched case–control study. BMC Public Health 2010;10:281.
15.Winkler C, Raab J, Grallert H, Ziegler AG. Lack of association of type 2 diabetes susceptibility genotypes and body weight on the development of islet autoimmunity and type 1 diabetes. PLoS One 2012;7(4):e35410.
16.Bingley PJ, Douek IF, Rogers CA, Gale EA. Influence of maternal age at delivery and birth order on risk of type 1 diabetes in childhood: prospective population based family study. Bart’s- Oxford Family Study Group. BMJ 2000;321(7258):420-4.
invalidates conclusions. BMJ 2001;322(7300):1489.
18.Okada H, Kuhn C, Feillet H, Bach JF. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol 2010;160:1-9.
19.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79-118
20.Majeed AA, Mea Hassan K. Risk Factors for Type 1 Diabetes Mellitus among Children and Adolescents in Basrah. Oman Med J 2011;26:189-95.
21.Craig ME, Nair S, Stein H, Rawlinson WD. Viruses and type 1 diabetes: a new look at an old story. Pediatr Diabetes 2013;14:149-58.
22.Galleri L, Sebastiani G, Vendrame F, Grieco FA, Spagnuolo I, Dotta F. Viral infections and diabetes. Adv Exp Med Biol. 2012;771:252-71.
23.Afonso G, Mallone R. Infectious triggers in type 1 diabetes: is there a case for epitope mimicry? Diabetes Obes Metab 2013;15(Suppl 3):82-8.
24.Cardwell CR, Stene LC, Joner G, et al. Cesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia 2008;51(5):726-735.
25.Stene LC, Magnus P, Lie RT, Søvik O, Joner G; Norwegian childhood Diabetes Study Group. Birth weight and childhood onset type 1 diabetes: population based cohort study. BMJ 2001;322(7291):889-92.
diabetes in pregnancy. Curr Diab Rep 2014;14:489.
27.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol 2013;208:249-54.
28.Aceti A, Santhakumaran S, Logan KM, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia
2012;55:3114-27.
29.Henry EB, Patterson CC, Cardwell CR. A meta-analysis of the association between pre-eclampsia and childhood-onset Type 1 diabetes mellitus. Diabetic Med 2011;28:900-5. 30.Peng H, Hagopian W. Environmental factors in the development of Type 1 diabetes. Rev
Endocr Metab Disord 2006;7:149-62.
31.Yu L, Chase HP, Falorni A, Rewers M, Lernmark A, Eisenbarth GS. Sexual dimorphism in transmission of expression of islet autoantibodies to offspring. Diabetologia
1995;38:1353-7.
32.Liu TC, Chen CS, Tsai YW, Lin HC. Taiwan's high rate of cesarean births: impacts of national health insurance and fetal gender preference. Birth 2007;34:115-22.
33.Chu KH , Tai CJ, Hsu CS, Yeh MC, Chien LY. Women's preference for cesarean delivery and differences between Taiwanese women undergoing different modes of delivery. BMC Health Serv Res 2010;10:138.
Table 1 Characteristics of cases and controls
Characteristics Cases Controls P-value a
n % n % Children , Total 632 100.0 6320 100.0 Age, yrs 0.994 0-4 429 67.9 4291 67.9 5-9 203 32.1 2029 32.1 MeanSD 4.01.9 4.02.0 Sex 0.964 Girl 327 51.8 3276 51.8 Boy 305 48.2 3044 48.2 Birth weight, g 0.003 >=2500 589 93.2 6055 95.8 <2500 43 6.7 265 4.2 Gestational age, wk 0.001 >=37 596 94.3 6115 96.8 <37 36 5.7 205 3.2 Events of certain conditions originating in the perinatal period b
0.753
No 577 91.3 5793 91.7
Yes 55 8.7 527 8.3
Prior history of admission for infection
<0.001
No 393 62.2 4475 70.8
Yes 239 37.8 1845 29.2
Population density of living areas at birth (people / km2) 0.1968 <=235 16 2.5 233 3.7 236-636 114 18.1 1267 20.1 637-1738 54 8.6 575 9.1 >1738 448 70.8 4245 67.1
Mothers, Total 259 100.0 2590 100.0
Age at delivery, yrs <0.001
<25 60 23.2 327 12.6 25-29 81 31.3 792 30.6 30-34 84 32.4 881 34.0 >=35 34 13.1 590 22.8 MeanSD 29.35.2 31.25.6 Mode of delivery <0.001 Vaginal birth 127 40.8 1813 69.9 Cesarean section 132 51.2 777 30.1 Preeclampsia No 257 99.2 2587 99.9 0.032 Yes 2 0.8 3 0.1
