Graduate Institute of Psychology College of Science
National Taiwan University Master Thesis
Visual- and Auditory-Based Episodic Memory
Feeling-of-Knowing in Individuals with Subjective Cognitive Decline
Yu-Hsuan Sun
Advisors: Mau-Sun Hua, Ph.D., Yu-Ling Chang, Ph.D.
108 1
January 2019
B R ; .
0
。 、
9 0
. 1
2 B
。 R
I 0
B
. .
R , .
R R
R
“Do not go gentle into that good night.
Rage, rage against the dying of the light.”
--Dylan Thomas
CONTENTS
Abstract……….….. 1
Introduction……….…... 4
Methods……….…. 10
Participants……….…. 10
Criteria for groups……….….. 11
FOK paradigm……….… 13
Neurocognitive measures……….… 14
Data collection……….… 16
Statistical analyses………... 16
Results……….... 17
Demogrphic characteristics………. 17
FOK paradigm test performances………. 18
Neurocognitive performances………. 23
FOK judgment and neurocognitive performances………. 24
Discussion……… 26
References……….. 33
Tables………. 51
Figures………... 58
Subjective Cognitive Decline Mild Cognitive Impairment
Alzheimer’s Disease
Feeling-of-Knowing
50 85
ABSTRACT
Background Subjective Cognitive Decline (SCD) in cognitively unimpaired
individuals has been recognized as a possible sign predicting future decline to mild
cognitive impairment (MCI) and Alzhemier’s Disease (AD). Individuals with SCD
showed atypical findings in brain regions that are associated with subjective feeling and
memory monitoring. Thus, the current study aimed to examine whether the performance
pattern on the episodic memory feeling of knowing (FOK) paradigm measures in
individuals with SCD is comparable to that of patients with MCI and AD, and whether
individuals with SCD exhibit different performance pattern on visual- and
auditory-modality FOK tests. Methods A total of 88 adult participants (aged 50 to 85),
including 4 groups, healthy control (HC), SCD, MCI and AD, were recruited in the
present study. Each participant received visually and aurally episodic memory
feeling-of-knowing (FOK) paradigm and a battery of neuropsychological tests. Results
On the visual FOK test, the performance scores were not significantly different between
SCD and HC, and between SCD and MCI while the score differences between SCD and
AD were remarkable. The HC’s performance significantly overpowered the two patient
groups. On the auditory FOK test, the performance scores between participants of HC
and SCD were not significantly different while the scores of both HC and SCD were
significantly different from the two patients groups. Conclusion. Based on the present
results of meta-memory functioning study, we suggest that individuals with SCD may
be placed on the stage between health aging and pathological aging. However, further
study on a large scale and different memory tests on this issue is necessary.
Keywords: subjective cognitive decline, feeling-of-knowing, memory monitoring, episodic memory, Alzheimer’s disease, mild cognitive impairment
INTRODUCTION
Alzheimer’s disease (AD), characterized by a primary deficit in episodic memory
that gradually progresses to a global impairment (Backman, Jones, Berger, Laukka, &
Small, 2004, 2005; Dubois et al., 2007; Weintraub, Wicklund, & Salmon, 2012), is the
most common cause of elderly dementia. Its neurodegenerative process is thought to
begin years before the symptoms surface (Jack et al., 2013; Villemagne et al., 2013). It
is thus crucial to identify people at risk for developing AD and provide early
intervention to slow down disease progression. Therefore, concepts such as “preclinical
AD” or “asymptomatic AD” have been proposed based on evident AD biomarkers in
cognitively normal people (Dubois et al., 2010; Sperling et al., 2011). However, in
addition to the AD biomarkers, recent studies have suggested that subjective cognitive
decline (SCD) in individuals with unimpaired performances on cognitive tests might
serve as a sign of preclinical AD (Jessen et al., 2014; Perrotin, Mormino, Madison,
Hayenga, & Jagust, 2012), predicting future memory decline (Koppara et al., 2015; van
Oijen, de Jong, Hofman, Koudstaal, & Breteler, 2007).
Emerging evidence suggests that SCD is related to AD in multiple domains. A
seven-year follow-up study reported that most individuals with SCD decline faster than
those without such concerns regarding cognitive and functional performances (Reisberg,
Shulman, Torossian, Leng, & Zhu, 2010). Moreover, greater self-reported concern
regarding SCD is significantly associated with Abeta deposition, one of the distinctive
neuropathological features of AD patients (Nelson et al., 2012), after controlling for
objective memory performance (Amariglio et al., 2015). One study also suggested that
the reduced confidence in one’s general memory performance is correlated with greater
Abeta deposition in the right medial prefrontal cortex, anterior cingulate cortex, and
precuneus and posterior cingulate cortex in cognitively normal individuals (Perrotin et
al., 2012). Similar finding in tau aggregation has been reported recently as well
(Swinford, Risacher, Charil, Schwarz, & Saykin, 2018). These regions, known as parts
of the default mode network (DMN) (Raichle, 2015), are recognized to be associated
with subjective experience and memory monitoring (Chua, Schacter, Rand-Giovannetti,
& Sperling, 2006). Functionally, individuals with SCD show abnormal activity in these
regions, leading to disintegrations between anterior and posterior regions as well as
hippocampal decoupling from the posterior DMN (Dillen et al., 2017; Erk et al., 2011;
Sheline et al., 2010). Similar connectivity dysfunctions have been observed in
individuals with dementia due to AD and those with high-risk mild cognitive
impairment (MCI) (Nellessen et al., 2015; Wang et al., 2015).
A growing body of literature has reported impaired memory monitoring, along
with salient deficits in episodic memory, in patients with AD (Dodson et al., 2011;
Galeone, Pappalardo, Chieffi, Iavarone, & Carlomagno, 2011; Souchay, Isingrini, & Gil,
2002), as well as in individuals with MCI (Galeone et al., 2011; Perrotin, Belleville, &
Isingrini, 2007; Souchay, 2007; Y.-L. Wang, Hua, Chang, & Lu, 2007). While some
researchers have reported that both individuals with AD and MCI exhibit a tendency to
overestimate their memory performance on tests (Galeone et al., 2011; Perrotin et al.,
2007), others suggest that the impaired memory monitoring of overestimation is limited
to the general memory performance in daily living (Gallo, Cramer, Wong, & Bennett,
2012). Moreover, recent studies have revealed that tasks involving self-related
information induced abnormal prefrontal activity in patients with AD and MCI (Genon
et al., 2014; Zamboni et al., 2013). A similar disadvantage regarding the processing of
self-related information in individuals with SCD has been reported, suggesting a
weakness in memory monitoring. One study reported that compared to their
counterparts of the same age, individuals with SCD tend to have lower confidence
regarding general memory performance (Perrotin et al., 2012). Moreover, research has
found discrepant memory-specific observations between individuals with SCD and their
informants; informants’ observations tend to be better at predicting cognitive and
functional declines (Slavin et al., 2015). However, no study directly measures the
memory monitoring function in SCD.
Regardless of the memory-related deficit, some studies suggest that the nature of
the materials that constitute memory may lead to different forgetting rates in patients
with AD and MCI (Ally, Hussey, Ko, & Molitor, 2013; Vallet et al., 2016). Vallet et al.
(2016) used learning items incorporating different abstraction levels of information and
recorded their forgetting rates in healthy controls (HCs) and individuals with AD and
MCI. They found that despite the fact that patients with AD tended to have the fastest
forgetting rate compared to the other two groups, an exceptionally fast rate for items
that embodied abstract visual features was revealed. Patients with MCI also exhibited a
faster decline rate in recognizing abstract visual items. Although contradictory findings
were reported by another research team, according to whom patients with AD and MCI
demonstrate better memory for pictures (Ally, 2012; Ally, Gold, & Budson, 2009; Ally
et al., 2013), it is possible that the difference was mainly due to the level of abstraction
of the stimuli used. Accumulative research has documented the fact that the atypical
neural activities in processing visual and auditory stimuli among patients with AD and
MCI stem not from fundamental elements processing but from the information
integration levels (Bender et al., 2014; Golden et al., 2015; Golden et al., 2016; Hao et
al., 2005; Kurimoto et al., 2012). Thus, in terms of a preference for visual or auditory
memory in patients with AD and MCI, the results might reflect their impaired functions
in dealing with memory composed of items at higher levels of abstraction.
