壹、中文摘要 本研究第一年主要是透過教學模 組的設計、試教與評估的過程,探討如 何提昇國小學生科學探究能力。依據文 獻的探討與教材內容的分析,設計出教 學模組的原型,並據以進行第一次試 教,透過教學省思來評估是否充份提昇 學生的科學探究能力,再修正教學模組 的原型;並根據修正後的教學模組新型 進行第二次試教,透過教學者之省思與 評量工具來評估學生科學探究能力表 現與科學興趣提昇的情形,最後根據小 組討論與專家意見,整合歸納出整套探 究式教學活動方案。 本 研 究 第 一 年 共 開 發 出 二 套 主 題,主題一設計與發展中年級「植物的 種植與成長」探究式教學模組,在高雄 縣某國小三年級 136 位學生進行第一次 試教,並在該國小四年級 66 位學生進 行第二次試教。在第一次試教時進行學 生科學學習興趣問卷施測,整體而言, 第一次模組原型教學確能提昇學生的 科學學習興趣。接著在第二次試教前、 後,亦進行學生科學學習興趣問卷與科 學探究能力學生自我評量表的施測,結 果顯示,第二次模組新型教學確能提昇 學生的科學學習興趣,並能顯著的提昇 學生的科學學習探究能力。主題二設計 與發展高年級「水溶液的祕密」探究式 教學模組,在屏東縣及高雄市二所國小 共 102 位學生進行試教與評估,同時經 由教學歷程分析與教學者省思開發出 「科學探究能力檢核表」與「科學 探究能力實作評量」,提供本研究第二 年進行正式實驗教學使用。 關鍵詞:自然與生活科技、行動研究、 科學探究能力、教學設計、國小學童 Abstract
This action research aimed to develop two inquiry-based instructional modules (IBIMs), which are the
plantation and growth of plant and the mystery of aqueous solution. The subjects for the study included 136 third graders and 66 fourth graders for the first module and 102 fifth graders for the second module.
Through analyzing qualitative and quantitative data collected while the two IBIMs were utilized in an elementary school, it was found that both modules can increase students’capability of scientific inquiry and interest in science learning. Based on the findings, it is proposed that these modules be adopted in the teaching of the domain of science and life-technology of the
nine-year-sequence curriculum. This paper concludes with suggestions for further research.
Keywods:Action Research,Capability of Scientific Inquiry,Elementary School, Instructional Design,Science &
貳、研究緣起
小中年級自然科教學之行動研究。屏 東師範學院教育數理研究所碩士論 文(未出版)。 黃湃翔(1995):科學探究的實驗室教 學。高雄:高雄文教,109,56-63。 楊榮祥(1985):生物科教學模式研究, 台北:高立圖書公司。 樊琳、李賢哲(2002):以「專題研究」 培養國小職前教師科學探究過程與教 材開發能力之研究。師大學報,47 (2),105-126。 歐陽鐘仁(1988):科學教育概論,台 北:五南書局。 蔡綺文(2003):國小自然科建構式與 食譜式探究教學比較之研究。國立高 雄師範大學科學教育研究所碩士論 文(未出版)。 魯慧敏(2005):提升國小低年級學生 觀察力之行動研究—以校園植物為 例。國立屏東師範學院數理教育研究 所碩士論文(未出版)。 顏弘志(2004):從建構主義看探究教 學。科學教育研究與發展,39,1-14。 顏瓊芬、黃世傑(1999):職前生物教 師進行開放式科學探究過程之研 究。科學教育,10,46-64。 二、英文部分
Anderson, J. R.(1983). Science teaching
and the development of thinking.
California : Wadsworth Publishing Company.
Ausubel, D.P.(1968) .Educational
psychology: A cognitive view. New
York: Holt, Rinehart & Winston. Bibens, R.F(2001). Using inquiry
effectively. Theory into practice.19
(2).87-92.
Bruner, J. S., Goodnow, J. J., & Austin, G. A.(1956). A Study of Thinking.
New York:John Wiley & Son. Byber, R. W. & Landes, N. M. (1988).
The biological sciences curriculum study ( BSCS ) . Science and Children,25 ( 8 ) ,36-37.Cambridge,
MA:Harvard University Press.
Carr, W. & Kemmis, S. (1986).Becoming critical: Education, knowledge and
action research. London : Falmer.
Dembrow, M P. & Molldrem-Shamel, J. (1997).Thinking about teaching
through inquiry, Reading Teacher,51(2),162-164.
Hand, B. (1996). Diagnosis of teachers’ knowledge bases and teaching roles when implementing constructivist teaching/learning approaches.
Improving teaching learning in science and mathematics. Teacher
college, Columbia University. 212-221.
Herbert Arnold Thelen (1981) . the
Classroom Society - the Co
(Hardcover). New York:John Wiley
& Son.
Karplus, R., & Their, H. D. (1967). A new
look at elementary school science.
IL: Rand McNally.
Keys, C. W.,& Bryan, L. A. (2001). Co-constructing inquiry-based science with teachers: Essential research for lasting reform. Journal
of Research in Science Teaching, 38(6), 631-645.
Lederman, N. G.. & Lederman J. S.(2002). Scientific Inquiry : How Is It Defined /Used in Curriculum Reform?載於九十一年南區中學科 學課程教材教 法及教 師智能研習 會,(pp7-10),教育部主辦。 Lawson, A. E. (1995). Science teaching
and the development of thinking.
California : Wadsworth Publishing Company.
Marzano, R. J., Brandt, R. S., Hughes, C. S., Jones B. F., Presseisen, B. Z., Rankin, S. C., & Suhor, c. (1989).
Dimensions of thinking—A framework for curriculum and instruction. Alexandria, VA: Association for Supervision and Curriculum Development.
Mattheis, F.E.& Nakayama, G(1989): Effects of a laboratory-centered inquiry program on laboratory skills, science process skills and understanding in middle grades students(ERIC
Document Reproduction Service No.ED307148)
Norman G. Lederman(2002). Scientic Inquiry: As A Means to Integrate Subject Matter?九十一年南區科學
課程教材教法及教師智能研習會手 冊,高雄師範大學(未出版)。
NRC. (1996a). The National Science
Education Standards. Washington, D.C.: National Academy Press.
NRC. (1996b) .The Role of Scientists in
the Professional Development of
Science Teachers . Washington, D.C.:
National Academy Press.
NRC. (2000). Inquiry and the National
Science Education Standards. A Guide for Teaching and Learning. Washington, D.C.: National Academy
Press. This report is also available online at http://www.nap.edu.
Palincsar, A. S.,Magnusson,S. J. gutter,& Vincent, M. (2002).supporting guided-inquiry instruction. Teaching
exceptional children. 34(3),88-91.
Schwab, J. J. (1962). The Teaching of Science as Inquiry. The Teaching of
Science.
Suchman, J. R.(1968). Learning through inquiry, in R.F. Allen(eds). Inquiry in
the Social Studies, NCSS.