History of admission for infection during pregnancy No 257 99.23 2576 99.46 Yes 2 0.77 14 0.54 Gestational diabetes mellitus <0.001 No 232 90.6 2535 97.9 Yes 27 10.4 55 2.1 Diabetes 0.035 No 250 96.5 2435 94.0 Type 1 1 0.4 1 0.04 Type 2 7 2.7 148 5.7
Type 1 & Type 2 1 0.4 6 0.2
Fathers, Total 373 100.0 3730 100.0
Age at delivery, yrs <0.001
<25 25 6.7 448 12.0
25-29 93 24.9 1210 32.4
30-34 152 40.8 1244 33.4
MeanSD 32.35.1 31.15.7
Diabetes 0.001
No 335 89.8 3511 94.1
Type 1 2 0.5 2 0.05
Type 2 34 9.2 213 5.7
Type 1 & Type 2 2 0.5 4 0.1
Table 2 Odds ratio of type 1 diabetes incidence in relation to children’s perinatal characteristics
Characteristics Crude estimates Adjusted estimates a
OR 95% CI OR 95% CI Birth weight, g >=2500 1.00 1.00 <2500 1.66 1.12-2.46 1.12 0.47-2.69 Gestational age, wk >=37 1.00 1.00 <37 1.83 1.17-2.86 1.61 0.69-3.73 Events of certain conditions originating in the perinatal period b No 1.05 0.78-1.40 0.86 0.62-1.20 Yes Prior history of admission for infection No 1.00 Yes 1.48 1.25-1.75 1.46 1.23-1.73 Population density of living areas at birth (people / km2) <=235 1.00 1.00 236-636 1.31 0.77-2.25 1.31 0.76-2.26 637-1738 1.37 0.77-2.44 1.37 0.77-2.44 >1738 1.54 0.92-2.57 1.54 0.92-2.58 P for trend=0.048 a Adjusted estimates were derived from the logistic regression model that simultaneously included all children’s characteristics of Table 2 as independent variables.
Table 3 Odds ratio of T1D among children relation to overall and specific infection
Type of infection a Cases, n=632 Controls, n=6,320 Adjusted estimates b
n % n % OR (95% CI)
No history of infection 393 62.2 4475 70.8 1.00
Any one of the following infections 239 37.8 1845 29.2
Infectious and parasitic diseases (001-139) 51 8.1 530 8.4 1.15 (0.79-1.66)
Acute respiratory infections (460-466) 92 14.5 636 10.1 1.74 (1.30-2.33)
Pneumonia and influenza (480-488) 96 15.2 613 9.7 1.80 (1.35-2.41)
Infections of kidney (590) or urinary tract (5990) 3 0.5 60 1.0 0.55 (0.13-2.30)
Infections of skin and subcutaneous tissue (680-686) 3 0.5 42 0.7 0.82 (0.19-3.47) Keratitis (370) and disorders of conjunctiva (372) 4 0.7 2 0.0 30.87 (3.18-299.35)
Acute otitis media (381, 382) 9 1.4 26 0.4 4.43 (1.72-11.42)
Bacteremia (7907) 1 0.2 3 0.0 5.05 (0.42-61.03)
Meningitis (320-322) 1 0.2 5 0.1 3.90 (0.40-37.86)
a Numbers in parenthesis are ICD-9-CM codes
b Adjusted estimates were derived from multiple logistic regression model with adjustment for all variables listed in Table 2. OR, odds ratio; CI, confidence interval
Table 4 Odds ratio of type 1 diabetes incidence in children in relation to perinatal characteristics of mothers and fathers
Mothers Fathers
Characteristics Crude estimates Adjusted estimate a Crude estimates Adjusted estimate a
OR 95% CI OR 95% CI OR 95% CI OR 95% CI
Age at delivery, yrs
<25 1.79 1.25-2.57 1.94 1.34-2.81 0.73 0.46-1.14 0.74 0.47-1.17 25-29 1.00 1.00 1.00 1.00 30-34 0.93 0.68-1.28 0.93 0.67-1.29 1.59 1.21-2.08 1.57 1.19-2.05 >=35 0.56 0.37-0.85 0.60 0.39-0.92 1.62 1.21-2.17 1.56 1.16-2.10 Mode of delivery Vaginal birth 1.00 Cesarean section 2.43 1.54-3.84 2.35 1.52-3.64 Preeclampsia No 1.00 Yes 6.71 1.12-40.35 4.68 0.60-36.49 History of admission for infection during pregnancy No 1.00
Yes 1.43 0.32-6.34 1.15 0.24-5.51 Gestational diabetes mellitus No 1.00 Yes 5.40 3.62-8.50 4.36 2.76-7.77 Diabetes No 1.00 1.00 1.00 1.00 Type 1 10.71 0.67-171.86 2.02 0.09-45.98 10.48 1.47-76.64 7.36 1.02-57.21 Type 2 0.48 0.21-1.01 0.50 0.21-1.17 1.67 1.15-2.44 1.54 1.04-2.26
Type 1 & Type 2 1.79 0.21-14.89 2.65
0.30-23.25
5.24
0.96-28.72
5.56 1.01-30.71
a Adjusted estimates were derived from logistic regression model that simultaneously includes all characteristics of children (Table 2) and those of mothers or fathers in the model.
Supplementary Figure 1. Flow Chart of Setting Study Cohort and Section of Cases and Controls.