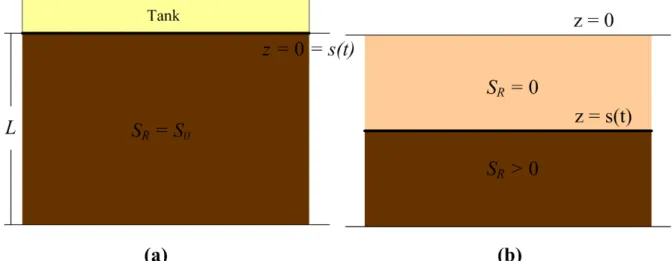國 立 交 通 大 學
環 境 工 程 研 究 所
碩 士 論 文
揮發性有機物在未飽和土壤中自然揮發之研究
A Study on Natural Evaporation of VOC in Unsaturated
Soils
研究生:俞仲豪
指導教授:葉弘德 教授
揮發性有機物在未飽和土壤中自然揮發之研究
A Study on Natural Evaporation of VOC in Unsaturated
Soils
研究生:俞仲豪 Student:Jung-Hau Yu
指導教授:葉弘德 Advisor:Hund-Der Yeh
國立交通大學
環境工程研究所
碩士論文
A ThesisSubmitted to Institute of Environmental Engineering College of Engineering
National Chiao Tung University in Partial Fulfillment of the Requirements
for the Degree of Master of Science in Environmental Engineering
September 2010 Hsinchu, Taiwan
揮發性有機物在未飽和土壤中自然揮發之研究
研究生:俞仲豪 指導教授:葉弘德
國立交通大學環境工程研究所
摘 要
地面儲存槽的洩漏,是土壤污染主要的污染源之一,發生滲漏後,部分揮發性有機 物(volatile organic compound, VOC)會以非水相液體(NAPL)殘存在土壤中,若經過一 段時間,氣相、液相、及吸附相的 VOC 會處於平衡狀態。若要整治受污染的土層,需先 移除滲漏的儲存槽,隨後 VOC 將會在自然環境下逸散至大氣中,此自然揮發的過程,值 得研究。VOC 存在土壤中,可分為純 VOC 和以多種化合物組成的複合 VOC 兩類,常見的 汽油儲存槽滲漏污染問題,則以複合 VOC 污染居多。在本文中,我們發展了一個數值模 式,描述複合 VOC 殘存相的揮發鋒面移動及地表下莫耳分率的空間分布,在模式中,考 慮於揮發鋒面及其上下三個區域,透過有限差分法及可移動格網,近似求解莫耳分率的 分佈。此外,考慮單一 VOC 污染的問題,將複合 VOC 模式簡化為單 VOC 模式,利用 Boltzmann's transformation,求得單 VOC 模式的解析解。本文考慮六個污染案例, 包括有無殘存相解之濃度分佈,以及不同土壤孔隙率、污染物、初始莫耳分率對揮發鋒 面和莫耳分率分布之影響。藉由計算 VOC 揮發鋒面的位置和移動速度,本研究發展的模 式,或可用來分析或評估受 VOC 污染的現地問題。
A Study on Natural Evaporation of VOC in Unsaturated Soils
Student:Jung-Hau Yu Advisor:Hund-Der Yeh
Institute of Environmental Engineering
National Chiao Tung University
Abstract
Leak of underground storage tank is one of major sources for the spill of volatile organic
compound (VOC) entering unsaturated soil. Once leak occurs, some VOCs may present in
soil as residual non-aqueous phase liquid (NAPL) phase. The gas, liquid, and absorbed
phases of VOC may reach equilibrium after a period of time in the soil. To clean up the
contaminated soil, the tank must be removed and the VOC can then evaporate to the
atmosphere. It is of interest to investigate the natural evaporation of NAPL. There are two
types of VOC contamination in soil. One is multi-component VOC while the other is
single-component VOC. Multi-component VOC, composed of several VOC components, is
often found in petroleum leaking problems. This study develops a two-component model
describing the mole fraction distributions of VOCs and migration of evaporation front of the
VOC in NAPL phase in a homogeneous soil system. The model considers three regions
moving grid approach. The model is also simplified to a single-component model which
deals with a one-component VOC contamination and solved analytically by Boltzmann’s
transformation. Six cases are considered including a comparison of the solutions for the
cases with and without the presence of residual NAPL phase and the assessment for the
influences of different soil porosity, chemicals, and initial mole fraction on the front location
and mole fraction. Finally, analytical expressions for the depth and moving speed of the
front are also developed for practical uses.
誌 謝
首先誠摯的感謝指導教授葉弘德,老師悉心的教導使我得以一窺地下水領域的深 奧,不時的討論並指點我正確的方向,使我在這兩年中獲益匪淺。老師對學問的嚴謹更 是我輩學習的典範。本論文的完成另外亦得感謝口試委員台灣大學吳先祺教授、逢甲大 學馮秋霞教授、及本校葉立明教授,有你們的指正及建議,使得本論文能夠更完整而嚴 謹。 感謝智澤、彥禎、士賓、博傑學長、雅琪、彥如、敏筠學姐、珖儀、其珊以及璟勝、 庚轅、昭志、國豪、裕霖學弟們,這些日子,實驗室裏共同的生活點滴,學術上的討論, 感謝眾位學長姐、同學、學弟的共同砥礪,你們的陪伴讓兩年的研究生活變得絢麗多彩, 你們對於我的幫助我銘感在心。 最後,將本論文獻給我最摯愛的家人。TABLE OF CONTENTS
摘要 ---I ABSTRACT ---II 致謝---IV TABLE OF CONTENTS ---V
LIST OF TABLES ---VII
LIST OF FIGURES ---VIII
NOTATION ---X
CHAPTER 1 INTRODUCTION ---1
1.1. Background ---1
1.2. Literature Review ---2
1.3. Objectives ---4
CHAPTER 2 METHEMATICAL MODEL ---6
2.1 Mathematical model: Two-component case ---6
2.1.1 Below the evaporation front ---9
2.1.2 Above the evaporation front ---10
2.1.3 At the evaporation front ---11
2.1.4 Boundary and initial conditions ---11
2.2.1 Finite difference approximation ---13
2.2.2 The solution procedure of the model ---15
2.3 Simplified model: Single-component case ---16
2.3.1 Below the evaporation front ---16
2.3.2 Above the evaporation front ---16
2.3.3 At the evaporation front ---17
2.3.4 The analytical solution of single-component model ---19
CHAPTER 3 RESULTS AND DISCUSSION ---22
3.1 Case 1: Different evaporation times in two models ---23
3.2 Case 2: Initial mole fraction ---24
3.3 Case 3: Soil porosity ---25
3.4 Case 4: Absence of the NAPL phase ---26
3.5 Case 5: Different chemicals ---27
3.6 Case 6: Effect of effective diffusion coefficient on moving speed of evaporation front---28
CHAPTER 4 CONCLUSIONS---29
LIST OF TABLES
Tables
Page
1 Soil chemical properties used in case studies--- 36
LIST OF FIGURES
Figures Page
1 Schematic diagram of VOC contamination problem--- 38
2 Flowchart of the solution procedure for two-component
model--- 39
3 Spatial distributions of mole fraction predicted by the
single-component and two-component models at various
evaporation times --- 40
4 The curves of the mole fraction versus depth at 100 day
with various initial mole fractions--- 41
5 NAPL phase saturation versus mole fraction of benzene---- 42
6 Normalized total concentration versus depth at 100 day for
different values of soil porosity --- 43
7 The curves of normalized total concentration versus depth
for single-component and simplified Jury et al.’s models at
8
9
10
11
Normalized total concentration versus time for
single-component and simplified Jury et al.’s models at the
depth of 1 m
---Normalized total concentrations of carbon tetrachloride
and toluene versus depth at 100 day
---The curves of the depths of evaporation front versus
evaporation time for carbon tetrachloride and toluene
---Depth and Moving speed of evaporation front versus time
for different effective diffusion coefficients ---45
46
47
48
NOTATION
The following symbols are used in this thesis: CT = total concentration
CL = liquid-phase concentration
CS = adsorbed-phase concentration
CG = gas-phase concentration
CT0 = saturated total concentration
CL0 = saturated liquid concentration
CG0 = saturated gas concentration
CS0 = saturated adsorbed concentration
CGP = equilibrium gas concentrations of pure component
CLP = equilibrium liquid concentrations of pure component
DGair = diffusion coefficient in air
DG = diffusion coefficient in gas phase in soil
DE = effective diffusion coefficient
f = mass fraction of organic compound in NAPL
i = number of component
u = mole fractions of organic compounds in the NAPL phase
u0 = initial mole fraction
φ = soil porosity
θR = volumetric content of NAPL
θL = volumetric content of liquid-phase
θG = volumetric content of gas-phase
θR 0 = initial volumetric content of NAPL
θL 0 = initial volumetric content of liquid-phase
θG 0 = initial volumetric content of gas-phase
SR = saturation of NAPL
SL = saturation of liquid-phase
SG = saturation of gas-phase
S0 = initial NAPL saturation
t = time
z = depth from surface
ρR = density of NAPL
ρb = soil bulk density
KH = Henry’s Law constant
KD = distribution coefficient
Koc = organic carbon partition coefficient
foc = soil organic carbon fraction
PP
0
= saturated vapor pressure of the VOC M = molecular weight of the VOC
T = absolute temperature L = depth of lower boundary
d = stagnant air boundary layer with thickness dz = initial grid size
dzr = grid size above the front dzN-r = grid size below the front
dt = time interval
Uf = moving speed of evaporation front
C = a constant parameter ηi = φSG +
(
φSW +ρbKDi)
KH i σ = ρRφ/CGiP i δ = ηi/DG i μ = σiM /i DG( )
u F = δ1u+μ1u[
C−δ1u−δ2(
1−u)
]
[
μ1u+μ2(
1−u)
]
h = Dair d G /CHAPTER 1 INTRODUCTION
1.1
BackgroundSubsurface contamination by volatile organic compounds (VOCs) has been one of
important issues to environmental problems recently. The pollution of VOCs is a common
problem in nowadays, and VOCs may harm human health in skin and the respiratory system.