To our knowledge, few studies have explored the memory-related characteristics
of individuals with SCD. Despite a self-reported experience of memory decline in such
individuals, no study has directly measured their memory monitoring functioning
through objective methods. Traditional cognitive tests for studying pre-clinical AD were
those used to diagnose dementia; therefore, it is possible that they lacked the sensitivity
to detect the subtle cognitive changes that correlate to AD pathology progression at the
preclinical stage (Mortamais et al., 2017). Such change might be more likely to be
detected by tasks conducted prospectively; that is, tasks that demand high execution
abilities (Bisiacchi, Tarantino, & Ciccola, 2008). The feeling-of-knowing (FOK)
paradigm (Hart, 1965) reflects the memory monitoring prospectively with respect to
subsequent memory recognition (Chua, Schacter, & Sperling, 2009). An imaging study
demonstrated that the FOK paradigm is correlated with activity in the prefrontal, medial
parietal, and hippocampal formation regions (Chua et al., 2009), which have been found
to exhibit atypical activity and salient Abeta deposition in individuals with SCD (Dillen
et al., 2017; Erk et al., 2011; Sheline et al., 2010). Moreover, previous studies have
suggested that the aging-related decline in memory monitoring is associated with
change in executive function (Isingrini, Perrotin, & Souchay, 2008; Souchay & Isingrini,
2004; Souchay, Isingrini, & Espagnet, 2000), whereas the declined performance in
patients with AD and MCI exhibits a correlation with episodic memory (Cosentino,
2014; Perrotin et al., 2007; Souchay et al., 2002). Regarding patients with MCI, a study
suggested that, along with the episodic memory deficit, the existence of executive
dysfunction might predict the decline from MCI to AD (Bisiacchi, Borella,
Bergamaschi, Carretti, & Mondini, 2008).
Thus, the current study aimed to examine 1) whether individuals with SCD share
similar performance pattern on episodic memory FOK measures with those with MCI
and AD, and 2) whether individuals with SCD exhibit different performance pattern on
visual- and auditory-based episodic memory FOK tests.
METHODS
PARTICIPANTS
A total of 100 participants (50 to 85 years old) were recruited from the Neurology
Clinics of the National Taiwan University Hospital (NTUH) or from the communities in
the present study. Exclusive criteria were applied to exclude individuals with alcohol or
substance abuse, intellectual disability, brain injury, stroke, endocrine dysfunction,
neurological disorders, or psychiatric disorders. All participants had a normal or
corrected-to-normal vision and hearing abilities. Participants with diagnoses of
dementia or mild cognitive impairment other than Alzheimer’s origin were excluded as
well. Twelve participants were excluded from further analyses due to other demented
origins (N = 6), psychiatric conditions (N = 2), intelligent disability (N = 1), and
non-diagnostic demented conditions (N = 3). A total of 88 participants were recruited in
the final analyses.
All participants received a thorough explanation of the research purpose and
signed an informed consent form. The Institutional Review Board (IRB) of the National
Taiwan University Hospital approved the current study. Detailed demographic data
were shown in Table 1.
(INSERT TABLE 1 HERE)
CRITERIA FOR GROUPS
Participants recruited from the Clinics, prior to participating in the study, firstly
received an examination by a physician who performed a medical history review,
Mini-Mental Status Examination (MMSE), and neurologic examination. Then, a
neuropsychologist conducted the neurocognitive assessment, including an interview
with participant’s informant for the Clinical Dementia Rating (CDR). The final
diagnosis was made upon a primary attending physician after reviewing all examination
results, including results of brain imaging, neuropsychological assessment, and lab
examinations. With respect to their episodic memory performance for research
classification purpose, Taiwan version of Wechsler Memory Scale-III (WMS-III) (Hua
et al., 2005) Logical Memory I and II were performed. Participants were later classified
into the following groups.
SCD Group. Individuals who performed normally across cognitive domains in
neuropsychological tests and had a subjective decline in memory within the last five
years (Jessen et al., 2014) were classified into the SCD group.
MCI Group. Individuals with episodic memory scores of approximately 1.0 SD or
greater below the mean in the general population were considered for possible memory
impairment (Albert et al., 2011). However, no algorithm was used to simply determine
the diagnosis of MCI; study coordinators, neuropsychologists, and physicians who had
examined the individual assigned the diagnosis based on their discussion regarding the
examinations and published criteria (Albert et al., 2011). For the purpose of the study,
only individuals with primary memory impairment were recruited in the MCI group.
AD Group. Individuals who had a CDR score of 0.5 and met the published criteria
of the National Institute on Aging and the Alzheimer’s Association (NIA-AA)
(McKhann et al., 2011) were classified into dementia due to AD.
HC Group. Individuals in the HC group volunteered from the communities.
Before attending the study, volunteers received a thorough neuropsychological
examination performed by a study coordinator to determine their neurocognitive
functions and other conditions. Information regarding medical history, family history,
and medication were collected during the process. Individuals who performed without
1.0 SD below age- and education-matched norms in all cognitive domains were
recruited and matched to the SCD group in terms of demographics.
FOK PARADIGM
The memory monitoring ability was assessed by FOK paradigm with a
recall-judgment-recognition fashion in episodic memory tests, in which studies
suggested that were better in revealing the impaired abilities of the AD patients
(Cosentino, 2014; Souchay, 2007). The episodic memory tests with FOK paradigm
were the Rey Complex Figure Test and Recognition Trials (RCFT) (Meyers & Meyers,
1995) for visual episodic memory, and the Word List subtest in the WMS-III for
auditory episodic memory. The FOK judgments were embodied after delayed recall
phase and before recognition for each presenting item. That is, participants were asked
to answer the FOK question of “Do you feel like you can accurately recognize the item”
in a binary fashion before giving the “Yes/No” answer for recognition. The traditional
FOK paradigm asked participants to judge their responses toward unrecalled items (Hart,
1965; Nelson, 1990). However, the current study following the FOK paradigm used by
Souchay et al. (2002) that participants were asked to make FOK judgments for each
item during the recognition phases. In this way, their responses, in combination of
recognition accuracy and FOK judgment, were coded into four categories for further
calculation of the Hamann coefficient (Schraw, 1995; Souchay et al., 2002); please refer
to Table 2 for the equation. Hamann coefficient was used to represent FOK accuracy.
(INSERT TABLE 2 HERE)
NEUROCOGNITIVE MEASUREMENTS
In consideration of the influences of episodic memory and executive function in
the FOK judgment, visual- and auditory-based tests relative to these functions were
selected.
Episodic Memory. Participants received the Visual Reproduction I and II, and the Verbal Paired Associates I and II subtest of the WMS-III for constructing the scores for
episodic memory. A study suggested that immediate and delayed recall performances in
episodic memory might involve different brain regions in the DMN (Huo, Li, Wang,
Zheng, & Li, 2018), scores were used separately to calculate into “Immediate Recall”
and “Delayed Recall” measures. In order to avoid the visuospatial deficit that interfered
participants’ performances on visual episodic memory, the Copy and the Discrimination
phases of the Visual Reproduction subtest were used as the reference.
Executive Function. Two subtests from the Wechsler Adult Intelligence
Scale-Third Edition (WAIS-III) were used as indicators of executive function; they
were the Matrix Reasoning and the Digit Span Backward.
General Intellectual Ability. In order to rule out the possibility that the
intellectual ability might interfere with participants’ learning ability, their IQ
performances on the WAIS-III or WAIS-IV were collected through their record of
recent neuropsychological examination. For those without previous examination record,
the project coordinator estimated their full-scale IQ by performances on the Similarities,
the Arithmetic, the Matrix Reasoning, and the Digit Symbol Substitution subtests from
the WAIS-III (Chen, Hua, Zhu, & Chen, 2008).