Yager, R. E. (1992). Viewpoint:What We Did Not Learn From The 60’s About Science Curriculum Reform. Journal
一、參加會議
第十八屆國際化學教育學術研討 會(18th
IUPAC International Conference On Chemical Education)於二零零四年 八月三日至八月八日在土耳其伊斯坦 堡(Istanbul, Turkey)召開,主辦單位 為土耳其的土耳其化學會。本次研討會 主題為「現代世界之化學教育」
(Chemical Education for the Modern World)。共有來自全球四十幾個國家和 地區,超過四百位各地的專家學者參與 此一盛會,發表 363 篇論文。論文題目 涵括:(1)認識化學與化工教育;(2) 發展之化學教育;(3)化學與社會;(4) 化學教師教育;(5)延續教育 (Continuing Education);(6)綠色化學 與親切環境的化學實驗;(7)國際化學 奧林匹亞;(8)微型(Micro-scale)化學; (9)化學教育之現代科技(Modern Technologies for Chemistry
Education);(10)聚合物;(11)大眾 了解的化學;(12)小學階段之科學教 育;(13)中學化學之教與學;(14)化 學教育研究的理論與方法學基礎 (theoretical and methodological basis)。
此外,大會並邀請世界級化學專家 學者做專題演講,論文發表會中並穿插 九場研習(workshop),現場並有展示 趣味化學(Fun Chemistry)及一些化學 實驗儀器、教科書、教學媒體及 CD-ROM 等。 整體而言,此次研討會主要在探討 面對二十一世紀的現代社會,尋求化學 教育的有效解決方案與因應策略。從大 學與中小學之制式的學校教育,到社會 大眾之非制式教育,化學工業以及環境 保護的綠色化學,從不同面向加以研 討,各國與會代表所發表之論文兼具理 論與實務。尤其是本屆化學教育論文在 多媒體教學軟體之應用、趣味化學以及 化學實驗儀器微型化與簡易化之設計 上更具特色。 二、與會心得與建議 本次會議首日開幕典禮後,即邀請 英 國 牛 津 大 學 資 深 化 學 教 授 Peter Atkins 針對化學教育之教學如何做好溝 通(Communicating Chemistry)提出一 些心得與大家分享,並強調如何將化學 與電腦(資訊科技)結合,將抽象概念 具體化,以使化學課程簡明易懂。我國 台灣師大邱美虹教授亦被大會邀請演 講,說明台灣中小學生之化學概念普查 情況,並報告國科會整合型研究計畫, 四年來之研究成果,主要發現是學生在 化學概念上之迷思概念和西方國家研 究發現相類似。 其 他 如 加 拿 大 ( The Kings university)Dr. Peter Mahaffy 發表形塑 化學教育,美國 The University of Texas, Dr. Joe J. Iagowski 介紹數位世界的化學 實 驗 , 南 非 University of The Witwatersrand, Dr. John D. Bradley 發 表以化學教育促進國家發展,澳洲 West Australia 大學,Dr. Robert B. Bucat 講述 化學教育研究之啟示, 委內瑞拉 de Oriente 大學,Dr. Mansoor Niaz 從科學 史哲觀點說明如何促進學生對化學概
出席第十八屆「國際化學教育學術研討會」心得報告
洪文東
照片一
照片三
照片五
A
St
udy
of
“Ac
i
d-Bas
e
”
and
“Oxi
dat
i
on-Re
duct
i
on”
Teaching Module Design and Performance Assessment on
Student Science Creativity and Problem Solving Ability
Wen-Tung Hung
Department of Natural Science Education
National Pingtung Teachers College
Pingtung(900), Taiwan, Republic Of China
(Paper presented at the 18
thInternational Conference on Chemical
Education, Augst 3-8, 2004, Istanbul, Turkey)
Abstract
I. Introduction
With the rapid changes in society, students must not only deal with the existing knowledge. They should also learn actively and develop problem-solving abilities. The general purpose of the nine-year sequence in the curriculum for Taiwan is therefore expand ed to focus on these abilities instead of merely passing on
information as usually outlined in standard or traditional sequences. The education goal should focus on training students to solve Current problems using acquired knowledge. Some educators see this problem-solving ability as high order thinking also cultivates creativity via the thinking process (Chen, 1995; Chiu, 1993; Chang, 1993; Parnes, 1967; Champagne, 1988; Sternberg, 1987; 1995).
Yager (1996), in promoting his STS science education reform, further indicats that the essence of science lies in scientific concepts and processes but the issue is how this is accomplished. One must comprehend science concepts via a process-scientific creativity is to be developed for daily problem solving use.
Science teaching in elementary schools today encourages students to probe the natural phenomena and their surroundings using observations and experiments. Students learn to observe, measure, and design experiments through structured exercises. Students learn the same scientific skills used by scientists. Using scientific experiments in elementary school science teaching is important. Therefore, the Ministry of Education (2001) emphasizes that the guidelines for the field of “Naturalscienceand living technology”in thenine-year sequencial curriculum is to foster basic independent thinking and problem-solving abilities.
Creativity, from thecognitivepsychology’spointofview,isthekey to problem solving. The creation process itself is a problem solving process. From the viewpoint of scientific logic, science creativity differs from general creativity primarily in that it emphasizes logical consistency during the scientific research process. C. L. Tseng(1999) pointed outthat“itisnotdiscovery and invention that changes human history and the spiritual-cultural property, rather, it is creativity.” (Hung, 1999). Creativity is the most important human resource needed for the 21st century.
This research wasdesigned based on the“CreativeProblem Solving”teaching model. In the“acid-base”and “oxidation-reduction”chemistry units, we hope to cultivateelementary schoolstudents’scientificcreativity during direct experiences with the problem-solving procedures. The researchers also assess student performance in scientific creativity and problem-solving, using an assessment tool they have developed . After post-experimental analysis and discussion, the researchers seek to determine an ideal education-model for subsequent studies.
According to above mentioned background and motivation, the main purpose of this study is to develop a teaching model for elementary chemistry, and an assessment tool for use in assessing scientific creativity and problem solving abilitiesin students.
Further details regarding purposes for the study include:
(i). Develop a teaching model for cultivating scientific creativity for elementary students based on AB and OR topics from the chemistry course.
(ii). Develop an assessment tool for scientific creativity and problem solving abilities to measure student understanding about the performance and connections between these abilities.
This study focuses on how students perform in terms of creativity using a new assessment tools. The experiments involved triangulation with description, calculation, and observation based on the subject teaching model. With analysis and evaluation, the researchers expect to describe teaching models that are ideal for cultivating science creativity via problem solving and effective tools for assessing problem solving and science creativity abilities.
The range of this study is based on the elementary school “acid-base” and “oxidation-reduction” chemistry units in grades 5 and of the Taiwan national curriculum. The teaching model was designed, demonstrated, and evaluated using the following contents:
a. Acidity, basicity: the acid-base nature of objects, acid-base indicators, and neutralization.
b. Oxidation-reduction: burning and extinguishing, the environmental drive for oxidation-reduction.