The VOCs may enter subsurface soil from a variety of sources such as surface spill and
leaking storage tank. They present in the unsaturated soil in different forms such as gas,
liquid, adsorbed, and non-aqueous phase liquid (NAPL) phases. After a long residing time,
the NAPL phase may be redistributed by water flow and turn into residual phase which is
essentially held by surface tension and may stay in the soil pores over a long period of time.
The transport mechanisms of VOC in the unsaturated soil generally include advection,
diffusion, dispersion, adsorption, volatilization, and chemical and biological reactions.
Often diffusion is the dominating mechanism under natural condition, especially, for gas
transport in a low-permeability soil. Advection is commonly ignored in studying organic
vapor and gas migration in unsaturated soils (e.g., Jury et al., 1983; Zaidel and Russo, 1994;
Yates et al., 2000). Falta et al. (1989) indicated that density driven advection is insignificant
if the magnitude of soil permeability is less than 1×10-11 m2. Massmann and Farrier (1992)
mentioned that the advection induced by atmospheric fluctuation is not substantial for gas
conditions.
Soil vapor extraction (SVE) is an in-situ remedial technology for removing volatile
constituents from unsaturated contaminated soils. In the cases of sparse VOC or low
permeability formation, the removal efficiency of volatile compounds in soils will be
significantly decreased. For this reason, diffusion may be the dominating mechanism for
VOCs to move to a high permeability zone or volatilize to atmosphere under natural condition
(Hoier et al., 2007). For nature evaporation, the upper boundary of the zone having NAPL
phase becomes a front which moves downward with increasing time. The evaporation front
of NAPL phase can therefore be defined as a moving boundary and the location of the moving
boundary naturally represents the depth of NAPL. Moving boundaries are time-dependent
and the position of the boundary has to be determined as a function of time and space (Crank,
1984). The moving boundaries occur mostly in heat-flow problems with phase changes and
in some diffusion problems.
1.2 Literature Review
Jury et al. (1983) presented an analytical solution for a single pesticide species without
NAPL phase undergoing first-order decay in an unsaturated zone. Sleep and Sykes (1989)
presented a two-dimensional finite element model to simulate VOC partitioning to gas, liquid,
solid and NAPL phases and its transport in unsaturated soils. They indicated that the
al. (1990) used a method developed by Jury et al. (1983) to study the effect of vapor phase
sorption on organic compounds transport. Their results showed that vapor phase sorption
affects liquid phase organic concentration and also retards the rate of chemical migration
toward the water table. Massmann and Farrier (1992) proposed a mathematical model to
describe the transport of organic compounds in NAPL, gas, liquid, and adsorbed phases and
used the finite difference approximation to simulate the movement of contaminants emanating
from two point sources. Zaidel and Zazovsky (1999) developed a mathematical model to
investigate the depletion of multi-component NAPL due to the evaporation and vapor
transport in a SVE process. Yates et al. (2000) presented an analytical model to study the
diffusion of organic vapors or other gases in layered soil systems.
Moving boundary problems are also called Stefan problems referred to Stefan’
investigations on the melting of the polar ice cap (Stefan, 1889a and b). Crank (1984) gave a
literature survey on the Stefan problems occurring in physical and biological sciences,
engineering, and decision and control theory, etc. Recently, studies involved moving
boundaries were applied in the areas such as heat conduction [e.g., Cheng, 2000],
solidification and melting processes [e.g., Feltham and Garside, 2001; Szimmat, 2002;
Caldwell et al., 2003; Lee and Marchant, 2004; Rattanadecho, 2006; Naaktgeboren, 2007;
Patnaik et al., 2009], dissolution [e.g., Quintard and Whitaker, 1995; Vazquez-Nava and
1.3 Objectives
The purpose of this thesis is to develop a two-component model to predict the mole
fraction distributions of VOC and the migration of evaporation front of NAPL phase after the
removal of the storage tank in a homogeneous unsaturated soil. To the best of our
knowledge, the existing models in simulating the natural evaporation of VOC in unsaturated
soil have the problem domains all with fixed boundaries. In contrast, the present model
considers a moving boundary to describe the downward move of evaporation front of NAPL
phase. The front, which migrates downward with time, is treated as a lower boundary while
the land surface is used as the upper boundary for the region above the front. For the region
below the front, the front becomes the upper boundary and the lower boundary is located at
some distance below the land surface. Based on the present model with these boundaries,
VOC mole fraction distributions and the front location are solved by the finite difference
method with a moving grid approach. This numerical model can predict the mole fraction
distributions between two components and the movement of the front, analyze evaporation
time of VOC, and assess the influences of initial mole fraction, soil porosity as well as
chemical volatility on VOC migration in the unsaturated soil. Moreover, a
single-component model is obtained from simplifying the two-component model and solved
analytically using Boltzmann’s transformation. Then, an analytical expression for the
CHAPTER 2 METHMETICL MODEL
This chapter presents the mathematical models and related solution procedures for VOC
transport in homogeneous and unsaturated soils.
2.1. Mathematical model: Two-component case
Figure 1(a) shows a leaking storage tank located on the top of land surface and filled
with VOC. Consider that the VOC leaking from the tank has four different phases (namely,
gas, liquid, adsorbed and residual NAPL phases) and distributes uniformly in the unsaturated
zone. Each phase has a saturated or equilibrium concentration and constant volumetric
content. The VOC has an initial NAPL saturation S0 in the soil. The saturation of each
phase denotes as the volume percentage in the soil pore and the sum of saturation of each
phase equals one. The evaporation front of the NAPL, denoted as s(t), initially stays right at
the land surface, i.e., z = s(t) = 0 where z is the vertical axis, and moves downward with
increasing time. Figure 1(b) shows the contamination scenario in which the gas phase VOC
begins to evaporate to atmosphere and the NAPL starts to vaporize to gas phase after the tank
being removed. Assume that the NAPL phase of VOC evaporates fully above the front and
the front migrates instantaneously when the evaporation occurs. In other words, the VOC
presents only in gas, liquid, and adsorbed phases and the NAPL saturation, SR, equals zero
between the land surface and the front. Below the front, the residual VOC still exists. At
of NAPL transferring to gas phase follows the conservation of mass.
The total concentration of VOC is the sum of the concentration of each phase; that is:
R R b S L L G G T C C C C = θ + θ + ρ +ρ θ (1) where CG, CL, and Cs represent the chemical concentrations in the gas, liquid, and adsorbed
phases, respectively, and ρb and ρR are the soil bulk density and density of NAPL,
respectively. The symbols θG, θL, and θR are the volumetric contents of gas, liquid, and
NAPL phases, respectively, and denote as
G G φS
θ = , θL =φSL, and θR =φSR (2)
where φ is the soil porosity and SG and SL are gas and liquid saturations, respectively.