DATA COLLECTION
Participants who had cardiovascular risk factors in groups other than HC,
Hachinski Ischemic Score (HIS) (Hachinski et al., 1975; Rosen, Terry, Fuld, Katzman,
& Peck, 1980) was used to rule out individuals with a score of 4 or greater. In
consideration of the influence of possible confounding variables, each participant was
asked to fill out the Taiwan Geriatric Depression Scale-15 (GDS-15) (Liao et al., 2004;
Liu et al., 1997; Sheikh, Yesavage, & Health, 1986). All participants were also asked to
report the cognitive decline as comparing to self, or to others.
STATISTICAL ANALYSES
Data were explored by scatterplot, the Shapiro-Wilk test and the Levene's test to
determine the analysis method. Different analysis methods were applied based on the
characteristic of data. All statistical tests were performed through SPSS version 25 on
the macOS system version 10.14.
RESULTS
DEMOGRAPHIC CHARACTERISTICS
Regarding to demographic characteristics among groups, analysis of variance
showed a main effect of age (F(3, 84) = 5.722, p = .001, d = .68) across four groups.
Post-hoc pairwise-comparison analyses using Scheffe indicated that HC was younger
than MCI (p = .029, d = .849) and AD (p = .01, d = 1.41), whereas SCD did not differ
significantly with other groups (p > .05). Due to the failure to meet the assumptions of
parametric methods, non-parametric method was used for comparing the years of
education between four groups. An independent-samples Kruskal-Wallis H test showed
that four groups did not differ significantly in years of education (H = 7.635, p = .054, d
= .483).
Performances in episodic memory and general cognitive tests. Analysis of
variance showed a main effect of the Logical Memory II (F(3,30) = 23.737, p < .001, d
= .92) across four groups. Post hoc pairwise-comparison analyses using Scheffe
indicated that the performance of AD was worse than HC (p < .001, d = 3.564), SCD (p
< .001, d = 4.02) and MCI (p = .012, d = 1.866), and MCI was worse than SCD (p
< .001, d = 1.496) and HC (p < .001, d = 1.515). The memory performance did not
differ significantly between HC and SCD (p = .728, d = .20).
Independent-samples Kruskal-Wallis H tests showed significant differences in the
MMSE (H = 26.752, p < .001, d = 1.256) and the FSIQ (H = 11.012, p = .012, d = .649)
among four groups. However, an independent-samples t-test indicated no difference in
the FSIQ between SCD and HC (t(61) = .391, p = .697, d = .099). No additional
analysis of the MMSE was done given its nature of cognitive screening. Please refer to
Table 1 for detailed information regarding demographic characteristics of four groups.
(INSERT TABLE 1 HERE)
FOK PARADIGM TEST PERFORMANCE
The proportion (%) of overall “yes” and “no” FOK judgments was computed to
determine whether four groups utilized the FOK category in a similar fashion.
Kruskal-Wallis H tests indicated no significant difference was found on the “yes” and
“no” judgment in the visual episodic memory among four groups (H = 2.258, p = .521,
d = .189), but significant in the auditory episodic memory (H = 9.083, p = .028, d
= .559). However, Post-hoc pairwise-contrast analyses using Dunn-Bonferroni method
for performance in the auditory episodic memory did not reveal any difference among
these groups.
Given the fact that no significant difference of “yes/no” preference in FOK
judgment among four groups, further analyses were done for exploring the group
differences in FOK performance. The one-way ANCOVA was conducted to compare
the visual FOK accuracy of four groups whilst controlling for age. Results indicated a
significant group difference on the visual FOK accuracy (F(3, 83) = 12.443, p < .001,
partial eta squared = .310). Post-hoc pairwise-comparison analyses using the
Dunn-Bonferroni procedure indicated that AD performed significantly worse than other
groups (AD-HC: p < .001, d = 10.987; AD-SCD: p < .001, d = 10.708; AD-MCI: p
> .05, d = 5.859); despite no significant difference between HC and SCD (p = .157, d =
1.232), SCD did not differ from MCI (p = .157, d = 3.385) while HC outperformed MCI
(p = .033, d = 4.263).
Regarding FOK performances on auditory-based episodic memory test, the
Kruskal-Wallis H test showed a significant difference (H = 37.613, p < .001, d = 1.674)
among four groups. Post-hoc pairwise-comparison analyses using Dunn-Bonferroni
method revealed that HC performed significantly better than MCI (p < .001, d = 1.991)
and AD (p < .001, d = 2.674), SCD also performed better than MCI (p < .001, d = 1.366)
and AD (p < .001, d = 2.145). Please refer to Table 3 for details.
(INSERT TABLE 3 HERE)
Subcomponents of FOK performance. The percentage of hits and misses relative
to yes/no FOK judgment was used as indicators of whether overestimation and
underestimation happen in the level of groups (Souchay et al., 2002). Given the
restriction of data pattern, the Kruskal-Wallis H test was used to detect differences at
the group level.
Results revealed a significant group difference for hits and misses on the “yes”
judgments of both visual and auditory episodic memory tasks (see Table 4). However,
further post-hoc pairwise-comparison analyses using Dunn-Bonferroni method
indicated different significant pattern in each condition. Comparisons on the auditory
task revealed a consistent pattern that patient groups made significant fewer hits for
“yes” judgment (AD-SCD: p = .001, d = 1.987; AD-HC: p < .001, d = 2.751; MCI-SCD:
p = .001, d = 1.267; MCI-HC: p < .001, d = 1.768) and more misses (AD-SCD: p = .019, d = 1.046; AD-HC: p = .003, d = 1.345; MCI-SCD: p = .019, d = .858; MCI-HC: p
< .001, d = 1.102) than HC and SCD. On the visual task, patient groups still made
significant fewer hits (AD-HC: p < .001, d = 2.287; MCI-HC: p = .005, d = 1.276) and
more misses (AD-HC: p = .004, d = 1.322; MCI-HC: p = .022, d = .788) than the HC,
but different pattern emerged while comparing to SCD. That is, no significant
difference was reported between MCI and SCD regardless of hits or misses; AD only
committed fewer hits than SCD (p = .001, d = 2.112), but no difference between for
misses (p = .44, d = 1.082).
While on the FOK “no” judgment, results showed significant group differences for
both hits and misses on the auditory task, but group difference was only reported for
misses (H = 9.4, p = .024, d = .574) on the visual task (see Table 4). However, further
post-hoc pairwise-contrast analyses on the auditory task showed that only AD made
more misses than HC (p = .019, d = 1.701). No group difference was found in other
conditions, including those for misses on the visual task.
(INSERT TABLE 4 HERE)
Individuals in each group that below the 5% performance in HC. In order to
examine whether the insignificance results between SCD and patient groups indicate
data homogeneity or difference that was statistically not detectable, a chi-square test
was performed. Individuals in the HC group were sorted based on their miss
performance on the visual FOK “yes” judgment. The percentage score of individual
who ranked at the five percentile was used in the following analyses as cutoff score.
Results showed that 12.1% of SCD, 33.3% of MCI, and 57.1% of AD were below that
cutoff score, 25. A likelihood ratio chi-squared test showed that performances in four
groups were not equally distributed, χ2 = (3, N = 88) = 14.743, p = .002, phi = .425.
Same procedure was applied on performance on the misses in the auditory FOK “yes”
judgment, and the cutoff score was 8.33. Results showed that 15.2% of SCD, 61.1% of
MCI, and 71.4% of AD were below the cutoff score. A likelihood ratio chi-squared test
showed that performances in four groups were not equally distributed, χ2 = (3, N = 88) =
29.698, p < .001, phi = .582.