The aim of this study was to blend the experimental spirit with specific teaching activities. The sample group was small because experimental assessment is time-consuming. No pre-tests were administered. The study used a post-test with the equivalent groups(as determined by previous success of studies in the experimental and controe grups, With teaching hour limitation, 8 weeks for the experiment, this study could only be used to assess student scientific creativity and problem-solving proficiency.
II. Review of Related Literature
Science is an organized and systematized knowledge system. It is a thinking method in the pursuit of specified knowledge about the natural universe. Driven by curiosity, human beings ponder and resolve problems that trouble them. From the philosophical and cognitive psychology points of view, Science is a creative activity and a reasonable method for interpreting natural phenomena. (Collette, A. T. & Chiappetta, E. L., 1994).
“Create” means “to bring into existence”(Gove, 1986). Psychologically speaking, creation is an ability that includes sensitivity, fluency, flexibility, originality, and elaboration (Guilford, 1986). To be successful on science requires professionals to be well equipped with these creative abilities.
The creative scientist utilizes suitable thinking patterns, including such as categorization, description, explanation, and prediction, for discovering new truths, new products, and new theories. The core of creativity lies within the operation of the mind. Most people are able to bring their creativity into full play if they perform normally. Humans have different degrees of creativity development and performance (Torrance, 1972; Sternberg & Lubart, 1995; Hung, 1997; 2001; 2002).
“Problem solving”,according to Chang,(Chang,C.H.,1997),in termsofbeing a mental process, is a thinking process created when one tries to achieve goals. Kahny (1986)views creativity as a way of retrieving answers using the knowledge and skills one has acquired. Mayer (1992) believes that creativity is a process of moving from a known description to a goal description. He belives that problem solving is a systematic procedure that moves toward a specific goal. Cheng (Cheng, C. M., 1993) regards“problem”astwo statesofconflictand controversy,oneisthepresentation state while the other is the goal state. The problem solving thinking process is a goal-oriented process.
problem. Based on cognitive experience and ability, one uses effective thinking strategies to solve the presented problem and to differentiate between goals.
Someresearcherson problem solving havestarted to emphasizethat“creativity” is one of the keys to problem solving (Hung, 2000a; Higgins, 1994). Guilford (1967) believes that creative problem solving is a complex thinking procedure. The procedures used for problem solving include operational thinking skills such as: cognition, memory, divergent thinking, convergent thinking, and evaluation, which are all covered inwhathecalled the“structure-of-intellectmodel”. In termsofthe characteristics of thought, Guilford believes that what has an intimate relationship with creation is divergent thinking and the transfer-factor. Some scholars even interpretGuilford’sdivergent thinking ability as creativity (Cheng, C. H. & Lin, C. H., 1973). Torrance (1970) once proposed three characteristics of divergent thinking in Guilford’sstructure-of intellect model. They are fluency, flexibility, and uniqueness. The end of the divergence and factor-transfer interaction is the source of uniqueness (Chen, 1994; Cheng, U. C., 1983; Guilford, 1977). Convergent thinking is needed in addition to divergent thinking. Convergent thinking is the reasoning and logical thinking that oneusesin perusing thecorrectanswersfrom one’smentaldatabase,i. e., known knowledge and experiences. During the creation and problem-solving procedures, the problem-finding to conclusion process must be evaluated to determine if the messages conveyed during instruction suitthestudent’sneeds(Kuo,1985). This kind of evaluation is a kind of critical thinking (Treffinger & Isaksen, 1992). The purpose of the Oxfordshire Skills Program is to improve the thinking ability of children. It includes critical thinking, creative thinking, reasoning thinking, and problem solving. The relationships are shown in Figure 1.
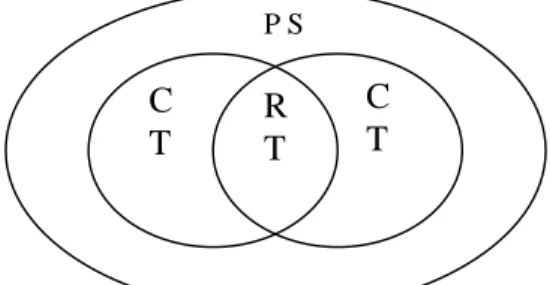
Figure 1: The problem solving and creativity relationship (Coles & Robinson, 1989). This researcher believes that the “problem-solving ability”mustinclude three kinds of thinking abilities: creation, discrimination, and reasoning. This researcher also suggests that student scientific creativity should be cultivated during problem solving procedures.
The course of science development can be seen as a continuing scientific problem solving process. The root of problem solving lies within the “mental operation”, that is, the mental process is the core of problem solving. Creative problem solving is a higher order mental reasoning activity. Looking at the history of scientific developments, the scientific research process involves continuous scientific creativity. Without creativity, many of the scientific research problems could not have been solved. From that, we see that there is an intimate relationship between problem solving ability and creativity. Therefore, we should be able to enhance student scientific creativity in a problem solving course. Yager (1996) pointed out that the core of science is too often only in the science concept and process domains. One has to reason the concepts through probing courses to bring creativity into full play. Furthermore, this process must be applied to daily life. As a result, this researcher modified Figure 1 into Figure 2:
Figure 2: The relationship between scientific creativity and problem solving abilities (modified from Hung, 2000a).
Scientific creative thinking ability grew out of existing scientific fields with new improvements and new concepts to generate new content (Hung, 1997). According to Guilford (1971), creative thinking is divided into fluency, flexibility, uniqueness, enrichment, and sensitivity. In a previous study, the creative thinking model in children (Hung, 1999; 2002), illustrated that the first three characteristics are more obvious than the others in elementary school students. The reason why scientific creativity is different from ordinary creativity is that, besides involving science related knowledge, suitable logical reasoning about the “thing”isessential.In Figure 2, this researcher used the scientific concept as the base and combined fluency, flexibility, and uniqueness to express student scientific creativity. This researcher also used scientific creativity as the foundation, adding“reasoning” and “criticalthinking”to fluency, flexibility, and uniqueness to form student “scientific problem solving ability”. In thenine-year“Naturalscienceand living technology”,curriculum the Ministry of Education pointed out that the purpose ofeducation isto foster“initiative probing” and “independentthinking and problem-solving abilities”(E.M.O.,2000). The new curriculum emphasizes these abilities. It is believed that there is an urgent need for teachers to use on the research on scientific creativity and problem solving ability and to contribute to it. Therefore, studies on cultivating student scientific creativity and problem solving abilities are needed.
The most distinct characteristic of the nine-year sequence curriculum is that there isflexibility in how thelessonsaretaught. The“Teaching module”refersto asetof teaching activities designed and operated with one theme. Each and every activity is related to the theme yet can be conducted independently. The teacher combines all of the teaching units according to his/her local needs (Yao, 2001). The teaching module was designed with diversified teaching activities based on the knowledge framework of theme-related concept and theory. The teaching module user chooses suitable teaching units and teaching strategies based on his/her own professional experiences and administers the teaching activities and assessments to the best teaching effect (Lai & Yang, 2001).