The equilibrium relationships between the gas and liquid phases as well as the liquid and
adsorbed phases may be expressed, respectively, as
L H G K C
C = and CS =KDCL =KocfocCL (3)
where KH is Henry’s Law constant, KD is the distribution coefficient, Koc is the organic carbon
partition coefficient, and foc is the soil organic carbon fraction. The equilibrium relationships
given in Equation (3) are linear and reversible and their coefficients are dependent on
different chemical characteristics and soil properties.
The equation of mass conservation for those four phases in the unsaturated soil is
[
+ + + +∇ =0 ∂ ∂ G R R b S L L G G C C J C t θ θ ρ ρ θ]
(4) where JG =−DG∇CG is the vapor flux and DG is the soil-gas diffusion coefficient. Atortuosity factor accounting for the reduced flow area and increased path length of diffusing
molecules in soil can be related to the soil porosity and the fluid content in the soil.
Millongton and Quirk (1961) defined that
⎟ ⎟ ⎟ ⎠ ⎞ ⎜ ⎜ ⎜ ⎝ ⎛ = 23 10 φ θG air G G D D (5)
where DGair is the air-gas diffusion coefficient.
Consider that VOC has multiple components. Substituting Equations (2) and (3) into
Equation (4), the mass-conservation equation in three dimension for those four phases in the
unsaturated soil becomes (Zaidel and Zazovsky, 1999)
(
)
[
]
(
)
) ( G Gi i R R Li Di b L Gi G D C t f S t C K nS C nS ∇ ⋅ ∇ = ∂ ∂ + ∂ + + ∂ ρ ρ φ (6) with∑
= j j i i p Gi Gi M f M f C C / / ,∑
= j j i i p Li Li M f M f C C / / (7) 1 1 =∑
= c N i i f (8)where and are equilibrium gas and liquid concentrations of pure component,
respectively, f is the mass fraction of organic compound in NAPL, i is the number of each
component, and N p G C p L C
concentration for pure component can be estimated from the idea gas law as T M P CP G = /ℜ 0 (9) where PP
0 is the saturated vapor pressure of the VOC, M is the molecular weight of the VOC,
is the ideal gas constant, and T is the absolute temperature. ℜ
To describe the behavior of volatilization of VOC from NAPL phase to gas phase, the
evaporation front is introduced as a moving boundary in the unsaturated soil. The problem
domain for VOC transport with a moving boundary in the soil can be divided into three
regions. The VOC transport equations in these three regions are described below.
2.1.1 Below the evaporation front
In this region, NAPL phase exists and the saturation of NAPL phase is greater than zero, i.e., SR >0. Equation (8) can then be expressed as
1 1 =
∑
= c N i i u (10)with representing mole fractions of organic compounds in the NAPL phase, i.e.,
. For a one-dimensional system, Equation (6) becomes:
i u P Gi Gi i C C u = /
(
)
2 2 / z u D t M u M u S t u i G j j i i R i i i ∂ ∂ = ∂ ∂ + ∂ ∂ σ∑
η (11)where ηi =φSG+
(
φSW +ρbKDi)
KH and . For a two-component VOC andbased on Equation (11), the mass-conservation equation for each component can be written
P Gi R i ρ φ/C
as:
(
)
2 1 2 2 2 1 1 1 1 1 1 1 / z u D t u M u M u M S t u G R ∂ ∂ = ∂ + ∂ + ∂ ∂ σ η (12)(
)
2 2 2 2 2 1 1 2 2 2 2 2 / z u D t u M u M u M S t u G R ∂ ∂ = ∂ + ∂ + ∂ ∂ σ η (13)Select u = u1 as a primary variable. The saturation of NAPL phase can be obtained from
Equation (12) as
(
)
[
]
[
(
)
]
(
u)
u u M u M u u C SR − + − + − − − = 1 1 1 2 1 2 1 2 1 μ μ δ δ (14)where δi =ηi/DG, μi =σiM /i DG, and C is a constant parameter computed by the initial NAPL saturation S0 and mole fraction u0 of component one as:
) 1 ( ) 1 ( ) 1 ( 0 2 0 1 0 2 0 1 0 0 2 0 1 u M u M u u S u u C − + − + + − + =δ δ μ μ (15)
Substituting Equations (10) and (14) into Equation (12) yields
( )
2 2 z u t u F ∂ ∂ = ∂ ∂ (16) where F( )
u =δ1u+μ1u[
C−δ1u−δ2(
1−u)
]
[
μ1u+μ2(
1−u)
]
.2.1.2 Above the evaporation front
In this region the NAPL phase fully evaporates. i.e., SR =0. For a two-component VOC, Equation (11) can be written for each component as follows:
2 1 2 1 1 z u t u ∂ ∂ = ∂ ∂ δ (17) 2 2 2 2 2 z u t u ∂ ∂ = ∂ ∂ δ (18)
where u1 and u2 represent normalized concentrations above the evaporation front,
2.1.3 At the evaporation front
Assume that the evaporation of NAPL phases occurs instantaneously and the sum of
mole fraction equals one, i.e., u1+u2 = 1. Combining Equations (12) and (13), the equation
for mass conservation at the front can be expressed as
t u t u z u D z u D t SR G G ∂ ∂ ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ − ∂ ∂ ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ − ∂ ∂ ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ + ∂ ∂ ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ = ∂ ∂ 2 2 2 1 1 1 2 2 2 2 2 1 2 1 σ η σ η σ σ (19)
Taking the limits of and , Equation (18) describing the front can then be
written as 0 → Δt Δz→0
(
)
(
)
t u u u u S Lim t t t t R t Δ − + − + − + − + − → Δ 2 2 2 2 1 1 1 1 0 0 σ η σ η s z u z u D z u z u D Lim z z G z z G s Δ ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ ∂ ∂ − ∂ ∂ + ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ ∂ ∂ − ∂ ∂ = − + − + → Δ 2 2 2 1 1 1 0 σ σ (20)where the superscripts t+ and t– of the mole fraction in Equation (20) denote the mole fraction
at the time slightly after and before the volatilization, respectively, and z+ and z– represent the
mole fraction at the region slightly below and above the front, respectively. Equation (20)
can be further simplified as
(
t t)
(
t t)
R z z G z z G S u u u u z u z u D z u z u D dt ds − − + − ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ ∂ ∂ − ∂ ∂ + ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ ∂ ∂ − ∂ ∂ = − + − + − + − + 2 2 2 2 1 1 1 1 2 2 2 1 1 1 σ η σ η σ σ (21)2.1.4 Boundary and initial conditions
considered to diffuse across a stagnant air boundary layer with thickness d and the gas phase
concentration is assumed equal to zero at the top of boundary layer. The flux diffusing to
the atmosphere can then be expressed as (Jury et al., 1983)
G T G G R C h z C D = ∂ ∂ , at z=0 (22)
where . If the thickness of air boundary layer near the surface is very small and
negligible, one may assume that d = 0. Equation (22) can then be expressed as d D h air G / = 0 = T C , at z=0 (23)
For a multicomponent VOC, the upper boundary conditions can be written from Equation (23)
as
( )
0, 2( )
0, 0 1 t =u t =u (24)
The conditions of the mole fractions at the lower boundary are denoted as:
( )
L t uou1 , = , u2
( )
L,t = 1−uo (25)where L is the depth of the lower boundary.
The initial NAPL saturation and the mole fractions of each component are assumed
spatially uniform; they are
( )
z,0 S0SR = (26)
( )
z uoThe evaporation front is initially located at the land surface and thus denoted as
( )
0 =0s (28)
2.2 The numerical method in solving the model
This section presents the numerical method used to solve the two-component model.