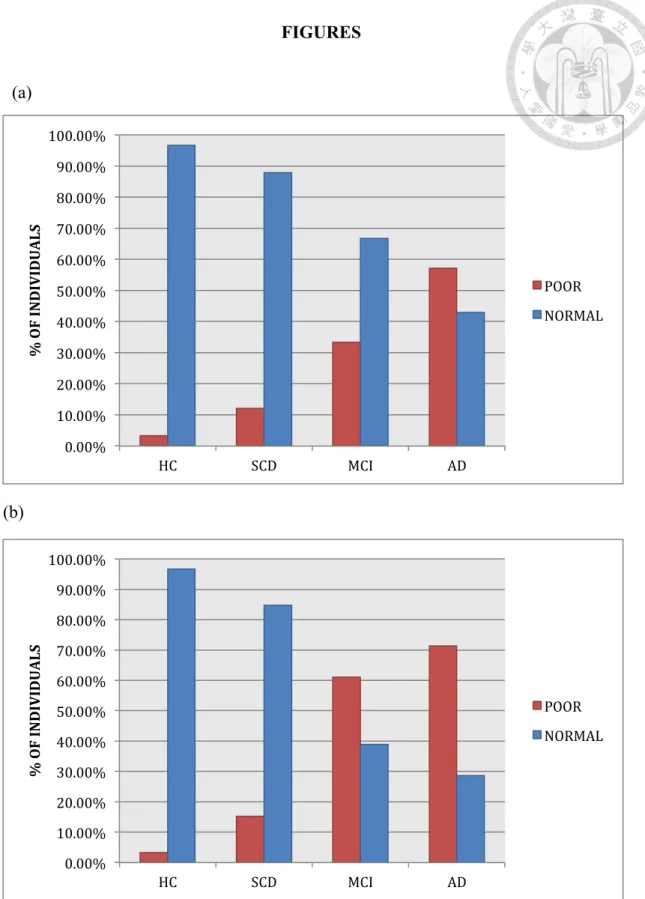
Figure 1 shows the participant proportion with “a poor-level performance,” which was based on a cut-off score below five-percentile rank of the HC group performance
score, distribution on both visual- and aural-FOK tests. On Figure 1a, based on such a
criterion, only AD group had a higher proportion of participants with poor-level
performance than normal-level performance; it was not the case for HC, SCD and MCI
groups. Nonetheless, the participant proportion with poor-level performance tended to
gradually increase from the HC, SCD, MCI to AD groups. Figure 1b shows that both
HC and SCD groups exhibited a lower proportion of participants with poor-level
performance scores on the auditory test while both patient groups evidenced the reverse
picture.
(INSERT FIGURE 1 HERE)
NEUROCOGNITIVE PERFORMANCES
Analysis of variance showed a main effect of group on delayed recall (F(3, 81) =
7.422, p < .001, partial eta squared = .216) and immediate recall (F(3, 81) = 4.831, p
= .004, partial eta squared = .152) measures after controlling age and the FSIQ.
However, no significant main effect of group was found in executive function measure
(F(3, 81) = .157, p = .925, partial eta squared = .152). Independent-samples t-tests
showed no significant difference between HC and SCD in all three neurocognitive
measures. Detailed information was in Table 5.
(INSERT TABLE 5 HERE)
FOK JUDGMENT AND NEUROCOGNITIVE PERFORMANCES
In order to examine the relationship between FOK judgment and neurocognitive
performance, correlations were calculated (see Table 6). Previous analyses revealed the
misses on the FOK “yes” judgment was sensitive in distinguishing HC from other
groups. Thus, special attention was paid on the relationships between misses on the
FOK “yes” judgment and three neurocognitive measures. Pearson’s r correlation was
performed. However, the relationship with neurocognitive measures did not examine in
the auditory-based FOK performance due to violation to the assumption of Pearson’s.
The misses on the “yes” judgment was negatively correlated with executive
function in both HC (r(30) = -.370, p = .044) and SCD (r(33) = -.420, p = .015); no
correlation was found in MCI (r(18) = .118, p = .641) and AD (r(7) = -.347, p = .445).
In addition to executive function, the misses was also negatively correlated with
immediate recall in HC (r(30) = -.572, p = .001). Contrary to the results of Souchay et
al. (2002), no correlation with memory score was found in all four groups.
Since literature has suggested that the aging-related decline of executive function
and episodic memory behaving similarly and being strongly correlated to each other
(McCabe, Roediger, McDaniel, Balota, & Hambrick, 2010), further correlations were
done to examine the relationship between executive function and other two measures.
Pearson’s r correlations showed that executive function measure was positively
correlated with immediate recall measure in both HC (r(30) = .782, p < .001) and SCD
(r(33) = .453, p = .008). A z-test was conducted (Eid, Gollwitzer, & Schmitt, 2017)
comparing the correlations in SCD and HC. The result was statistically significant (z =
2.119, p = .017, one-tailed) that the HC group showed a stronger correlation between
executive function and immediate recall when compared to SCD. Positive correlations
between executive function and delayed recall were also found in both HC (r(30) = .694,
p < .001) and SCD (r(33) = .409, p = .018). However, no significant correlation difference was reported (z = 1.588, p = .056, one-tailed).
(INSERT TABLE 6 HERE).
DISCUSSION
The present study examined memory monitoring performance in individuals with
SCD by applying the FOK paradigm in episodic memory tests, which examined
whether individuals with SCD exhibit differences across different modalities.
Individuals with SCD did not exhibit differences on the overall performance of
making memory-monitoring judgment as compared to healthy elders in the present
study. This finding supports a previous study wherein individuals with SCD judged
their memory performance no worse than did healthy elders (Perrotin et al., 2012).
However, the current study revealed the difference between individuals with SCD and
healthy elders while comparing them to patients with MCI and AD. While healthy
elders consistently exhibited significantly better memory monitoring performances than
did patients across domains, individuals with SCD only excelled on the auditory-based
test. That is, despite no statistical difference was reported between healthy elders and
individuals with SCD, our results also demonstrated insignificant differences between
individuals with SCD and patients with MCI and AD on visual-based test. A possible
explanation for the aforementioned results is that the subtle cognitive changes in
individuals with SCD were compensated for by other neurocognitive mechanisms (Erk
et al., 2011), leading to a decline that was not detectable when compared to healthy
elders (Koppara et al., 2015). This is in line with the cognitive decline depicted in the
study of Jessen et al. (2014); the slope of cognitive decline did not steeply drop during
the preclinical phase. In other words, our finding might suggest individuals with SCD
lying at the intermediate position between healthy elders and patients with MCI, as
Figure 1a showed a gradually increasing trend in the proportion of poor performance
from healthy elders, individuals with SCD, to pathological patients. Moreover, a recent
study has addressed the relationship between SCD and MCI from a different perspective.
It stated that the boundary between MCI and SCD is artificial in nature, and thus the
issue requires further study establishing an optimal distinction (Molinuevo et al., 2017).
Our results support the idea that auditory-based tests are better in the context of
detecting episodic memory deficits (Albert et al., 2011; Mortamais et al., 2017).
However, our discoveries in the visual-based test contradict previous findings of the
picture superiority effect in patients with AD and MCI (Ally, 2012; Ally et al., 2009;
Embree, Budson, & Ally, 2012). The reasons might be multifold. First, the visual
stimuli we used in this study were highly abstract, without concrete general concept that
was familiar to participants. The figure placed a high demand on information processing
(Shin, Park, Park, Seol, & Kwon, 2006), differing from concrete pictures used in
previous studies. Therefore, instead of an unequal performance caused by test modality,
it is possible that the difference was created by the level of abstraction embodied in the
information (Vallet et al., 2016). Recent research has also indicated that patients with
mild AD exhibit a relatively intact cued performance when the cues are focused on
distinctive conceptual information related to the target item (Deason, Hussey, Flannery,
& Ally, 2015). Second, our study mainly focused on the accuracy of monitoring
memory prospectively in relation to subsequent recognition. Despite a previous study
reporting that patients with MCI demonstrate a coherent performance on rating their
confidence and recognizing presented picture is new or old (Embree et al., 2012), our
results from the comparison with healthy elders provide evidence that MCI patients’
ability to deal with visual items is not superior than auditory item at memory monitoring.
Third, the tests selected for the FOK paradigm might have been of varying levels from
their cognitive substrates to test procedures. For example, RCFT requires attentive
learning during encoding phase (Shin et al., 2006) whereas Word list subtest of
WMS-III uses semantically-associated learning during encoding (Chang et al., 2018).
Thus, these memory tests require different cognitive abilities while processing provided
stimuli. Moreover, it is unlikely that these tests were comparable given the fact that they
use different approaches to measure the memory performance other than visual versus
auditory stimulus difference only. Therefore, the discrepant results between our study
and previous literature might need further studies to clarify given the possibility that
FOK performances in two selected tests might actually reflect different cognitive
components.