Thespiritofthecurriculum in “Naturalscienceand living technology” sharesa common notion with Yager’s(1992;1996)STS teaching concept. Thisresearcher regards a teaching module as a set of activities, that stem from probing and resolving questions related to society and daily life, leading to probing and resolving related sub-questions. Social topics and living events utilizing flexible and diverse teaching methods to cope with the teaching goals and hours are provided. For example, experimental design, hands –on activities, research reports, field studies, or visiting other institutions. Therefore, the teaching module is a set
of unifying teaching activities built on student involvement and centered on SCK
P
C1 R C2
SCK:scientific concept and knowledge
events from real life. The topic, style, living contents, and methods are diverse and meet student learning desires.
Performance assessments were performed using a setting constructed similar to the applicable setting to evaluate what the students known and can do in new situations. Assigning certain homework is a way of evaluating the learning condition. Most of the assessment materials are associated with real life. Solving problems is emphasized to encourage students to apply what they learned from school in their own lives. (Yu, 1997; T. Wu & B. Hung, 1998; Chen & Y. Wu, 1991; Airasian, 1966; Aschbacher, 1991). A chart of the performance assessment characteristics collected from scholars Lin, Y,H. (2000) and B.Y. Wang (1995) is offered in Table 1.
Table 1. The characteristics of student performance assessment Performance assessment
Example National Assessment of Educational Progress、The Practical English Skill Test in Japan, the English Ability Test of Taiwan, Physical Strength Test.
Response method There are more than one correct answers. The range and depth
of the questions
The range of the questions is wide. It evaluates different knowledge skills at the same time to assess the application of higher order thinking, judgment, and strategy.
Time limitation There is a time limitation. Testing
environment
The test is given in an imaginary environment or a manufactured studio.
Testing method Observe the performance or the product of the behavior.
Scoring method It is not easy to mark because the procedures and conclusions have equal standing.
Applicable environment
The assessment during teaching or the subject that stresses technique performance.
Strength 1. Students can demonstrate the knowledge and skills in real or imaginary settings.
2. Would be able to ask students for higher order thinking and problem solving abilitues.
3. Focuses not onlyon theend results,butalso the“getting it” process.
4. Students need to unify the knowledge and skills learned and illustrate them it with integrity which would reflect the individual differences from different angles.
Shortcoming 1. The reliability of measure is a question. The score might represent only the evaluator’s own opinion.
2. Performance assessment is time consuming, which affects the reliability due to the limitation of the sample size. 3. Because of the diversity of assessment, the assessing
standard need to be designed with caution to meet the constructive reliability.
5. The cost is high; designing and assessment time are both long; more labor needed in scoring; preserving and recording the data are both time and money consuming.
Table 1 shows that, even though performance assessments costs more, they evaluate higher order thinking, judgment, and strategy application. Therefore, the spirit of this assessment was used in this study to perform the post-test, evaluation, and modification.
III.
Design
Thegoalofthisresearch isto increaseelementary schoolstudent’sscientific creativity through problem solving. From the documents found, we determine that creative problem solving (CPS) is the best model for scientific problem solving among all the other problem solving procedures. According to this theme, the investigator revised the CPS courses into fact finding, problem finding, idea finding, solution finding and solution acceptance. The revised module was called RCPS. RCPS wasused to design the“Acid-Base”teaching module. CPS wasused forthe “Oxidation-Reduction”teaching module.
There is no standardized testing tool for student’s scientific creativity in existence. The testee must have sufficient fundamental knowledge about the science to bring his/her scientific creativity into use. The aim of this research to is develop an assessment tool for evaluating scientific creativity and problem solving abilities.
1. Designing teaching module:
This research designed a teaching module based on the “Acid-Base” and “Oxidation-Reduction”chemistry unitsin the5th
and 6th grades. The“Acid-Base” topic includes three teaching units: (1) acid-base properties of aqueous solutions, (2) hands-on the natural indicator, and(3) acid and base mixtures. The oxidation-reduction module includes two units;(1)the magic of color change, and(2) the mystery of fire. The teaching goals are as follows:
◎ Acid-Base activity 1: acid-base properties of aqueous solutions (80 minutes)
i. From the learning activity, students learn that the aqueous solution has a hidden danger. Protection is needed before using this solution.
ii. Understand the basic properties of aqueous solutions.
iii. Understand how to define acid, base, or neutral aqueous solutions. ◎ Acid-Base activity 2: hands-on the natural indicator (80 minutes) i. Learn to make natural indicator out of certain plants.
ii. During the discussion, explain the reason why certain plants can be used as indicators.
iii. Be able to determine several ways to extract plants from the existing tools. ◎ Acid-Base activity 3: acid and base mixtures (80 minutes)
i. Learn to know the hidden dangers of the aqueous solutions around us.
Especially solutions that are commonly used around the home, like vinegar and hydrochloric acid.
ii. From actual examples, discuss the theory and phenomena of neutralization iii. From the experiment, learn to turn strong acids and bases into mild or
neutralized solutions.
ii. Be able to construct a factor connection, and design an experiment to prove it.
iii. Be able to find the factors influencing the pace of oxidation. For example, temperature,water,acid environment… etc.
iv. Be able to create a way to speed up the oxidation.
v. Understand that under different circumstances, the speed of oxidation would be different.
vi. Provoking students’scientificcreativity,problem solving ability,and team spirit during the activity.
◎ Oxidation-reduction activity 2: the mystery of fire (120 minutes) i. Be able to understand the basic elements in burning.
ii. Be able to observe and discover the quality change in all materials before, during and after burning.
iii. Be able to create a fast and convenient procedure to finish the experiment. iv. Be able to categorize objects according to their burning quality.
v. Provoking students’scientificcreativity,problemsolving ability, and team spirit during the activity.
To match the research requirement, this study designed teaching activities based on CPS to foster creativity in problem solving. Adjustment would be made according to different features of the contents with the different topics to elevate the learning effects. Due to the space limitation of this paper, the investigator listed only the lesson plans for the acid and base mixtures from Acid-Base activity 3 and the mystery of fire from Oxidation-reduction activity 2.
Table2. Lesson Plan forscientificcreation of“acid-baseofaqueoussolution” (Activity 3: acid and base mixtures)
Settings Stages (RCPS) Students activity
T ime
Assessment
The teacher prepares self-made indictor, vinegar, egg, egg shell, and some aqueous solutions.
◎ Students, think of what are those aqueous solutions that might be consumed or used at home?
Students report:
they might have used detergent, soap, vinegar, toilet washer, salt water…etc.
they might have drank juice, sugared water, orsoftdrinks… etc.
5 Be able to name the aqueous solutions used.
◎ Do those aqueous solutions cause any safety concern?
◎ We have learned the acid-base nature of aqueous solution. Can you tell which of them are acid, or base?
Teacher puts vinegar and eggshells into the beaker and covers it with a glass lid. Then, displaying the vinegar-egg that’s already made.
(To keep the smell of
vinegar from troubling
students)
Should be safe!
We have made somesolutionsof…..