2.2.1 Finite difference approximation
The equations of describing VOC transport in the three regions are solved separately by
the finite difference method. An interpolative moving grid approach (Javierre et al. 2006) is
adopted to handle the moving boundary problem. The total number of nodes within the
problem domain is equal to N and r is the nodal number assigned at the evaporation front
beginning from the land surface. Accordingly, the number of grids from the land surface to
the front is r-1, the number of grids below the front is N-r, and the initial grid size dz is equal
to L/(N-1). The grid sizes above and below the front defined as dzr and dzN-r, respectively,
need to be re-estimated after each move of the front. To avoid introducing large truncation
error, the grid sizes dzr and dzN-r should be close to dz. If the front move to a location
between the nodal numbers initially assigned as j and j+1, the new grid sizes of dzr and dzN-r
are then estimated by s/(r-1) and (L-s)/(N-r), respectively, where r = j+1. The backward
difference relative to time and central difference relative to space are used to approximate the
region below the front obtained from Equation (16) is
( ) ( )
2 1 1 1 ) ( 2 r N n j n j n j n j n j dz u u u dt u F u F − − + − − + = − , s )(t < z≤∞ (29)where n is the number of time step, and dt is the time interval. The difference equations for
the mole fractions of the two components in the region above the front obtained from
Equations (17) and (18) are, respectively:
2 ) 1 ( 1 1 ) 1 ( 1 1 1 1 1 ) ( 2 r n j n j n j n j n j dz u u u dt u u − + − + − = − δ , 0≤z<s(t) (30) 2 ) 1 ( 2 2 ) 1 ( 2 1 2 2 2 ) ( 2 r n j n j n j n j n j dz u u u dt u u − + − + − = − δ , 0≤z<s(t) (31)
Finally, the difference equations for the mole fractions of the two components at the
front also derived from Equations (17) and (18) with different grid sizes above and below the
front are, respectively, expressed as
r N r r N r n r r N r n r r N n r r n r n r dz dz dz dz u dz dz u dz u dz dt u u − − − − − + − + + − + = − ⎟ ⎠ ⎞ ⎜ ⎝ ⎛ ) ( ) ( 2 1 ) 1 ( 1 ) 1 ( 1 1 1 1 1 δ , z=s(t) (32) r N r r N r n r r N r n r r N n r r n r n r dz dz dz dz u dz dz u dz u dz dt u u − − − − − + − + + − + = − ⎟ ⎠ ⎞ ⎜ ⎝ ⎛ ) ( ) ( 2 2 ) 1 ( 2 ) 1 ( 2 1 2 2 2 δ , z=s(t) (33)
Substituting Equation (10) into Equation (21), the difference equation for the front location
(
nr nr)
(
nr nr)
R r n r n r r N n r n r G r n r n r r N n r n r G n n S u u u u dz u u dz u u D dz u u dz u u D dt s s − − + − ⎟ ⎟ ⎠ ⎞ ⎜ ⎜ ⎝ ⎛ − − − − + ⎟ ⎟ ⎠ ⎞ ⎜ ⎜ ⎝ ⎛ − − − = − − − − − + − − + − 1 1 1 2 2 1 1 1 1 1 ) 1 ( 2 1 ) 1 ( 1 1 2 ) 1 ( 1 1 1 ) 1 ( 1 1 1 1 σ η σ η σ σ (34)2.2.2 The solution procedure of the model
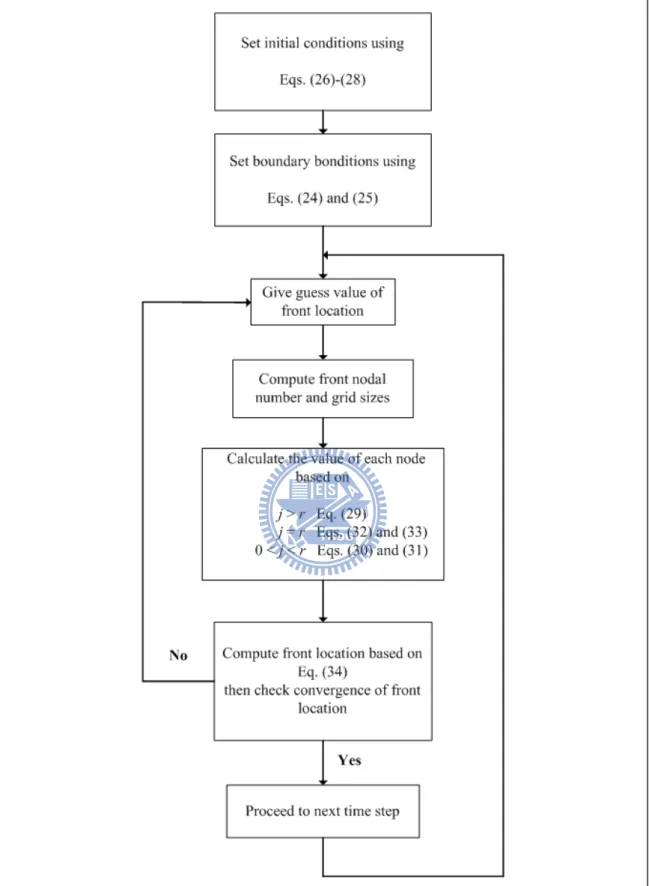
Because the location of the evaporation front s(t) is unknown, a trial and error procedure
is therefore taken to find the front location. The steps of the procedure are listed below and
the related flowchart is shown in Figure 2:
1. Give the initial front location (Equation (28)) and nodal values of mole fraction based on
the boundary conditions (Equations (24) and (25)) and initial conditions (Equations (26) and
(27)).
2. Guess front location after the start of evaporation.
3. Determine the nodal number for the front and the grid sizes based on the front location.
4. Solve the solutions for the region below the front (Equation (29)), for the region above the
front (Equations (30) and (31)), and at the front (Equations (32) and (33)).
5. Compute the front location based on Equation (34).
6. Proceed to next time step if the location of the front converges, i.e., the difference between
two succeeding estimations of the front location is less than a very small value, e.g., 10-10 m;
2.3 Simplified model: Single-component case
This section presents a single-component model with the governing equation simplified
from the two-component model. The domain of the single-component model is also divided
into three regions based on the front location. In a one-dimensional homogeneous and
unsaturated soil system, Equation (6) representing the VOC transport can be written as
0 2 2 = ∂ ∂ − ∂ ∂ z C D t C G G T (35)
2.3.1 Below the evaporation front
In this region, the NAPL has not vaporized to gas phase yet. The VOC concentrations
in each phase are the initial saturated concentrations and the total concentration can be
expressed as 0 0 0 0 R R b P S L P L G P G T T C C C C C = = θ + θ + ρ +ρ θ (36) where CSP is the saturated VOC concentrations for pure component in adsorbed phases, CT0 is
the initial total concentration, and θG0, θL0, and θR0 are the initial volumetric contents,
respectively.
2.3.2 Above the evaporation front
In this region, NAPL phase of the VOC completely vaporizes to gas phase; therefore, the VOC presents only in gas, liquid, and adsorbed phases, i.e., CT = CGθG+ CLθL+CSρb. Jury et
Accordingly, one may introduce the following equation based on Equations (1) and (3): S S L L G G T R C R C R C C = = = (37)
where the ratios are RG =θG+θL KH +ρbKD KH , RL =θGKH +θL +ρbKD, and
b D L D H G S K K K R =θ +θ +ρ .
With Equation (37), Equation (35) can be written as
0 2 2 = ∂ ∂ − ∂ ∂ z C D t C T E T (38)
where denotes as effective diffusion coefficient. Equation (38) describes the
VOC transport in gas and liquid phases between the land surface and evaporation front. In
reality, the volatilization of NAPL occurs right at the evaporation front which will be
discussed in the next section.
G G E D R
D = /
2.3.3 At the evaporation front
The evaporation front moves downward while the NAPL vaporizes to the gas phase.
Assume that the transformation of the contents between these two phases occurs
instantaneously and the liquid volumetric content is unchanged, i.e., θL = θL0. In addition,
the VOC concentrations in each phase are still saturated,i.e., CG = CGP, CL = CLP, and CS =
CSP. The total concentration at the front can therefore be expressed as
0 T T C
C = (39)
θR equals soil porosity φ, i.e., θG+θL+θR =φ. Assumes φ does not change with time,
the transformation of volumetric content with respect to time among each phase is conserved.