In comparison with healthy elders, further analyses suggest a discrepant
relationship between memory monitoring and neurocognitive functions in individuals
with SCD. Unlike the finding in patients with AD (Souchay et al., 2002), executive
function was negatively correlated with the overestimation of accuracy in both healthy
elders and individuals with SCD. This finding supports previous studies that found
memory monitoring performance measured by the FOK paradigm to be associated with
executive function in aging-related decline (Isingrini et al., 2008; Souchay & Isingrini,
2004). However, a negative correlation between the immediate recall score indicating
learning functioning and the accuracy overestimation of the FOK task was only evident
in our HC group. As the learning index indicated participants’ ability to learn new
information, reflecting a partial characteristic of episodic memory (Albert et al., 2011),
our results might suggest that individuals with SCD has a tendency to less use memory
resources in proceeding memory monitoring compared to healthy elders. Such findings
appear to be in line with a recent proposal suggesting that within-person variability
across cognitive domains is more valuable in predicting late-life cognitive decline
(Salthouse & Soubelet, 2014). However, further follow-up studies on this issue are
needed.
Several limitations were noted in the current study. First, our study used a
relatively small sample size in each group, particularly the patient groups. In order to
obtain sufficient information to examine differences between groups, it is advised that
future studies involve larger sample sizes. Second, we are aware of the debate about the
influence of recruiting sites for individuals with SCD (Perrotin et al., 2017;
Rodriguez-Gomez, Abdelnour, Jessen, Valero, & Boada, 2015). Thus, information
regarding depressive mood, medical records, and judgment regarding one’s own
memory decline were collected to eliminate possible confounding variables. Third, it is
likely that our results were biased by participants’ response preference in the FOK
paradigm. In other words, all participants tended to state “yes,” firmly assured of their
following accuracy, which the base rate for “yes” judgment was enlarged enough to
show variation. However, this tendency was observed across groups, and no significant
difference was reported between groups. Thus, this is unlikely to have led to the final
results. Another similar statistical limitation was from our data distribution. That is, the
selected auditory episodic memory test had items with high familiarity or high semantic
association to help memorizing. According to our data, it is clear that cognitively
normal participants almost excelled in every trial in the auditory-based test, leading to a
violation of the parametric assumption. This makes data analysis problematic as some
useful kits could not be performed. Fourth, our study requires extra caution while
explaining the FOK test results between SCD and HC given the fact that no direct
differences were observed. It is possible that the insignificance, other than the gradual
decline during the AD pathology, is rooted from the visual stimulus item lacking in
sensitivity differentiating SCD from HC. Future study on this issue is merited.
However, to our knowledge, the current study is the first to use an objective
method to examine how individuals with SCD monitor their memory. Despite the fact
that there was no significant difference in comparison with healthy elders, our results
suggest that individuals with SCD are at the intermediate position between normal
aging and pathological aging. This finding is in line with a recent hypothesis depicting
AD as a continuum (Jack et al., 2018). Moreover, a recent study simulated the AD
disease progression through data-driven model and found multifactorial interactions,
rather than linear cascade event, are responsible for the progression (Veitch et al., In
press). In addition, out study might provide an objective measure targeting individuals
with SCD who might be in risk for pathological change. Future follow-up study on this
issue is thus needed.
REFERENCES
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., . . .
Phelps, C. H. (2011). The diagnosis of mild cognitive impairment due to
Alzheimer's disease: Recommendations from the National Institute on
Aging-Alzheimer's Association workgroups on diagnostic guidelines for
Alzheimer's disease. Alzheimers Dement, 7, 270-279.
doi:10.1016/j.jalz.2011.03.008
Ally, B. A. (2012). Using pictures and words to understand recognition memory
deterioration in amnestic mild cognitive impairment and Alzheimer's disease: A
review. Current Neurology and Neuroscience Reports, 12, 687-694.
doi:10.1007/s11910-012-0310-7
Ally, B. A., Gold, C. A., & Budson, A. E. (2009). The picture superiority effect in
patients with Alzheimer's disease and mild cognitive impairment.
Neuropsychologia, 47, 595-598. doi:10.1016/j.neuropsychologia.2008.10.010 Ally, B. A., Hussey, E. P., Ko, P. C., & Molitor, R. J. (2013). Pattern separation and
pattern completion in Alzheimer's disease: Evidence of rapid forgetting in
amnestic mild cognitive impairment. Hippocampus, 23, 1246-1258.
doi:10.1002/hipo.22162
Amariglio, R. E., Mormino, E. C., Pietras, A. C., Marshall, G. A., Vannini, P., Johnson,
K. A., . . . Rentz, D. M. (2015). Subjective cognitive concerns, amyloid-beta,
and neurodegeneration in clinically normal elderly. Neurology, 85, 56-62.
doi:10.1212/WNL.0000000000001712
Backman, L., Jones, S., Berger, A.-K., Laukka, E. J., & Small, B. J. (2004). Multuple
cognitive deficits during the transition to Alzheimer's Disease. Journal of
Internal Medicine, 256, 195-204.
Backman, L., Jones, S., Berger, A.-K., Laukka, E. J., & Small, B. J. (2005). Cognitive
impairment in preclinical Alzheimer's Disease: A meta-analysis.
Neuropsychology, 19, 520-531. doi:10.1037/0894-4105.19.4.520.supp
Bender, S., Bluschke, A., Dippel, G., Rupp, A., Weisbrod, M., & Thomas, C. (2014).
Auditory post-processing in a passive listening task is deficient in Alzheimer's
disease. Clinical Neurophysiology, 125, 53-62. doi:10.1016/j.clinph.2013.05.026
Bisiacchi, P. S., Borella, E., Bergamaschi, S., Carretti, B., & Mondini, S. (2008).
Interplay between memory and executive functions in normal and pathological
aging. Journal of Clinical and Experimental Neuropsychology, 30, 723-733.
doi:10.1080/13803390701689587
Bisiacchi, P. S., Tarantino, V., & Ciccola, A. (2008). Aging and prospective memory:
The role of working memory and monitoring processes. Aging Clinical and
Experimental Research, 20, 569-577.
Chang, H. T., Chen, T. F., Cheng, T. W., Lai, Y. M., & Hua, M. S. (2018). Arbitrary
and semantic associations in subjective memory impairment and amnestic mild
cognitive impairment among Taiwanese individuals: A cross-sectional study.
Journal Formosan Medical Association, 117, 427-433.
doi:10.1016/j.jfma.2017.05.014
Chen, H.-Y., Hua, M.-S., Zhu, J.-J., & Chen, Y.-H. J. (2008). Selection of factor-based
WAIS-III tetrads in the Taiwan standardization sample: A Guide to Clinical
Practice. Chinese Journal of Psychology, 50, 91-109.
Chua, E. F., Schacter, D. L., Rand-Giovannetti, E., & Sperling, R. A. (2006).
Understanding metamemory: Neural correlates of the cognitive process and
subjective level of confidence in recognition memory. Neuroimage, 29,
1150-1160. doi:10.1016/j.neuroimage.2005.09.058
Chua, E. F., Schacter, D. L., & Sperling, R. A. (2009). Neural correlates of
metamemory: A comparison of feeling-of-knowing and retrospective confidence
judgments. Journal of Cognitive Neuroscience, 21, 1751-1765.
doi:10.1162/jocn.2009.21123
Cosentino, S. (2014). Metacognition in Alzheimer's Disease. In S. M. Fleming & C. D.
Frith (Eds.), The cognitive neuroscience of metacognition (pp. 389-407). New
York, NY, US: Springer-Verlag Publishing.
doi.org/10.1007/978-3-642-45190-4_17
Deason, R. G., Hussey, E. P., Flannery, S., & Ally, B. A. (2015). Preserved conceptual
implicit memory for pictures in patients with Alzheimer's disease. Brain and
Cognition, 99, 112-117. doi:10.1016/j.bandc.2015.07.008
Dillen, K. N. H., Jacobs, H. I. L., Kukolja, J., Richter, N., von Reutern, B., Onur, O.