(Students answer
according to real situation)
1 5
Be able to tell the acid-base nature of commonly used aqueous solutions. Facts finding (provoking motivation)
◎ Guess what would happen inside the breaker?
Wow! How strange the egg became!
(Students answer
according to real situation)
5
◎ How come the egg and eggshell turned out that way inside the vinegar?
* Is it the vinegar?
◎ Vinegar, which is not a strong acid, could turn egg into this within a short period of time. The erosion of strong acid and base is even stronger and more dangerous. Is there a way to turn them into mild acid or base or neutralize them?
Is there a way to do this?
(Allow students some time to think)
5 Be able to think from observing.
◎ When the doctor said that there is too much acid in your stomach, what would the property of the medicine given be to decrease the amount of acid in your stomach?
Dissolve the medicine into water, and test the acid-base of it. (base)
Could it be that the base-medicine reduced the amount of stomach acid. We could use a base to weaken a strong acid, or even neutralize it. Or we could use acid to weaken a strong base….
(Let students talk
freely.)
1 0
Be able to reason out the theory of weakening acid or base.
◎ Let’stestand seeif your idea works. Starting the experiment on mixing the acid and base solutions. Use the materials to make a
Students follow the guidance of the teacher. Categorizing some of the solutions. Mixing the solutions according to the
indicator. Guide the students in performing the “mixing”experiment.
indicator. Observing the changes in color of the indicators, recording it and discussing it.
Each group present the records from their experiment:
when strong acid meets base, there would be some transforming. Until the indicator changes color, we know that it has become base.
When the indicator returns to the original color, the solution is neutral.
( Let the students report what they see)
* Teacher unifies and sorts out the informations.
Students present what they seen:
there are some dangerous strong acids, or base, solutions in our life, use with extreme caution.
We could mix some acid with base, and alter the nature of them.
1 5
Table3. Lesson Plan for“oxidation-reduction”creativeproblem solving (Activity 2: the mystery of fire)
Setting Stages Children Activity Equip ment
T ime
◎ Children! What are the requirements for combustion?
◎Teacher performs the result of combustion. Ex. twig, alcohol. Students report: - Oxygen - flammable materials - Heating procedure Little wooden piece, alcohol, a piece of fabric, copper wire, spoon, aluminum foil basin, 5
◎ Have you ever burned anything? Have any of your family members? What is the appearances of flammable materials?
- Roast pork, burning Hell money, burning the straw.
- Solid,liquid….
1 0
◎ What are the differences before, during, and after combustion?
- Turning dark, turning hard
◎ What is the smell? - Stinky smell? smells good?
Teacher issues experiment equipment:
equipment list.
◎ Do you know what a measuring cylinder is? What would happeen to them after the burning?
- wood - cotton balls - should be darker
◎ Children! Some are flammable, some are fire-resistant. You would need to burn them and record the properties and details on the record sheets. You could use the equipment on the teacher’s table. What could you do to speed up the experiment?
Thinking time:
what is the trait for No. 1? -- what is the trait for No. 2?
- first,do ….. then,do…. Activit y records 1 2 ◎ Children! Now start! Please record every single detail, and draw
- fill out the record. - Categorize according to traits.
(Group activity sheet 2 )
4 0
◎ Each group writes down the experimental methods and conclusions on the blackboard, and presents the results.
◎ Children! Which of the results do you think are reasonalbe? Which one do you not agree with? Which one is the best? And why?
◎ Each group delivers the results from their discussion on the stage.
Students observe the experimental design and results of other groups.
Each group discusses and records their results onto a projection slide. The discussion is presented on the stage.
◎ Cleaning up. 1
0 3. Designing the performance assessment
This performance assessment was administrated based on the previous concept of “acid-base” and “oxidation-reduction” teaching module concepts. It tests the differences between scientific creativity and problem solving ability after teaching the student either module or the regular teaching process. Here is the assessment plan:
i. Subject: grade 5 and 6 students in elementary school.
ii. Topic: (1) Acid-base: test unknown aqueous solution with natural
indicator to determine the acid-base nature of the solution. (2) Oxidation-reduction: observe the combustion phenomenon, describe and categorize the characteristics.
iii. Time: 20 minutes with each topic.
iv. Assessment method: Part I –directly observe the students and evaluate their operation performance. Part II –evaluate according to the records made during the operation process.
v. Equipment: pre-made acid-base indicator, unknown aqueous solution, flammable materials, and other tools.
vi. Assessment content:
(1). Acid-base activity: (See the experimental procedure assessment sheet in Attachment I.)
Children! There is purple cabbage juice, all the experiment tools, and three cups of known aqueous solutions on the table. They are marked as: ammonia water (base), juice (acid), and water (neutral). Please use the purple cabbage juice as the indicator and determine what color the cabbage juice turns to in acid, base, and neutral solutions. Display the results on the board.
There are three unknown solutions on the table. Please use the results above to finish the second list.
What should we do with the leftovers if we want to use them to water the plants without harming them after the experiment? Please finish this task!
A written test follows, the questions are: (See the experimental record sheet in Attachment II.)
What do you think the character of the indicator should be? Why? Have you noticed any special phenomenon during the procedure?
Do you have any suggestions for improving this activity?
(2). Oxidation-reduction activity: (See the experimental produre assessment sheet in Attachment III.)
Children! Please burn the materials provided, and use the equipment to observe the traits of the materials before, during and after combustion. (activity sheet 1). Please write as detailed a description as possible. Then, categorize these materials in your own way. (activity sheet 2).
vii. Assessment standard:
(1). Acid-base activity: There are three assessment tools to evaluate the fluency (T), propriety (P), and uniqueness (U).
fluency (T): grading according to the answers the students wrote. One complete answer is one point.
Propriety (P): grading according to the correct answers written by the students. Unrelated answers are eliminated. One correct answer is one point.
Uniqueness (U): selecting from the correct answers, grading according to unique answers given by the students, with a unique-ratio of U/P.
The scores in the assessment sheet include uniqueness and completion. The uniquenessassessmentisgraded with “uniqueness”and “distinction”,onepointeach. The completion assessment is graded using the features completed in the experiment. Two points for completed, one for partially completed, and none for incomplete.
(2). Oxidation-reduction activity:
(i) Experimental procedure. (See the experimental procedure assessment sheet in Attachment III.)
Check the student’s performance during the procedure. The number of points gained is indicated on the back of the question. The divergent thinking portion is graded using only uniqueness. Convergent thinking is counted separately in each sub-part of the assessment. This will be the score for that part. This part of the score would be graded using the procedure record. The total problem solving score is the total for divergent and convergent thinking. Any special performance or behavior by the students would alter this score. Besides that, special performance and some other behaviors not listed in the sheet, the questions raised by students during the experiment, or the replies from observers could be marked and recorded after the 4th section. The methods was used to grade uniqueness. The examiner could grade this using the ratio to all other methods, two points for less than 1/5, one point for 1/5 ~ 1/3, none for over 1/3. The conclusion for the second question would be graded according to the uniqueness for the conclusion, the same as question 1. Observers collected all of the records after the experiment and then graded them.