Thus, t t t R L G ∂ ∂ − = ∂ ∂ + ∂ ∂θ θ θ (40)
Since ∂θL/∂t =0, one can get ∂θG/∂t=−∂θR/∂t from Equation (40). In addition, the bulk density ρb does not change with time, i.e., ∂ρb/∂t =0. Therefore, one can obtain the
following relationship from Equation (1)
(
)
t C t C P R G R T ∂ ∂ − = ∂ ∂ ρ θ (41)With Equation (41) and taking the limits of Δt→0 and Δz→0, Equation (35) describing
the front can then be written as
(
)
z z C z C D Lim t C Lim T T E z R R P G R t Δ ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ ∂ ∂ − ∂ ∂ = Δ − − − + → Δ − + → Δ 0 0 θ θ ρ (42)where the superscripts + and - denote the volumetric content at the time slightly after and
before the volatilization, respectively, and the concentrations at the region slightly below and
above the front, respectively. Consider that the volatilization occurs instantaneously,
therefore equals and equals zero after evaporation. The VOC
concentrations in each phase are the initial saturated concentrations below the front and the
concentration gradient of gas phase below the front is naturally equal to zero, i.e.,
+ R θ 0 R θ − R θ 0 = ∂ ∂ + z
orders (Falta et al., 1989, Tables 1 and 4), the term related to CGP on the left-hand side of
Equation (42) is thus negligible. Accordingly, Equation (43) representing the front z = s(t)
can be expressed as z C D dt ds T E R R ∂ ∂ = − ρ θ0 (43)
2.3.4 The analytical solution of single-component model
Consider that the VOC is saturated or in equilibrium state in different phases and
uniformly distributed in the unsaturated soil initially. The mathematical model describing
the single-component VOC transport in the soil consists of Equation (38) as the governing
equation, Equations (39) and (43) as the lower boundary conditions, and Equation (23) as the
upper boundary condition.
Based on Boltzmann’s transformation, a new variable is defined as ξ = z 2 DEt . Equation (38) can then be transformed to an ordinary differential equation as
0 2 2 2 = + ξ ξ ξ d dC d C d T T (44)
The solution of Equation (44) can be obtained as (Carslaw and Jaeger, 1959)
( )
B erfA
CT(ξ)= ⋅ ξ + (45) where erf(ξ) is the error function and A and B are unknown coefficients. Substituting Equation (45) into Equation (23), the result for the concentration distribution is
( )
⎟⎟ ⎠ ⎞ ⎜ ⎜ ⎝ ⎛ × = t D z erf A t z C E T 2 , (46)Substituting Equation (39) into Equation (46), the evaporation front s(t) and coefficient A
can then be obtained, respectively, as
t t s( )=α (47) ⎟ ⎟ ⎠ ⎞ ⎜ ⎜ ⎝ ⎛ = E T D erf C A 2 0 α (48)
where α is an unknown constant depending upon the soil parameters and contaminant
characteristics.
The time of vanish of NAPL can be solved by Newton’s method (Yeh, 1987) from
Equation (47) when the front reaches a target location below the land surface designated by
the environmental or legal requirement. In addition, the moving speed of the evaporation
front can also be obtained after taking the derivative of Equation (47) with respect to time and
the result is t Uf 2 α = (49) Substituting Equations (46), (47) and (48) into Equation (43) yields
⎟ ⎟ ⎠ ⎞ ⎜ ⎜ ⎝ ⎛ ⎟⎟ ⎠ ⎞ ⎜⎜ ⎝ ⎛ − = E E E T E R R D erf D D C D 2 4 exp 2 2 0 0 α π α α ρ θ (50)
The unknown constant α can then be easily determined from Equation (50) by Newton’s
method. Note that the normalized total concentration is defined as CT(z,t)/CT0, representing
CHAPTER 3 RESULTS AND DISCUSSION
Leaks of petroleum fuels from the underground storage tanks are common problems for
soil contamination. The petroleum spills are often associated with aromatic hydrocarbons
such as benzene, toluene, ethyl benzene, and various xylene isomers (BTEX). In this section,
the hydrocarbons of benzene and toluene are chosen to simulate their transport and mole
fraction distributions in unsaturated soils using the two-component model. In the past,
Carbon tetrachloride was commonly used as coolant in industry or produced as the fire
extinguishers. Carbon tetrachloride is highly toxic; a small amount of this chemical residing
in the soil may pose severe environmental problems. The carbon tetrachloride in the
unsaturated soil is considered as a target VOC and analyzed using the single-component
model.
Six cases are considered to address the issues in regard to the evaporation rate,
evaporation front movement, mole fraction, and concentration distributions of VOC for the
present models. Case 1 is to compare the mole fractions of toluene at various evaporation
times predicted by single-component and two-component models. Case 2 examines the
effect of initial mole fraction on the evaporation and the changes of the mole fraction
distributions of benzene and toluene. Case 3 investigates the effect of soil porosity on
vaporization of carbon tetrachloride from NAPL phase to gas phase while case 4 addresses
the presence of NAPL phase. Case 5 studies the migrations of evaporation front for different
contaminants, namely carbon tetrachloride and toluene. Case 6 discusses the effect of
effective diffusion coefficient of carbon tetrachloride on the moving speed of evaporation
front. The values of the soil chemical properties are listed in Table 1 and the properties of