A., . . . Fink, G. R. (2017). Functional disintegration of the default mode
network in prodromal Alzheimer's Disease. Journal of Alzheimer’s Disease, 59,
169-187. doi:10.3233/JAD-161120
Dodson, C. S., Spaniol, M., O'Connor, M. K., Deason, R. G., Ally, B. A., & Budson, A.
E. (2011). Alzheimer's disease and memory-monitoring impairment:
Alzheimer's patients show a monitoring deficit that is greater than their accuracy
deficit. Neuropsychologia, 49, 2609-2618.
doi:10.1016/j.neuropsychologia.2011.05.008
Dubois, B., Feldman, H. H., Jacova, C., Cummings, J. L., Dekosky, S. T.,
Barberger-Gateau, P., . . . Scheltens, P. (2010). Revising the definition of
Alzheimer's disease: A new lexicon. The Lancet Neurology, 9, 1118-1127.
doi:10.1016/S1474-4422(10)70223-4
Dubois, B., Feldman, H. H., Jacova, C., DeKosky, S. T., Barberger-Gateau, P.,
Cummings, J., . . . Scheltens, P. (2007). Research criteria for the diagnosis of
Alzheimer's disease: Revising the NINCDS–ADRDA criteria. The Lancet
Neurology, 6, 734-746. doi:10.1016/s1474-4422(07)70178-3
Embree, L. M., Budson, A. E., & Ally, B. A. (2012). Memorial familiarity remains
intact for pictures but not for words in patients with amnestic mild cognitive
impairment. Neuropsychologia, 50, 2333-2340.
doi:10.1016/j.neuropsychologia.2012.06.001
Erk, S., Spottke, A., Meisen, A., Wagner, M., Walter, H., & Jessen, F. (2011). Evidence
of neuronal compensation during episodic memory in subjective memory
impairment. Archives Of General Psychiatry, 68, 845-852.
Galeone, F., Pappalardo, S., Chieffi, S., Iavarone, A., & Carlomagno, S. (2011).
Anosognosia for memory deficit in amnestic mild cognitive impairment and
Alzheimer's disease. International Journal of Geriatric Psychiatry, 26, 695-701.
doi:10.1002/gps.2583
Gallo, D. A., Cramer, S. J., Wong, J. T., & Bennett, D. A. (2012). Alzheimer's disease
can spare local metacognition despite global anosognosia: Revisiting the
confidence-accuracy relationship in episodic memory. Neuropsychologia, 50,
2356-2364. doi:10.1016/j.neuropsychologia.2012.06.005
Genon, S., Bahri, M. A., Collette, F., Angel, L., d'Argembeau, A., Clarys, D., . . . Bastin,
C. (2014). Cognitive and neuroimaging evidence of impaired interaction
between self and memory in Alzheimer's disease. Cortex, 51, 11-24.
doi:10.1016/j.cortex.2013.06.009
Golden, H. L., Agustus, J. L., Goll, J. C., Downey, L. E., Mummery, C. J., Schott, J.
M., . . . Warren, J. D. (2015). Functional neuroanatomy of auditory scene
analysis in Alzheimer's disease. Neuroimage: Clinical, 7, 699-708.
doi:10.1016/j.nicl.2015.02.019
Golden, H. L., Agustus, J. L., Nicholas, J. M., Schott, J. M., Crutch, S. J., Mancini, L.,
& Warren, J. D. (2016). Functional neuroanatomy of spatial sound processing in
Alzheimer's disease. Neurobiology of Aging, 39, 154-164.
doi:10.1016/j.neurobiolaging.2015.12.006
Hachinski, V. C., Iliff, L. D., Zilhka, E., Du Boulay, G. H., McAllister, V. L., Marshall,
J., . . . Symon, L. (1975). Cerebral blood flow in dementia. Archives of
Neurology, 32, 632-637.
Hao, J., Li, K., Li, K., Zhang, D., Wang, W., Yang, Y., . . . Zhou, X. (2005). Visual
attention deficits in Alzheimer's disease: an fMRI study. Neuroscience Letters,
385, 18-23. doi:10.1016/j.neulet.2005.05.028
Hart, J. T. (1965). Memory and the feeling-of-knowing experience. Journal of
Educational Psychology, 56, 208-216.
Hua, M., Chang, B., Lin, K., Yang, J., Lu, S., & Chen, H. (2005). Wechsler Memory
Scale Third Edition (WMS-III) Manual for Taiwan. Taipei, Taiwan: The Chinese
Behavioral Science Corporation.
Huo, L., Li, R., Wang, P., Zheng, Z., & Li, J. (2018). The default mode network
supports episodic memory in cognitively unimpaired elderly individuals:
Different contributions to immediate recall and delayed recall. Frontiers in
Aging Neuroscience, 10, 6. doi:10.3389/fnagi.2018.00006
Isingrini, M., Perrotin, A., & Souchay, C. (2008). Aging, metamemory regulation and
executive functioning. Progress in Brain Research, 169, 377-392.
doi:10.1016/S0079-6123(07)00024-6
Jack, C. R., Jr., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S.
B., . . . Contributors. (2018). NIA-AA Research Framework: Toward a
biological definition of Alzheimer's disease. Alzheimer’s & Dementia, 14,
535-562. doi:10.1016/j.jalz.2018.02.018
Jack, C. R., Jr., Knopman, D. S., Jagust, W. J., Petersen, R. C., Weiner, M. W., Aisen, P.
S., . . . Trojanowski, J. Q. (2013). Tracking pathophysiological processes in
Alzheimer's disease: An updated hypothetical model of dynamic biomarkers.
The Lancet Neurology, 12, 207-216. doi:10.1016/S1474-4422(12)70291-0
Jessen, F., Amariglio, R. E., van Boxtel, M., Breteler, M., Ceccaldi, M., Chetelat, G., . . .
Subjective Cognitive Decline Initiative Working, G. (2014). A conceptual
framework for research on subjective cognitive decline in preclinical
Alzheimer's disease. Alzheimer’s & Dementia, 10, 844-852.
doi:10.1016/j.jalz.2014.01.001
Koppara, A., Wagner, M., Lange, C., Ernst, A., Wiese, B., Konig, H. H., . . . Jessen, F.
(2015). Cognitive performance before and after the onset of subjective cognitive
decline in old age. Alzheimer’s & Dementia (Amsterdam), 1, 194-205.
doi:10.1016/j.dadm.2015.02.005
Kurimoto, R., Ishii, R., Canuet, L., Ikezawa, K., Iwase, M., Azechi, M., . . . Takeda, M.
(2012). Induced oscillatory responses during the Sternberg's visual memory task
in patients with Alzheimer's disease and mild cognitive impairment. Neuroimage,
59, 4132-4140. doi:10.1016/j.neuroimage.2011.10.061
Liao, Y., Yeh, T., Yang, Y., Lu, F., Chang, C., Ko, H., & Lo, C. J. T. J. o. P. (2004).
Reliability and validation of the Taiwan geriatric depression scale. Taiwanese
Journal of Psychiatry, 18, 30-41.
Liu, C., Wang, S., Teng, E., Fuh, J., Lin, C., Lin, K., . . . Yang, Y. J. P. M. (1997).
Depressive disorders among older residents in a Chinese rural community.
Psychological Medicine, 27, 943-949.
McCabe, D. P., Roediger, H. L., McDaniel, M. A., Balota, D. A., & Hambrick, D. Z.
(2010). The relationship between working memory capacity and executive
functioning: Evidence for a common executive attention construct.
Neuropsychology, 24, 222-243. doi:10.1037/a0017619
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr., Kawas,
C. H., . . . Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer's
disease: Recommendations from the National Institute on Aging-Alzheimer's
Association workgroups on diagnostic guidelines for Alzheimer's disease.
Alzheimer’s & Dementia, 7, 263-269. doi:10.1016/j.jalz.2011.03.005
Meyers, J. E., & Meyers, K. R. (1995). Rey Complex Figure Test and recognition trial
professional manual. Odessa, FL, US: Psychological Assessment Resources.
Molinuevo, J. L., Rabin, L. A., Amariglio, R., Buckley, R., Dubois, B., Ellis, K. A., . . .