(ii) Experiment record. (The experiment record sheet seen in attachment IV.) - Divergent thinking. Fluency is counted based on the display of all traits during the procedure. One point is issued for each trait. Each flexibility category was valued at one point. Uniqueness is counted using special features that go unnoticed during the procedure. One point is issued for each unique item.
The total for the scientific problem solving assessment is the actual performance and performance record score. The scientific creativity score is the divergent operation and record total.
IV.
Methods
This study investigated how students perform in terms of their creativity and problem solving abilities. The assessment tools used in this study were developed by the researchers. Based on the teaching model for chemistry in the elementary school natural science class, this experiment conducted post-test with the control and experimental groups to compare the outcomes. With description, calculation and observation the researchers analyzed and evaluated the data looking forward to finding a teaching model ideal for cultivating science creativity via problem solving and effective assessment tools for evaluating student problem solving and science creativity abilities.
1. Study sample
The subjects were grade 5 and 6 students from the Kaohsiung and Pingtung areas. Confined by the labor, material, and school administration resources, it was not possibleto run alargescaleresearch study. In the“oxidation-reduction” section the control group was 20 6th graders from an elementary school in Kaohsiung County. The experimental group was 19 (4 ineffective samples were eliminated) 6th graders from anotherclassatthe sameschool. In the“acid-base”section thecontrol group was 28 5th graders from a school in Pingtung county. The experimental group was 26 students from another class from the same grade at same school. The teachers from these classes participated in this experiment with full understanding of the notion, purpose, and experimental steps.
2. Research Tools
A performance evaluation was used as the assessment tool (seen in attachment 1 ~ 4). The tools, developed by the researchers, assessed the scientific creativity and problem solving abilities using “acid-base”and “oxidation-reduction”. A scientific creativity and problem solving ability written test designed by Hung (2001) was used for criteria-validity test.
3. Research Steps
i. Design teaching plans for teachers and primary work sheets for students based on 2 topics:“acid-base”and “oxidation-reduction”.
ii. Based on the“performanceassessment”,design an assessmenttoolfor scientific creativity and problem solving ability for elementary school students. iii. The research group discussed and revised the teaching activity plans and
learning activity plans to correspond with the performance assessment testing tools.
iv. Scientific creativity and problem solving ability assessment tools were designed for the “acid-base” and “oxidation-reduction” instruction units. Teaching modules for these topics (each unit is expected to finish within 6 sessions) were proposed.
v. Develop pre-test and evaluation methods for student scientific creativity via problem solving.
vi. Discuss and remodel the assessment tools. Evaluate and confirm the content validity of the research tools.
viii. Determine the scientific creativity and problem solving ability of the
students during teaching activity. Perform post-tests using the assessment tool.
ix. Choose control groups from grade 5 (28 students) and grade 6 (20 students), to conduct post-test with the assessment tool.
x. Process and analyze the data.
xi. Propose complete teaching modules for the “acid-base” and “oxidation-reduction”instruction. Prescribeateaching pattern forcreativeproblem solving. Prescribe a performance assessment tool for student scientific creativity and problem solving abilities.
xii. Present conclusions and suggestions according to the research findings.
V. Results
The “acid-base” and “oxidation-reduction” modules were used experimental teaching. Performance assessment and written tests were conducted with the experimental and control groups. The results were test results (quantity) and experimental teaching observations(quality).
1. Test result analysis
i. The reliability and validity of the assessment tool used for the “oxidation-reduction”instruction results.
Two researchers separately evaluated the collected student divergent and convergent thinking data. The students’ procedural performance was evaluated using the“experimentalprocedureassessmentsheet”.
In the “procedure assessment” portion the correlation between the evaluators was .97 (p<.01). This means that the two evaluators agree with the procedural assessment grade. The correlation between the “divergent thinking” assessors was .96 (p<.01). This significant correlation shows that the divergent think procedural assessment grading was concordant. The convergent thinking correlation was .98 (p<.01), which is significant. The reason the convergent thinking assessment was slightly higher than that for divergent thinking was that the convergent thinking answers were clearer. However, the “uniqueness” assessment varied. The researchers regard this as one of the characteristics of uniqueness. The assessor recognition of uniqueness is not objective. The correlation for experimental procedure was .87 (p<.01). This is because the procedure performances were recorded differently. Some assessors recorded more, some less. Hence, thisw correlation was slightly lower than that for the divergent and convergent thinking.
effectively determined. That is why these two assessments did not exhibit a higher correlation.
ii. Thereliability and validity ofthe“acid-base”assessmenttool.
The internal concordance (Cronbach α) of the scientific creativity assessment tools was .86 for the experimental group and .91 for the control group. The internal concordance for the problem solving ability assessment tools was .77 for the experimental group and .62 for the control group, which is satisfactory.
The grader correlation for the scientific creativity procedural assessment tools was significantly high. The correlation coefficient, r, for the experimental group graders was 094 (p<.01), and .96 (p<.01) for the control group. The correlation for the graders using the problem solving ability procedural assessment tools was significantly high. The correlation coefficient, r, for the experimental group graders was 093 (p<.01) and .93 (p<.01) for the control group. In the total procedural assessment score for the experimental group graders was 095 (p<.01), and .96 (p<.01) for the control group. Both scores were also highly significantly correlated. The above data shows that the graders evaluated each item in the assessment tools concordantly.
Further more, the criteria-validity for the procedural assessment and written scientific problem solving ability test was .78 (p<.01) and .62 (p<.01) for scientific creativity. Both scores were significantly correlated at the above-medium degree. Therefore, the assessment tools for this experiment were validated.
iii. Theconclusion fortheexperimental“acid-base”teaching
(1) To understand if there were differences in learning between the experimental control groups, the researchers ran a t-test with the natural science and academic achievement sample scores. See table 4.
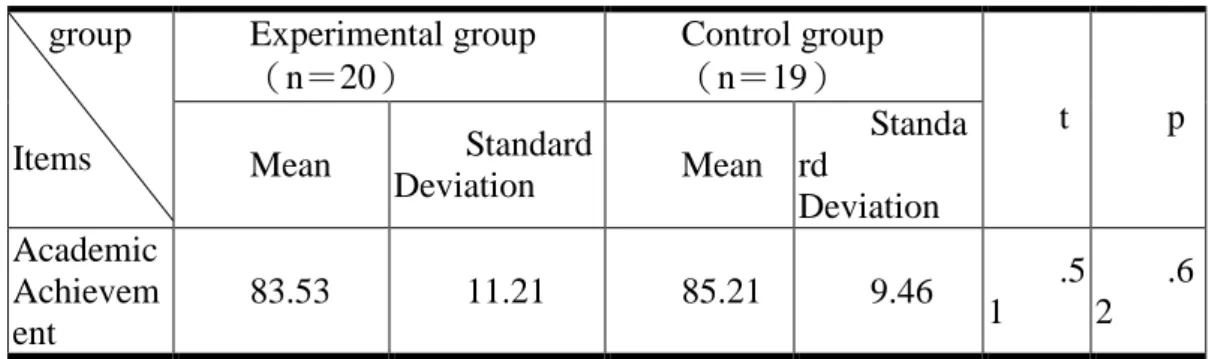
Table 4. The academic Achievement scores Experimental group
(n=20)
Control group (n=19) group
Items Mean Standard
Deviation Mean Standa rd Deviation t p Academic Achievem ent 83.53 11.21 85.21 9.46 .5 1 .6 2
As show in Table 4, no significant differences occurred (p<.05). Generally speaking, a similarity in general learning performance occurred in both groups. The learning performance in natural science for both groups was tested using the t-score. The results are shown in Table 5.