benzene, toluene, and carbon tetrachloride are given in Table 2 for these six cases. Note that
the depth of the lower boundary L is chosen as 5 m, the total number of nodes N is 10000, and
the time interval dt is 0.1 sec in the case study when adopting the finite different
approximation for the two-component model.
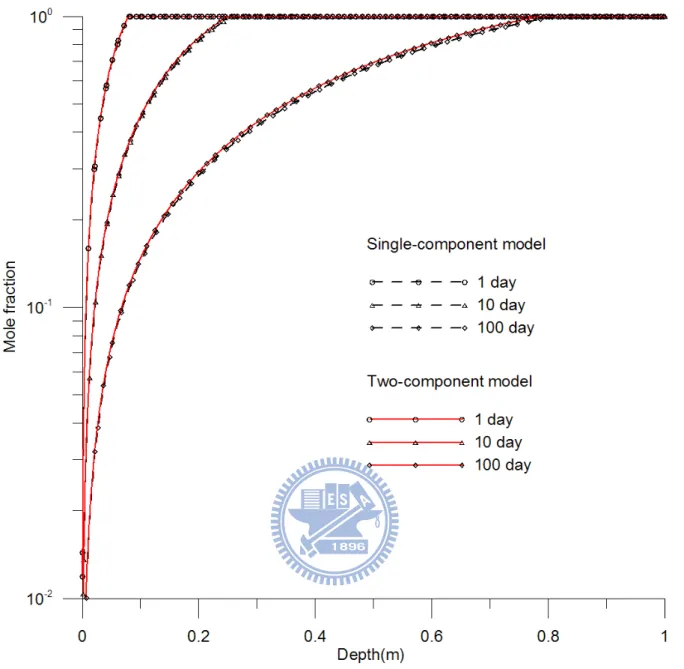
3.1 Case 1: Different evaporation times in two models
This case uses the same assumptions for both single-component and two-component
models and considers that toluene is the only VOC found in the soil, i.e., u0 = 1. Figure 3
shows the mole fraction distributions of toluene versus depth predicted by the
single-component and two-component models at various evaporation times. The dashed line
denotes the solution of single-component model while the solid line represents the results
predicted by the two-component model. Moreover, the symbols of rhombus, triangle, and
circle represent the mole fractions at times 1, 10, and 100 day, respectively. This figure
shows the front locations at various evaporation times and at the front the mole fraction equals
its initial value for the single-component VOC. The figure also shows that the curves
two-component model with the present numerical approach match well with those estimated
based on the analytical solution of the single-component model. The moving speeds of the
front Uf estimated by equation (49) are 8.296×10-2, 2.624×10-2, and 8.296×10-3 m/day at times
1, 10, and 100 day, respectively, indicating that the moving speed decreases rapidly at early
time and then slowly as time increases
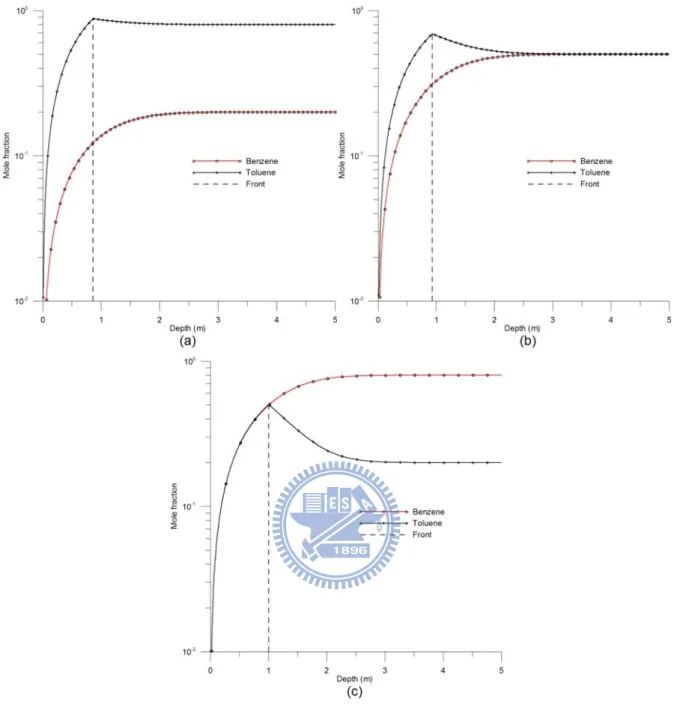
3.2 Case 2: Initial mole fraction
In this case, benzene is considered to be component one and toluene is component two in
the two-component model. Figures 4(a) - 4(c) show the mole fraction distributions of
benzene and toluene versus depth when the initial mole fractions of component one are 0.2,
0.5, and 0.8, respectively, at 100 day. The evaporation front of the NAPL with u0 = 0.2, 0.5,
and 0.8 reaches 0.860, 0.931, and 1.002 m below the surface, respectively. In addition, at
the front u1 = 0.123 and u2 = 0.877 when u0 = 0.2, u1 = 0.310 and u2 = 0.690 when u0 = 0.5,
and u1 = 0.498 and u2 = 0.502 when u0 = 0.8. The figures show that the depth of the front
increases with the initial mole fraction of benzene, representing the moving speed of the front
depends on the initial mole fraction. The mole fraction of benzene increases with depth until
reaching u1 = u0; on the other hand, the mole fraction of toluene increases above the front but decreases below the front until reaching u2 =1 u− 0. These results indicate that at the front, the mole fraction of benzene decreases as time increases while that of toluene increases with
the front. In fact, both components reach their initial values occurred at certain distances
below the front. Such a phenomenon can be attributed to the fact that benzene has higher
evaporation efficiency than toluene. Therefore, the mole fractions of benzene and toluene
change with depth, although the NAPL below the front does not evaporate. Figure 5 shows
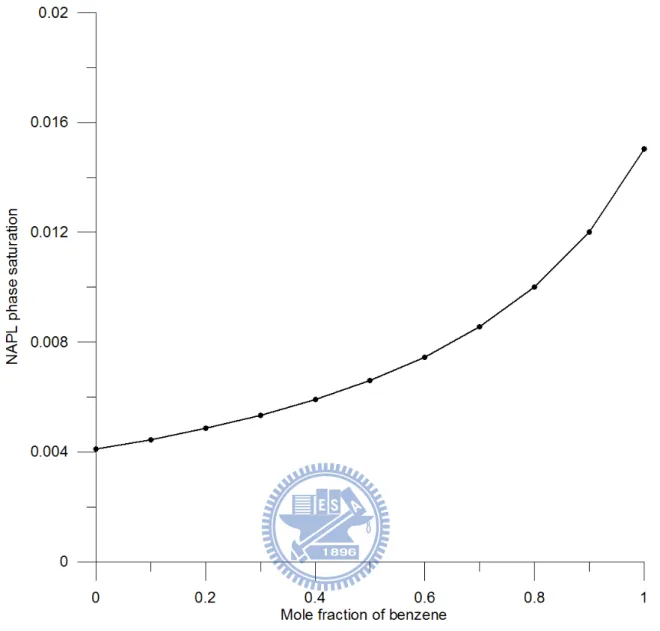
the curve of NAPL phase saturation, calculated from Equation (14), versus mole fraction of
benzene. The figure demonstrates that SR equals 0.004 when u0 = 0 and 0.015 when u0 = 1.
This result indicates that SR changes with mole fraction and varies slightly below the front.
3.3 Case 3: Soil porosity
This case examines the effect of soil porosity on the concentration distribution of carbon
tetrachloride using the single-component model. The total evaporation time is considered to
be 100day. Note that the saturation S of each phase is constant and the volumetric contents
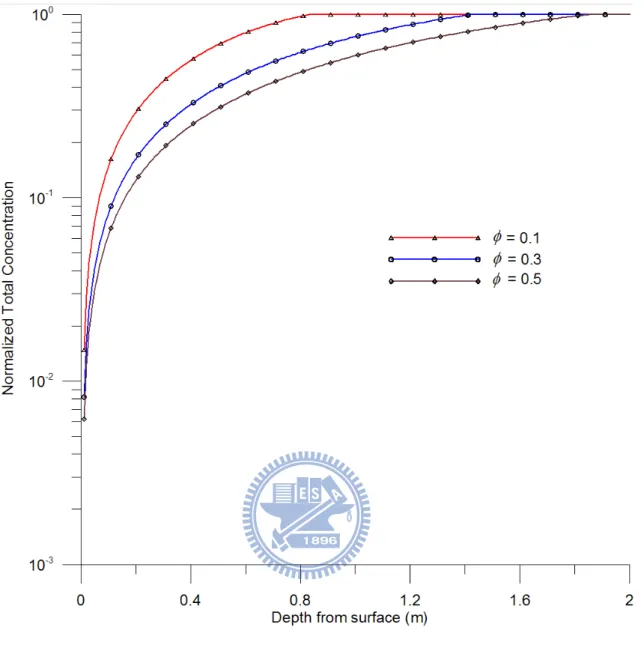
θ of gas, liquid, and NAPL change in equal proportion with the soil porosity. Figure 6 shows the predicted normalized total concentration versus depth at 100 day for the porosities
of 0.1, 0.3, and 0.5. It is apparent from Figure 4 that the vaporization increases moderately with soil porosity. In addition, the evaporation front of the NAPL with φ = 0.1, 0.3, and 0.5 are at 0.831, 1.432, and 1.860 m below the land surface, respectively, and the moving
speeds of the front are 4.154×10-3, 7.158×10-3, and 9.301×10-3 m/day, respectively, indicating
that the moving speed of the front increases with soil porosity although different amounts of
3.4 Case 4: Absence of the NAPL phase
In this case, the effects of presence and absence of NAPL on the distribution of carbon
tetrachloride in the soil are compared and studied. Jury et al. (1983) considered the scenario
that there were three phases of VOC presented in the soil with neglecting the NAPL phase.
Their analytical model included the mechanisms of diffusion, soil water advection, and
first-order decay. They used a diffusive flux as the upper boundary condition at the land
surface and zero total concentration at infinite depth as the lower boundary condition. Jury
et al.’s model is simplified by neglecting the water phase advection and decay and thus called
simplified Jury et al.’s modelhereafter.