Subjective Cognitive Decline Initiative Working, G. (2017). Implementation of
subjective cognitive decline criteria in research studies. Alzheimer’s & Demeniat,
13, 296-311. doi:10.1016/j.jalz.2016.09.012
Mortamais, M., Ash, J. A., Harrison, J., Kaye, J., Kramer, J., Randolph, C., . . . Ritchie,
K. (2017). Detecting cognitive changes in preclinical Alzheimer's disease: A
review of its feasibility. Alzheimer’s & Dementia, 13, 468-492.
doi:10.1016/j.jalz.2016.06.2365
Nellessen, N., Rottschy, C., Eickhoff, S. B., Ketteler, S. T., Kuhn, H., Shah, N. J., . . .
Reetz, K. (2015). Specific and disease stage-dependent episodic memory-related
brain activation patterns in Alzheimer's disease: A coordinate-based
meta-analysis. Brain Structure and Function, 220, 1555-1571.
doi:10.1007/s00429-014-0744-6
Nelson, P. T., Alafuzoff, I., Bigio, E. H., Bouras, C., Braak, H., Cairns, N. J., . . . Beach,
T. G. (2012). Correlation of Alzheimer disease neuropathologic changes with
cognitive status: a review of the literature. Journal of Neuropathology &
Experimental Neurology, 71, 362-381. doi:10.1097/NEN.0b013e31825018f7
Nelson, T. O. (1990). Metamemory: A theoretical framework and new findings. In B. H.
Ross (Ed.) Psychology of learning and motivation (Vol. 26, pp. 125-173).
Cambridge, MA, US: Academic Press.
Perrotin, A., Belleville, S., & Isingrini, M. (2007). Metamemory monitoring in mild
cognitive impairment: Evidence of a less accurate episodic feeling-of-knowing.
Neuropsychologia, 45, 2811-2826. doi:10.1016/j.neuropsychologia.2007.05.003
Perrotin, A., La Joie, R., de La Sayette, V., Barre, L., Mezenge, F., Mutlu, J., . . .
Chetelat, G. (2017). Subjective cognitive decline in cognitively normal elders
from the community or from a memory clinic: Differential affective and imaging
correlates. Alzheimer’s & Dementia, 13, 550-560.
doi:10.1016/j.jalz.2016.08.011
Perrotin, A., Mormino, E. C., Madison, C. M., Hayenga, A. O., & Jagust, W. J. (2012).
Subjective cognition and amyloid deposition imaging: A Pittsburgh Compound
B positron emission tomography study in normal elderly individuals. Archives of
Neurology, 69, 223-229. doi:10.1001/archneurol.2011.666
Raichle, M. E. (2015). The brain's default mode network. Annual Review of
Neuroscience, 38, 433-447. doi:10.1146/annurev-neuro-071013-014030
Reisberg, B., Shulman, M. B., Torossian, C., Leng, L., & Zhu, W. (2010). Outcome
over seven years of healthy adults with and without subjective cognitive
impairment. Alzheimer’s & Dementia, 6, 11-24. doi:10.1016/j.jalz.2009.10.002
Rodriguez-Gomez, O., Abdelnour, C., Jessen, F., Valero, S., & Boada, M. (2015).
Influence of sampling and recruitment methods in studies of subjective cognitive
decline. Journal of Alzheimer’s Disease, 48, 99-107. doi:10.3233/JAD-150189
Rosen, W. G., Terry, R. D., Fuld, P. A., Katzman, R., & Peck, A. (1980). Pathological
verification of ischemic score in differentiation of dementias. Annals of
Neurology, 7, 486-488. doi:10.1002/ana.410070516
Salthouse, T. A., & Soubelet, A. (2014). Heterogeneous ability profiles may be a unique
indicator of impending cognitive decline. Neuropsychology, 28, 812-818.
doi:10.1037/neu0000100
Schraw, G. (1995). Measures of feeling of knowing accuracy: A new look at an old
problem. Applied Cognitive Psychology, 9, 321-332.
Sheikh, J. I., Yesavage, J. A. J. C. G. T. J. o. A., & Health, M. (1986). Geriatric
Depression Scale (GDS): Recent evidence and development of a shorter
version. Clinical Gerontologist: The Journal of Aging and Mental Health, 5,
165-173.
Sheline, Y. I., Raichle, M. E., Snyder, A. Z., Morris, J. C., Head, D., Wang, S., &
Mintun, M. A. (2010). Amyloid plaques disrupt resting state default mode
network connectivity in cognitively normal elderly. Biological Psychiatry, 67,
584-587. doi:10.1016/j.biopsych.2009.08.024
Shin, M. S., Park, S. Y., Park, S. R., Seol, S. H., & Kwon, J. S. (2006). Clinical and
empirical applications of the Rey-Osterrieth Complex Figure Test. Nature
Protocols, 1, 892-899. doi:10.1038/nprot.2006.115
Slavin, M. J., Sachdev, P. S., Kochan, N. A., Woolf, C., Crawford, J. D., Giskes, K., . . .
Brodaty, H. (2015). Predicting cognitive, functional, and diagnostic change over
4 years using baseline subjective cognitive complaints in the Sydney Memory
and Ageing Study. The American Journal of Geriatric Psychiatry, 23, 906-914.
doi:10.1016/j.jagp.2014.09.001
Souchay, C. (2007). Metamemory in Alzheimer's Disease. Cortex, 43, 987-1003.
doi:10.1016/s0010-9452(08)70696-8
Souchay, C., & Isingrini, M. (2004). Age related differences in metacognitive control:
Role of executive functioning. Brain and Cognition, 56, 89-99.
doi:10.1016/j.bandc.2004.06.002
Souchay, C., Isingrini, M., & Espagnet, L. (2000). Aging, episodic memory
feeling-of-knowing, and frontal functioning. Neuropsychology, 14, 299-309.
doi:10.1037/0894-4105.14.2.299
Souchay, C., Isingrini, M., & Gil, R. (2002). Alzheimer's Disease and
Feeling-of-Knowing in episodic memory. Neuropsychologia, 1442, 1-11.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., . . .
Phelps, C. H. (2011). Toward defining the preclinical stages of Alzheimer's
disease: Recommendations from the National Institute on Aging-Alzheimer's
Association workgroups on diagnostic guidelines for Alzheimer's disease.
Alzheimer’s & Dementia, 7, 280-292. doi:10.1016/j.jalz.2011.03.003
Swinford, C. G., Risacher, S. L., Charil, A., Schwarz, A. J., & Saykin, A. J. (2018).
Memory concerns in the early Alzheimer's disease prodrome: Regional
association with tau deposition. Alzheimer’s & Dementia (Amsterdam), 10,
322-331. doi:10.1016/j.dadm.2018.03.001
Vallet, G. T., Rouleau, I., Benoit, S., Langlois, R., Barbeau, E. J., & Joubert, S. (2016).
Alzheimer's disease and memory strength: Gradual decline of memory traces as
a function of their strength. Journal of Clinical and Experimental
Neuropsychology, 38, 648-660. doi:10.1080/13803395.2016.1147530
van Oijen, M., de Jong, F. J., Hofman, A., Koudstaal, P. J., & Breteler, M. M. (2007).
Subjective memory complaints, education, and risk of Alzheimer's disease.
Alzheimer’s & Dementia, 3, 92-97. doi:10.1016/j.jalz.2007.01.011
Veitch, D. P., Weiner, M. W., Aisen, P. S., Beckett, L. A., Cairns, N. J., Green, R.
C., . . . Alzheimer's Disease Neuroimaging, I. (In press). Understanding disease
progression and improving Alzheimer's disease clinical trials: Recent highlights
from the Alzheimer's Disease Neuroimaging Initiative. Alzheimer’s & Dementia.
doi:10.1016/j.jalz.2018.08.005
Villemagne, V. L., Burnham, S., Bourgeat, P., Brown, B., Ellis, K. A., Salvado, O., . . .
Lifestyle Research, G. (2013). Amyloid beta deposition, neurodegeneration, and
cognitive decline in sporadic Alzheimer's disease: A prospective cohort study.