Table 5. Independent- analysis score for natural science from both groups Experimental group
(n=20)
Control group (n=19) Group
Items Mean SD Mean SD
t p natural science 84.79 10.09 87.89 5.05 1. 22 . 23 After analyzing the data, we found no significant differences in the science learning performance for both groups (p<.05). Therefore, both groups showed similar science learning performance.
the experimental and control groups was assessed. The data comparison is presented in Table 6.
Table 6. Experimental and control group independent analysis Experimental group
(n=20)
Control group (n=19) Group
Items Mean SD Mean SD
t p operation 51.95 19.69 27.70 12.69 -4.60 . 000 ** p<0.01
From the above descriptive analysis, the experimental group has a higher mean than the control group. When the independent variable is used, t-test in Table 7, we found that the differences in mean score reached the significant level (p<.05). Conversely, the experimental group performed better on the after theme-teaching activity than the control group without theme-teaching activity. The experimental group also performed significantly better in creativity related divergent thinking than the control group (p<.05).
Table 7. Divergent thinking independent analysis Experimental group (n=20) Control group (n=19) Group Items Mean SD Mea n SD t p divergent thinking 30.47 37 12.33 05 15.15 7.922 -4. 64 .00 ** p<0.01
(3). The gender differences in performance
There was not much difference in mean gender performance. Further analysis is shown in Table 8. There was no significant differences in the procedural performance and divergent thinking in both groups.
Table 8. Independent performance analysis from both genders Boy
(n=8)
Girl
(n=11) Group
Items Mean SD Mean SD
t p
divergent
thinking 31.13 9.69 30.00 14.40 .19 .85 operation 53.75 14.96 50.64 23.16 .33 .74 iv. “Acid-base”experimentalteaching activity results.
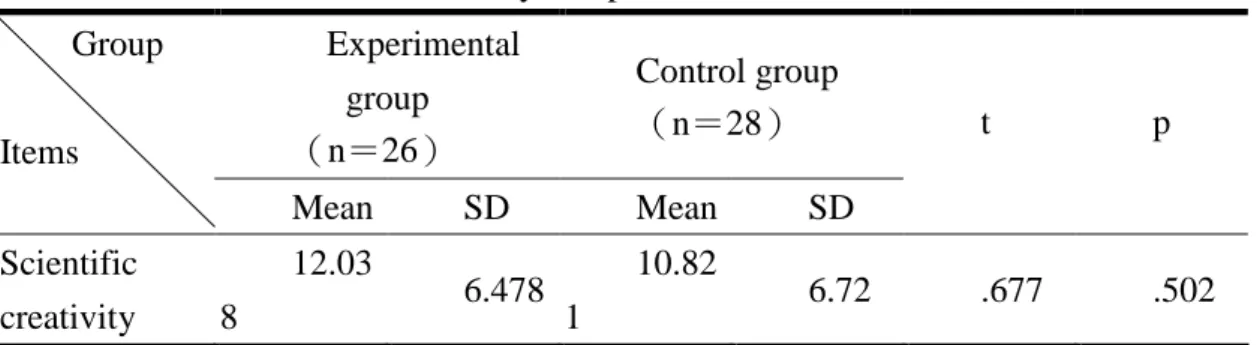
(1). Comparison of scientific creativity between the experimental and control groups
Table 9. The scientific creativity comparison Experimental group (n=26) Control group (n=28) Group Items Mean SD Mean SD t p Scientific creativity 12.03 8 6.478 10.82 1 6.72 .677 .502 The result shows that there were no significant differences in student scientific creativity before and after teaching (p<.05). However the mean score shows progress. The reason could be that the sample size was not big; the labor, material resources, and time were sufficient; and the assessment was limited to post-test with the same groups.
(2). Problem solving ability comparison between the experimental and control groups
The mean and SD scores for the student answers and performance were run with the t-test to compare the differences in both groups. The result is shown in Table 10.
Table 10. Problem solving ability comparison Experimental group (n=26) Control group (n=28) Group Items Mea n SD Mea n SD t p Problem solving ability 24.4 04 7.50 7 11 1.17 9 9.331 .000 ***p<.001
The result shows that there significant differences exist between the problem solving ability of students before and after teaching (p<.001). This shows that the teaching module developed by this research does promote students problem solving ability.
(3). The differences between boy and girl performance.
The samples were divided into gender groups, the result analyzed, the differences compared, and t-test was run. See the result in Table 11.
Total 32.9 29 16.3 18 26.2 73 9.99 2 1.679 .104 The result shows that the boys out-scored the girls in mean scientific creativity score, problem solving ability and total mean score. After the t-test, the result shows no significant differences in scientific creativity, problem solving ability, or total score. Confined by the labor and material resources, and the unrepeatable nature of the procedural assessment, this research could use only small scale post-test within the samegroup. From previousresearches,wefound thatthedeveloped “acid-base”and “oxidation-reduction”themeteaching in thisstudy produced better effects than other regular teaching units. Therefore further research and discussion on this subject are required.
2. Experimental teaching observation
Researchers observed and wrote down the experimental procedure results as follows:
i. Thediscovery and reflection of“oxidation-reduction”.
(1).In oneoftheteaching activities,“themagicofcolorchange”,theentire class was divided into 5 groups, in which, three had comparison groups. The other two did not (groups 1 and 2). Each group was designed with 7, 2, 3, 7, 3 experiments, indicating that some of the students were eager to prove their ideas through experiments. However, under the general teaching training, students tended to have insufficient control and logical understanding of the variables. Some of the experimental designs were duplicated, for example, the air-contact group 3 and the comparison group were the same. Most students had problems controlling the time. For example, apples react faster in air and in alcohol than coated with vinegar (group 1, 3, and 4). Some of the apples coated with ammonia water also reacted faster than those coated with vinegar (group1 and 5). Generally speaking, most of the experimental groups worked very hard to solve the problems and looked for different variables. They were logical and tried to record as many details as possible. In the entire experiment, the acceleration of air, water and acid to oxidation were considered. However, the students did not take the idea of increasing the air-contact area in the apples to increase the oxidation speed into consideration. Group 2 has the most detailed records among all other groups.
(3). There are 4 in 23, about one sixth of the students had difficulties in classifying uncertain objects. Those 4 data were discarded because the samples could not classify the characteristics of the objects. Those that did make the classification table could only describe the object characteristics before and after burning in the simplest way. It was the same with the control groups. Generally, therewerelessthan threecategories. “Classification”isan areathateducatorsmust put more effort on in the future.
ii. The discovery and reflection of“acid-base”.