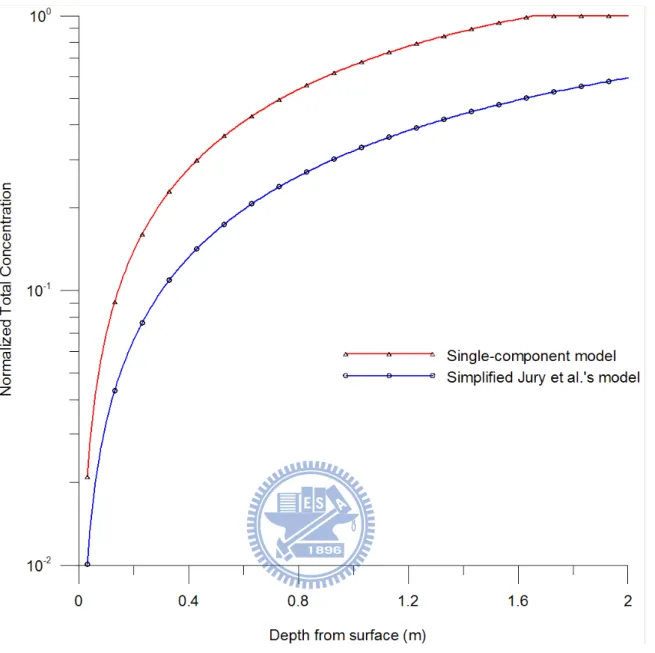
Figure 7 shows the curves of normalized total concentration of carbon tetrachloride
versus depth predicted by two different VOC transport models at t = 100 day. The solid line
with triangle symbol represents the normalized total concentration distribution predicted by
the present single-component model while the solid line with circle predicted by simplified
Jury et al.’s model. Figure 7 indicates that the normalized total concentration of VOC
predicted by the present model is significantly higher than that of simplified Jury et al.’s
model. Although NAPL occupies only one-percent of volume in the soil pores, the NAPL
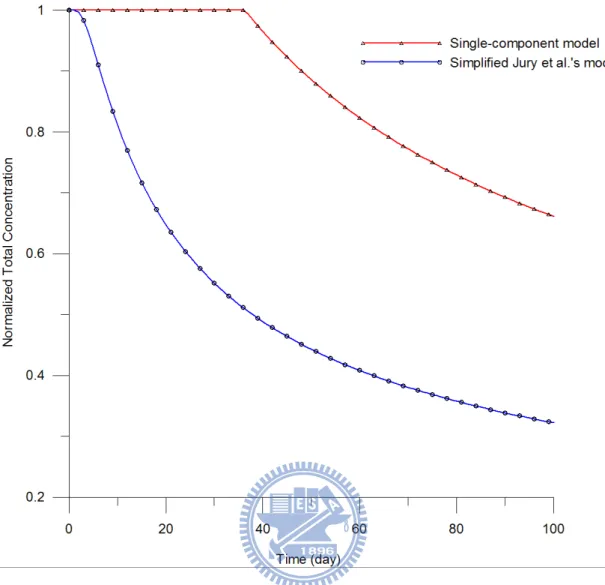
however affects the total concentration distribution and transport capability greatly. Figure 8
exhibits the predicted distribution of normalized total concentration versus time for the
the total concentration of carbon tetrachloride decreases quickly after 2 day predicted by
simplified Jury et al.’s model and after 37 day by the single-component model indicating that
the presence of NAPL has significant impact on the VOC transport.
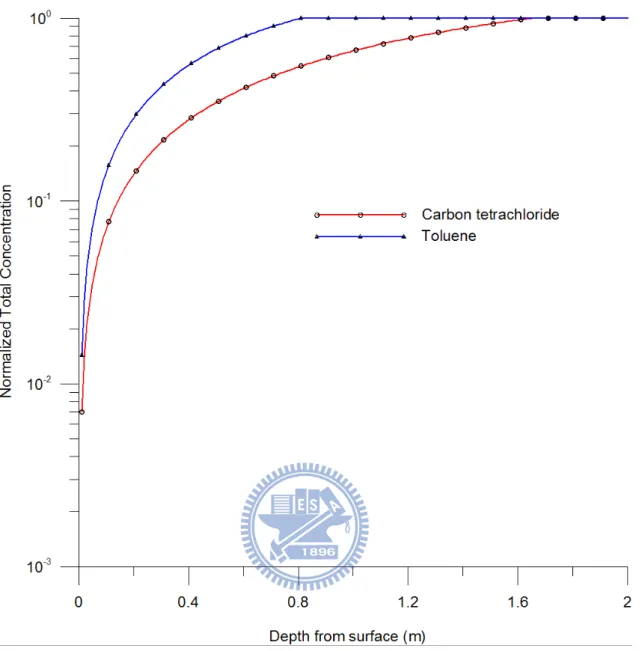
3.5 Case 5: Different chemicals
In this case, both carbon tetrachloride and toluene are considered to reside in the
unsaturated soil. Table 2 shows that toluene has less molecular weight and liquid density
and lower saturated vapor pressure and Henry’s law constant than those of carbon
tetrachloride. Figure 9 shows the curves of normalized total concentration versus depth for
carbon tetrachloride and toluene at 100 day predicted by the single-component model for each
chemical. The solid lines with circle and triangle represent the concentration distributions of
carbon tetrachloride and toluene, respectively. The vaporization of toluene is significantly
lower than that of carbon tetrachloride; the depths of the evaporation front of carbon
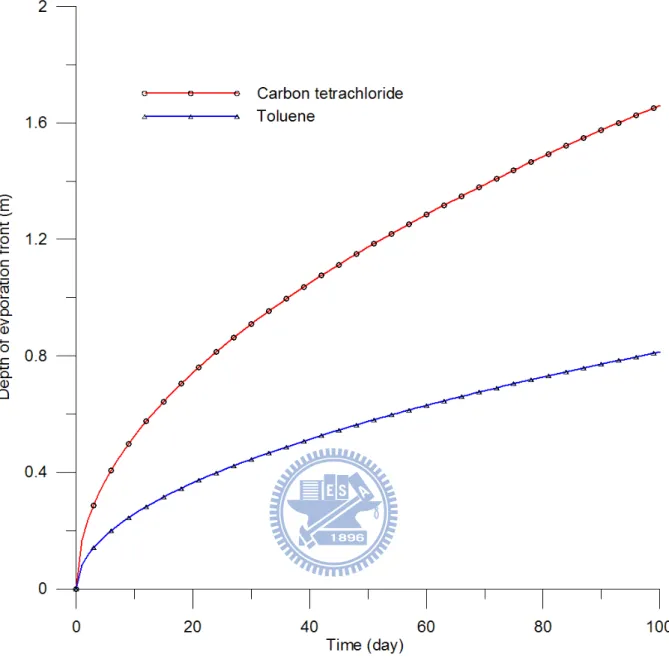
tetrachloride and toluene equal 1.659 m and 0.813 m, respectively, at 100 day. Figure 10
shows that the depths of the front versus evaporation time for both chemicals. This figure
indicates that the fronts at 50 day and 100 day reach the depths of 1.173 m and 1.659 m,
respectively, for carbon tetrachloride and 0.575 m and 0.813 m, respectively, for toluene.
Obviously, the migration of the front of carbon tetrachloride is significantly faster than that of
toluene due to lower vapor pressure and Henry’s Law constant value of toluene. Such a
and liquid phases. In addition, the diffusion coefficients of gas and liquid phases of both
chemicals differ by four orders of magnitude. Thus the diffusion of liquid phase is
significantly lower than that of gas phase.
3.6 Case 6: Effect of effective diffusion coefficient on moving speed of evaporation front
Equation (49) shows the moving speed of evaporation front Uf which in fact represents
the evaporation rate of NAPL in soil. This case investigates the effect of the soil chemical
properties on Uf based on the single-component model. The DE is a function of soil porosity,
volumetric content of each phase, air diffusion coefficient, soil bulk density, Henry’s Law
constant, and organic carbon fraction. Obviously, different soil chemical properties will
affect the value of DE. Figure 11 shows the curves of the depth and moving speed of the
front versus evaporation time for DE = 10-10, 10-9, 10-8, and 10-7 m2/s. The depth of the front
increases with time and DE greatly while the moving speed is maximal when VOC begins to
CHAPTER
4 CONCLUSIONS
This thesis presents a two-component model to describe the mole fraction distributions
of VOC in the unsaturated soil. In the model, zero-concentration is chosen as the upper
boundary condition and a moving boundary representing the evaporation front of NAPL is the
lower boundary in the region where the NAPL evaporates fully. In the region below the
front, the NAPL phase prevails. The upper boundary of this region is the evaporation front
and the lower boundary is relatively far away from the front and thus chosen at infinity. The
model is solved by the finite difference method with a moving grid approach. This
numerical model is applied to predict the mole fraction distributions between two components
and the movement of the front in the soil. In addition, the numerical model is also used to
analyze evaporation time of VOC and assess the influences of initial mole fraction, soil
porosity as well as chemical volatility on VOC migration. The two-component model is
further simplified to a single-component model, which is solved analytically based on
Boltzmann’s transformation. In addition, analytical expressions are also developed for the
front and its moving speed as functions of evaporation time, characteristics of soil and VOC,
and volumetric content of each phase.
Both two-component and single-component models have been used to study the problems
of the evaporation rate, evaporation front movement, mole fraction, and concentration
following conclusions can be drawn:
1. The VOCs such as benzene and toluene usually have different transport efficiencies. The
predicted results from the two-component model indicate that the initial mole fraction of
each component has affects on the location of evaporation front and the mole fraction
distributions. As the result, the depth of the front increases with the initial mole fraction
for benzene but decreases with that for toluene.
2. The NAPL distributions after evaporation for single-component and two-component VOCs
are different. For the single-component case, the total VOC concentration at or below the
front is always equal to the initial concentration; however, for two-component case the
mole fractions of VOC at the front will change with time base on different evaporation
efficiencies of two components. In other words, both the mole fractions and NAPL phase
saturation change with depth below the front for a two-component VOC.