The Lancet Neurology, 12, 357-367. doi:10.1016/S1474-4422(13)70044-9
Wang, P., Zhou, B., Yao, H., Zhan, Y., Zhang, Z., Cui, Y., . . . Jiang, T. (2015).
Aberrant intra- and inter-network connectivity architectures in Alzheimer's
disease and mild cognitive impairment. Scientific Reports, 5, 14824.
doi:10.1038/srep14824
Wang, Y.-L., Hua, M.-S., Chang, W.-N., & Lu, C.-H. (2007). Episodic memory
Feeling-of-Knowing in early demented Ppatients with Alzheimer's Disease.
Chinese Journal of Psychology, 49, 365-382.
Weintraub, S., Wicklund, A. H., & Salmon, D. P. (2012). The neuropsychological
profile of Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 2,
a006171. doi:10.1101/cshperspect.a006171
Youden, W. J. (1950). Index for rating diagnostic tests. Cancer, 3, 32-35.
Zamboni, G., Drazich, E., McCulloch, E., Filippini, N., Mackay, C. E., Jenkinson,
M., . . . Wilcock, G. K. (2013). Neuroanatomy of impaired self-awareness in
Alzheimer's disease and mild cognitive impairment. Cortex, 49, 668-678.
doi:10.1016/j.cortex.2012.04.011
TABLES
Table 1
Demographic Characteristics of Four Groups
Variables HC
(N = 30)
SCD (N = 33)
MCI (N = 18)
AD (N = 7)
Female, No. (%) 24 (80) 20 (61) 9 (50) 3 (43)
Age, Mean (SD)* 63.37 (7.55) 66.09 (6.44) 69.72 (7.35)a 73.43 (4.65)a Education, Mean (SD) 14.20 (3.25) 14.61 (3.36) 12.5 (2.81) 14.14 (2.73) Education,
Median (Range) 15 (6-20) 16 (6-20) 12 (6-18) 16 (9-16) Estimated full scaled IQ,
Mean (SD)
119.27 (12.91) 118.06 (11.6) 111.11 (9.18) 107.43 (11.75)
Estimated full scaled IQ,
Median (Range)* 121.5 (49) 120 (50) 114.5 (29) 109 (35) MMSE, Mean (SD) 28.73 (1.41) 28.82 (1.1) 27.11 (2.22) 23.57 (1.51) MMSE, Median
(Range)*
29 (24-30) 29 (26-30) 27.5 (22-30) 23 (22-26)
Logical Memory,
Mean (SD)* 13.93 (3.07) 13.36 (2.52) 9.17 (3.29)a,b 6.75 (.957)a,b GDS, Mean (SD) 1.03 (1.19) 2.12 (1.56) 1.33 (1.03) .86 (1.47)
GDS, Median (Range) 1 (0-5) 2 (0-5) 1 (0-4) 0 (0-4)
Note. *significant difference between groups; a significantly different from HC; b significantly different from SCD.
Table 2
The data array and the equation for the Hamann Index Conditions Recognition performance
Correct Incorrect
FOK ‘Yes’ judgment a b
FOK ‘No’ judgment c d
Hamann Index = [(a+d)-(b+c)]/[(a+d)+(b+c)]
53
) of FOK Judgment and Episodic Memory Performance on Visual- and Auditory-Based Episodic Memory Performances HC (N = 30) SCD (N = 33) MCI (N = 18) AD (N = 7) Visual Auditory Visual Auditory Visual Auditory Visual Auditory 95.83 (62.5-100) 100 (62.5-100) 95.83 (54.17-100) 100 (70.83-100) 93.75 (58.33-100) 95.83 (33.33-100) 70.83 (37.5-100)
75 (16.67-100) 4.17 (0-37.5)
0.00 (0-37.5) 4.17 (0-45.83) 0.00 (0-29.17) 6.25 (0-41.67) 4.17 (0-66.67) 29.17 (0-62.5)
25 (0-83.33) 79.17 (50-95.83)
95.83 (62.5-100) 79.17 (45.83-100) 95.83 (66.67-100) 66.67 (37.5-87.5)a75.00a,b (33.33-100) 41.67a,b (25-66.67)
62.50a,b (12.5-79.17) 12.50 (0-33.33)
0.00 (0-16.67) 16.67 (0-37.5) 0.00 (0 -29.17) 20.83a (8.33-41.67) 18.75a,b (0-50) 33.33a (12.5-45.83)
20.83a,b (0-33.33) 4.17 (0-29.17)
0.00 (0-33.33) 0.00 (0-29.17) 0.00 (0 -25.00) 2.08 (0 -29.17) 0.00 (0-50) 20.83 (0-29.17)
8.33 (0-50) 0.00 (0-8.33)
0.00 (0-4.17) 0.00 (0-20.83) 0.00 (0 -12.50) 4.17 (0 -16.67) 0.00 (0-16.67) 8.33 (0-33.33) 8.33a (0-33.33)
54
(Continued) ) of FOK Judgment and Episodic Memory Performance on Visual- and Auditory-Based Episodic Memory Performances HC (N = 30) SCD (N = 33) MCI (N = 18) AD (N = 7) Visual Auditory Visual Auditory Visual Auditory Visual Auditory 0.63 (.17-.92) 0.96 (.33-1) 0.58 (.25-1) 0.92 (.42-1) 0.42a (.08-.75) 0.50a,b (0-1) 0.08a,b (-.08-.33)
0.33a,b (-.08-.58) RCFT Word list RCFT Word list RCFT Word list RCFT Word list 9.74 (4.33)
13.23 (2.10) 9.91 (3.90) 11.94 (1.97) 6.13 (3.58) 8.50 (2.09) 1.99 (1.33)
7.00 (0.00) 10.12 (3.31)
12.40 (1.45) 8.32 (3.25) 11.64 (2.42) 5.20 (3.67) 7.61 (3.13) 1.30 (1.02)
5.29 (1.60) . Median (Range) was reported if not specified. *FOK performance showed significant difference between groups; a significantly different b significantly different from SCD.
Table 4
Results of Subcomponents in FOK Performance
Variables FOK ‘yes’ judgment FOK ‘no’ judgment
Visual Auditory Visual Auditory
Hits
H 26.631 37.345 2.182 8.787 p < .001** < .001** .535 .032*
d 1.251 1.663 .198 .544
Misses
H 16.261 24.888 9.4 12.502 p .001** < .001** .024* .006**
d .866 1.187 .574 .714 Note. * significant at the level of p < .05; ** significant as the level of p < .01.
Table 5
Neurocognitive Performances of Four Groups
Performances HC
(N = 30)
SCD (N = 33)
MCI (N = 18)
AD (N = 7) Executive Function 9.73
(2.26)
9.42 (1.67)
8.36 (1.53)
8 (1.68) Matrix Reasoning 13.57
(3.17)
13.03 (2.663)
12.06 (3.019)
11.29 (3.352) Backward Digit Span 5.9
(1.626)
5.82 (1.685)
4.67 (.97)
4.71 (.951)
Immediate recall 12.6
(2.9)
11.89 (2.46)
9.58 (2.79)
6.58 (1.99) Visual Reproduction I 12.6
(2.472)
11.67 (2.847)
9.78 (2.777)
7 (3.098)
Word-Pairs I 12.6
(3.892)
12.12 (3.11)
9.39 (3.363)
6.17 (1.941)
Delayed recall 12.05
(2.91)
11.45 (2.19)
7.89 (2.33)
7.42 (3.75) Visual Reproduction II 11.47
(3.246)
10.52 (2.83)
7.11 (1.967)
9.5 (7.609)
Word-Pair II 12.63
(3.499)
12.39 (2.989)
8.63 (3.162)
5.33 (.516) Note. Mean (SD) was reported if not specified.
Table 6
Pearson’s r correlation between the misses (%) in the “yes” judgment of visual FOK test and neurocognitive measures
Variables HC
(N = 30)
SCD (N = 33)
MCI (N = 18)
AD (N = 7) Executive function -.370* -.420* .118 .347
Learning -.572** -.292 -.150 -.368
Memory -.311 -.225 -.134 .464
Note. *significant at the level of p < .05; ** significant at the level of p < .01.