(1). The acid-base unit in elementary school science class has been the one that produces the least interest. We often use a table for students to memorize the acid-base property of the objects and forget to teach the technical and natural way.
(2). Most elementary school teachers emphasize only recognition of acid-base. They do not stress the importance and application of it in life. The consequence is a lack of student learning motivation. Leaning on memorization, neglecting the operational skills during learning and experiment causes lack of student motivation. Based on this, these researchers chose this unit to perform the experimental teaching. We communicated with elementary teachers in the research group about the creative problem solving module and design.
(3). From the student reaction we could see that students react more with creative problem solving modules and exhibit greater learning interest with experiment participation. Before the experiment, students had no idea about the acid-base property of the liquid. They classified the solutions in their own way. The proposed method is better than the old method of telling them right from the start and making the liquid interact with the indicators. Students develop classification ability on their own in the creative problem solving module. They determine the way to classify objects and learn how to use the indicator to tell the acid-base property of the liquid during the process.
(4). The “liquid acid-base”teaching activities were separated into three units, they are (1)acid-base properties of aqueous solutions, (2)hands-on the natural indicator, and(3)mixtures of acids and bases. The first introduction was “the sorting methods”, then, the acid-base properties of aqueous solutions were presented. The learning procedure stems from the “classification”, then moves to introduce the sorting methods, and gradually build the acid and base concept. The researchers participated and observed that students were curious about the purple cabbage juice turning yellowish-green or red after mixing with a clear solution. Seeing this transformation increased the students’interest in learning. From the work sheets from each group, we could see that the students brainstormed all sorts of different ideas about classifying. This also shows that students participated more than usual. Student interest was raised higher than before and the group reports were much better than before. From the student performance, the researchers believe that the creative problem-solving module is the way to evoke and cultivate student scientific creativity and problem solving ability. The proposed method includes students to observe, probe, and seek the solutions.
VI. Conclusion
assessment sample size was not large due to the time, labor and material limitations. Therefore, post-teaching performance was easily influenced by individual sample factors. The following study should increase the sample size for better reliability. Properly condensing and revise the theme unit does help the course to move faster and smoothly. This also conforms to the administrative requirement for short period science camp activities.
Although, procedural assessment was not suitable for pre-, and post-tests, the experimental effect would stand out if pre-, post-, and “postponetests”were administrated in the future.The procedural assessment tool used in this research, was designed according to the theme-teaching module. The required level of reliability and validity was reached in both scientific creativity and problem solving ability. Therefore, this offers other researchers another angle different from the traditional method to assess related topics or abilities.The experimental group that received experimental treatment in the “oxidation-reduction” teaching module performed significantly better than the control group receiving ordinary class teaching.The experimentalgroup thatreceived experimentaltreatmentin the“acid-base”teaching module performed significantly better than the control group receiving ordinary class teaching.
The researchers determined from the performance -comparison that the group performance was better than individual performance in general. Individual performance was related to the degree of involvement in the team. From the end results for both “oxidation-reduction” and “acid-base”, the researchers found no significant differences in both genders in the scientific creativity and problem solving ability.
The primary goal of this study was to develop teaching modules and related procedural assessment tools. Confined by labor and finance limitations, the experimental teaching went on with one class each from grade five and grade six. After the experiment teaching and testing, the data collected from the small sample were carefully analyzed and used as the foundation for future model adjustment. Because the sample size was small, the researchers could not say for sure that this teaching module would yield the same or better results with students from other areas or with a larger sample size. Our suggestion is that, in follow-up studies, the experiment should be conducted with more through research plans and a large sample size to determine if more significant results can be achieved.
Cultivating observation ability, experimental procedure and scientific process skills in elementary school students is important. These skills are closely connected to scientific creativity and problem solving ability. Therefore, these researchers wish to run the scientific intelligence assessment in a more diverse way. The student scientific creativity and problem solving ability assessment tool should become a complete set with a mature pencil-and-paper test. The evaluation of such abilities would then become more concrete. The suggestion is that in a future study, the assessors should try to use more diverse assessment methods for the entire scope of elementary school student scientific creativity and problem solving abilities.
solving ability.
Acknowledgement
I would like to acknowledge the financial support of the National Science Council in Taiwan, R. O. C. (Grant NSC-90-2511-S-153-013). Special appreciation is acknowledged to Mr. Lee, C.O., Mr. Huang, C.W., Mr. Chang, F.F., Mr. Lin, Y.H., and Ms. Lee, C.Y. for their help in discussion, design, experiment teaching, and running the experiment.
VII.
References.
Airasian P. W. (1996). Assessment in the classroom. New York: McGraw-Hall.
Aschbacher, P.R.(1991). Performance assessment: state activity, interest, and concerns.
Applied measurement in Education, 4(4)275-288.
Champagne,A.B.(1988). Definition and Assessment of The High-order Cognitive
Skills. National Association for Research in Science Teaching, Research
Matter…To theScienceTeacher.
Chang, Y. C. (1993). Thinking skills and teaching. Taipei: Psychology.
Chang, Y. C. (1983). The impact of teacher’s questioning to student’s creative
thinking ability. Taipei: Education plan of Ministry of Education. Cheng, C. M. (1993). Cognitive Psychology. Taipei: Laureate.
Chia,F.M.translateDewey,John’s The way we think. (1992). Taipei: Wu-Nan. Lai, C. H. & Yang, C. C. (2001). Developmental research of elementary school
science resource teaching module. National Taipei Normal University News: 14, 673-704.
Chiu, M. H. (1993). Learning of comparison and scientific concept. Education research: 1 (6), 79-90.
Coles,M.. & Robinson,W.D.(1989). Teaching thinking, Bristol:The Bristol Press. Collette, A. T. & Chiappetta E. L. (1994). Science Instruction in the Middle &
Secondary Schools. New York : Macmillan Publishing Company.
Gove, P. B (1986). Webster’s3rd InternationalDictionaryoftheEnglish Language. Springfield Massachusetts : G. & C. Berrian Company Publishers.
Guilford, J. P.(1967). The nature of human intelligence. New York : McGraw-Hill. Guilford,J. P.(1971).Creative and its Cultivation. New York :Haper and Row. Guilford,J. P.(1977).Way beyond the IQ. Buffalo, New York:Creative Education
Foundation.
Hung, W. T. (2002). Connection between creative thinking and scientific creativity.
National Pingtung Normal University News: 16, 355-394.
Hung, W. T. (2001). Cultivate scientific creativity through problem solving ability: Chemistry learning activity module and teaching activity design. National Science Council: NSC-87-2511-S-153-006.
Hung, W. T. (2000a). Cultivate scientific creativity through the procedure of problem solving. National Pingtung Normal University Science Education: 11, 52-62.
Hung, W. T. (1999). Research sub-plan of elementary school students’ scientific
creativity trait and thedevelopment:creativechildren’sthinking trait. National
Science Council: NSC-87-2511-S-153-015.