3. The migration distance of the evaporation front of NAPL increases with evaporation time
and soil porosity. As a result, the NAPL phase will vaporize to gas phase and vanishes
slowly with increasing time.
4. The normalized total concentration of VOC in the case without the presence of NAPL will
be significantly lower than that with the presence of the NAPL. Even if the volumetric
5. Gas phase diffusion is the dominating transport mechanism for VOC migration in the
unsaturated soil. Since Toluene has lower values of vapor pressure and Henry’s Law
constant than those of carbon tetrachloride, the migration of the evaporation front of
toluene is therefore significantly slower than that of carbon tetrachloride.
6. Both the depth and moving speed of evaporation front increase with the effective diffusion
coefficient. Moreover, the depth of the front increases with time while the moving speed
References
Baehr, A. L., 1987. Selective transport of hydrocarbons in the unsaturated zone due to
aqueous and vapor phase partitioning. Water Resour. Res. 23 (10), 1926-1938.
Caldwell, J., Kwan, Y.Y., 2003. On the perturbation method for the Stefan problem with
time-dependent boundary conditions. Int. J. Heat Mass Transfer. 46, 1497–1501.
Cheng, T.F., 2000. Numerical analysis of nonlinear multiphase Stefan problem. Comput. Struct. 75, 225-233.
Carslaw, H. S., Jaeger, J. C., 1959. Conduction of heat in solids. 2nd ed., Clarendon, Oxford,
Corapcioglu, M.Y., Baehr, A.L., 1987. A compositional multiphase model for groundwater
contamination by petroleum products. Water Resour. Res. 23(1), 191-200.
Crank, J., 1984. Free and moving boundary problems. Oxford Univ. Press, New York,
Falta, R. W., Javandel, I., Pruess, K., Witherspoon, P. A., 1989. Density-driven flow of gas
in unsaturated zone due to evaporation of the volatile organic compounds. Water Resour. Res. 25(10), 2159-2169.
Feltham, D.L., Garside, J., 2001. Analytical and numerical solutions describing the inward
solidification of a binary melt. Chem. Eng. Sci. 56, 2357-2370.
Fetter, C.W., 1993. Contaminant hydrogeology. Prentice Hall, New Jersey.
Experimental investigation of pneumatic soil vapor extraction. J. Cont. Hydrol. 89,
29-47.
Javierre, E., Vuik, F.J., Vermolen, S., Zwaag, van der, 2006. A comparison of numerical
models for one-dimensional Stefan problems. J. Comput. Appl. Math. 192, 445-459.
Jury, W. A., Spencer, W. F., Farmer, W. J., 1983. Behavior assessment model for trace
organics in soil: I. Model description. J. Environ. Qual. 12 (4), 558-564.
Jury, W., A., Russo, D., Streile, G., EL Abd H., 1990. Evaluation of volatilization by organic
chemicals residing below the soil surface. Water Resour. Res. 26 (1), 13-20.
Lee, M.Z.C., Marchant, T.R., 2004. Microwave thawing of cylinders. Appl. Math. Modell.
28, 711–733.
Massmann, J., Farrier, D., 1992. Effects of atmospheric pressure on gas-transport in the
vadose zone. Water Resour. Res, 28(3), 777-791.
Millongton, R. J., Quirk, J. M., 1961. Permeability of porous solids. Trans. Faraday Soc. 57,
1200-1207.
Naaktgeboren, C., 2007. The zero-phase Stefan problem. Int. J. Heat Mass Transfer. 50,
4614–4622.
Patnaik, S., Voller, V.R., Parker, G., Frascati, A., 2009. Morphology of a melt front under a
Perry, R. H., Green, D.W., Maloney, J.Q., 1997. Perry’s chemical engineers’ handbook. 7th
ed., McGraw-Hill, New York.
Pinder, G. F., Abriola, L. M., 1986. On the simulation of nonaqueous phase organic
compounds in the subsurface. Water Resour. Res. 22 (9), 109S-119S.
Purlis, E., Salvadori, V.O., 2009. Bread baking as a moving boundary problem. Part 1:
Mathematical modelling. J. Food Eng. 91, 428–433.
Purlis, E., Salvadori, V.O., 2009. Bread baking as a moving boundary problem. Part 2:
Model validation and numerical simulation. J. Food Eng. 91, 434–442.
Quintard, M., Whitaker, S., 1995. The mass flux boundary condition at a moving fluid-fluid
interface. Ind. Eng. Chem. Res. 34, 3508-3513.
Rattanadecho, S., 2006. Simulation of melting of ice in a porous media under
multipleconstant temperature heat sources using a combined transfiniteinterpolation and
PDE methods. Chem. Eng. Sci. 61, 4571–4581.
Sazhin, S.S., Krutitskii, P.A., Gusev, I.G., Heikal, M.R., 2010. Transient heating of an
evaporating droplet. Int. J. Heat Mass Transfer. 53, 2826–2836.
Shoemaker, C. A., Culver, T. B., Lion, L. W., Peterson, M. G., 1990. Analytical models of
the impact of two- phase sorption on subsurface transport of volatile chemicals. Water Resour. Res. 26 (4), 745-758.
Sleep, B. E., Sykes, J. F., 1989. Modeling the transport of volatile organics in variably
saturated media. Water Resour. Res. 25 (1), 81-92.
Stefan, J., 1889. Sber. Akad. Wiss. Wien. 98, 473-484.
Stefan, J., 1889. Sber. Akad. Wiss. Wien. 98, 965-983.
Szimmat, J., 2002. Numierical simulation of solidification processes in enclosures. Heat and Mass Transfer. 38, 279-293.
Vazquez-Nava, E., Lawrence, C., Thermal dissolution of a spherical particle with a moving
boundary. Heat Transfer Eng. 30(5), 416–426.
Yates, S. R., Papieri, S. K., Gao, F., Gan, J., 2000. Analytical solutions for the transport of
volatile organic chemicals in unsaturated layered systems. Water Resour. Res. 36(8),
1993-2000.
Yeh, H. D., 1987. Theis' solution by nonlinear least-squares and finite- difference Newton's
method, Ground Water. 25(6), 710-715.
Zaidel, J., Russo, D., 1994. Diffusive transport of organic vapors in the unsaturated zone
with kinetically-controlled volatilization and dissolution: analytical model and analysis. J. of Contam. Hydro. 17, 145-165.
Zaidal, J., Zazovsky, A., 1999. Theoretical study of multicomponent soil vapor extraction:
Table 1
Soil chemical properties used in case studies
Property Symbol Value
Soil porosity φ 0.4
Initial NAPL saturation SR0 0.01
Initial liquid saturation SL0 0.3
Initial gas saturation SG0 0.69
Air diffusion coefficient (m2/s) a DGair 5×10-6
Water diffusion coefficient (m2/s) a DLwater 5×10-10
Temperature (oC) T 20
Soil organic carbon fraction foc 0.0125
Soil bulk density (g/m3) a ρb 1.59×106
Depth of the lower boundary (m) L 5
a
Table 2
Properties of carbon tetrachloride, toluene, and benzene (Perry et al., 1997)
Carbon tetrachloride Toluene Benzene
Property Symbol Value
Molecular weight (g/mole) M 153.8 92.1 78.1 NAPL density (g/m3) ρR 1.584×106 8.62×105 8.79×105 Saturated vapor pressure (kPa) P 12.13 2.9 10.3
Henry’s law constant KH 0.958 0.26 0.22
Organic carbon partition coefficient
(m3/g)
Figure 1. Schematic diagram of VOC contamination problem, where (a) VOC reaches
equilibrium between each phase, and (b) VOC begins to evaporate after the tank is
Figure 3. Spatial distributions of mole fraction predicted by the single-component and
Figure 4. The curves of the mole fraction versus depth at 100 day when the initial mole
Figure 6. Normalized total concentration versus depth at 100 day for different values of soil
Figure 7. The curves of normalized total concentration versus depth for single-component
Figure 8. Normalized total concentration versus time for single-component and simplified
Figure 9. Normalized total concentrations of carbon tetrachloride and toluene versus depth at
Figure 10. The curves of the depths of evaporation front versus evaporation time for carbon
Figure 11. Depth and Moving speed of evaporation front versus time for different effective